Abstract
Hypertrophic olivary degeneration is a result of a primary lesion damaging the dento-rubro-olivary pathway. It is a transynaptic form of degeneration and is unique, causing hypertrophy rather than atrophy of the inferior olivary nucleus. We report a case of bilateral hypertrophic olivary degeneration following surgical excision of a posterior fossa epidermoid cyst and review the relevant literature.
Case report
A 57-year-old man presented to the neurology outpatient clinic with a six-month history of slurred speech. He also noted that his handwriting had become unsteady. His medical history was unremarkable. At presentation, the positive findings were dysarthria, diminished right finger–nose co-ordination and an unsteady heel–toe gait, with stumbling to the left.
Initial MRI (Figure 1) revealed a large posterior fossa mass that showed high signal on T2 and diffusion sequences, with incomplete nulling of cerebrospinal fluid (CSF) signal on fluid-attenuated inversion recovery (FLAIR) sequence and no contrast enhancement. This was consistent with a diagnosis of an epidermoid cyst.
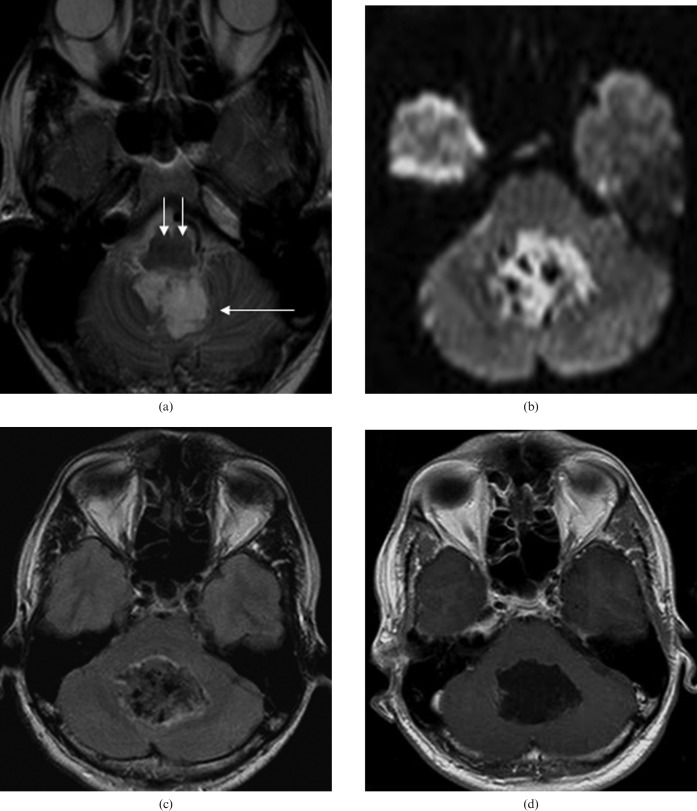
Figure 1.
Initial MRI examination reveals a large posterior fossa mass: (a), axial T2 image — the lesion is hyperintense (long white arrow) and the medulla has a normal appearance (small white arrows); (b), there is restricted diffusion; (c), there is incomplete nulling of cerebrospinal fluid signal within the lesion on fluid-attenuated inversion recovery (FLAIR) images; (d), there is no abnormal contrast enhancement. Appearances are consistent with an epidermoid cyst.
The mass was debulked via a posterior fossa craniotomy and confirmed histologically to be an epidermoid cyst. Post-operatively there was no new neurological deficit and the patient was treated by the local speech and language service for his dysarthria. Six months later he continued to demonstrate staccato speech and, following ear, nose and throat review, was found to have marked palatal myoclonus. MRI was repeated and showed abnormal high T2 signal in the anterolateral medulla bilaterally at the location of the inferior olivary nuclei (Figure 2). There was no contrast enhancement.
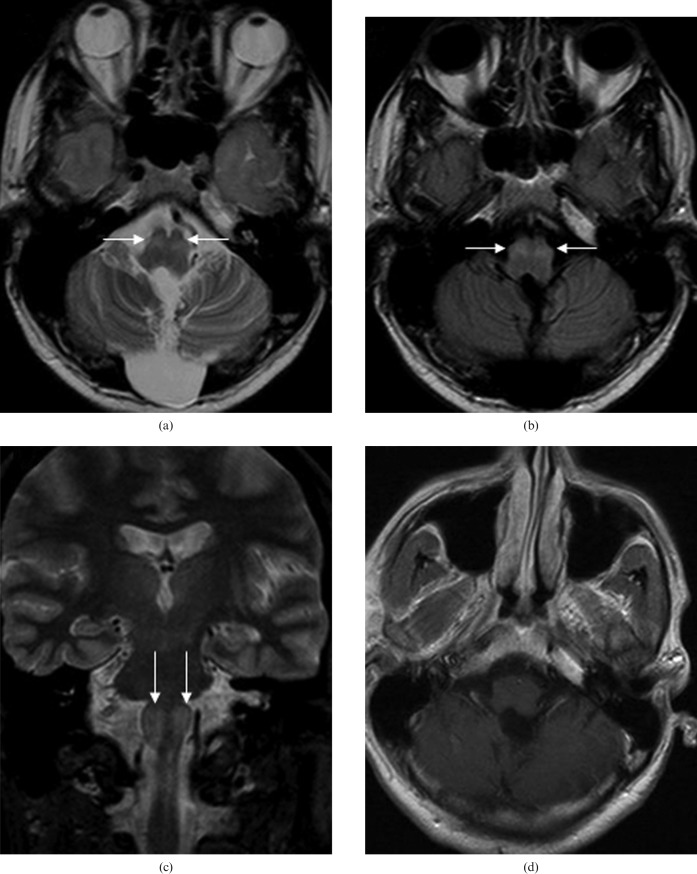
Figure 2.
MRI examination performed six months following surgery: (a), (b), (c), there is increased signal in the inferior olivary nucleus bilaterally (white arrows); (d), there is no abnormal contrast enhancement.
On follow-up imaging two years later, the abnormal signal persisted (Figure 3). Findings are consistent with bilateral hypertrophic olivary degeneration (HOD). The patient continues to receive speech therapy and has shown some improvement.
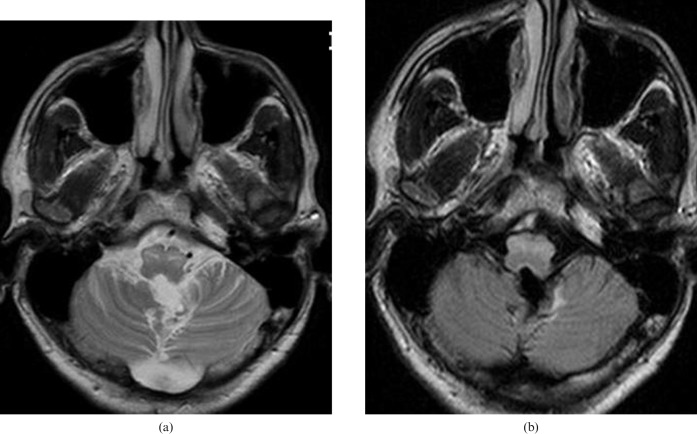
Figure 3.
Follow-up MRI performed two years after surgery. There is persistence of high signal in the inferior olivary nuclei.
Discussion
The dento-rubro-olivary pathway was described by Guillain and Mollaret [1] and is referred to as “the triangle of Guillain and Mollaret”. The triangle is defined by three anatomical structures: the dentate nucleus in the cerebellum; the contralateral red nucleus (at mid-brain level); and the contralateral inferior olivary nucleus (ION) in the medulla.
The afferent pathway to the olives originates in the dentate nucleus and travels through the superior cerebellar peduncle to enter the contralateral red nucleus — the dentatorubral tract. It then traverses downwards through the central tegmental tract to connect the red nucleus with the contralateral ION. Efferent fibres from the ION then cross superiorly through the inferior cerebellar peduncle back to the dentate nucleus — the olivocerebellar tract.
Disruption of this triangle results in three possible patterns of presentation. If the lesion is located in the tegmentum of the cerebellum and involves the central tegmental tract, degeneration occurs in the ipsilateral ION. When the primary lesion is in the dentate nucleus or the superior cerebellar peduncle, the contralateral ION is involved. This is due to decussation of the dentatorubral fibres. A paramedian lesion affecting both the central tegmental tract and the superior cerebellar peduncle results in bilateral degeneration. Although the pathway has been described as a triangle, it is the involvement of the afferent, not efferent, fibres that causes HOD.
Pathological changes within the olivary nucleus include neuronal cell body enlargement, cytoplasmic vacuolation, fibrillary gliosis, demyelination and astrocytic proliferation [2]. Kitajima et al [3] have shown how these pathological changes correlate to areas of increased T2 signal on MRI.
Goto et al [2] have documented the sequence of pathological changes in HOD. They describe six phases of pathological changes over a time frame ranging from immediate onset to several years: no olivary change; degeneration of olivary amiculum; mild olivary hypertrophy; olivary enlargement; olivary pseudohypertrophy; and olivary atrophy.
Bribamer et al [4] describe three stages of olivary changes on MRI: no olivary changes in the acute phase; increased signal on T2 and proton density images corresponding to olivary hypertrophy; and persistence of some high signal on T2 images with resolution of hypertrophy. Increased signal intensity on T2 and proton density images without olivary hypertrophy appears to occur within six months of ictus. Maximum hypertrophy of ION and signal intensity changes persist until 18 months, followed by resolution of hypertrophy and some persistent high signal within the ION [5].
Clinical presentation of HOD includes palatal myoclonus, dentatorubral (or Holmes') tremor and ocular myoclonus. Palatal myoclonus has been described as a rhythmic involuntary movement of the oropharynx, including the soft palate and uvula [6]. It develops nearly a year following initial ictus, although it does not always correlate with olivary hypertrophy. Severe myoclonus of the cervical muscles and the diaphragm has been reported and can precede palatal myoclonus. The clinical presentation reflects involvement of both the central tegmental tract and the dentatorubral pathway.
A variety of lesions involving the dentatorubro-olivary pathway have been implicated in the clinical presentation and MRI features of HOD. They include primary haemorrhage, cavernous haemangioma, infarction and trauma [7–10]. There are very few reported cases in the literature describing MRI changes in the ION following surgery of a primary lesion in the brain stem or cerebellum [11]. Most of the cases have been reported following surgery for pontine cavernous haemangioma. One recent paper describes changes in the ION following resection of a low-grade cerebellar tumour [12].
To the best of our knowledge there has been no previous report of HOD following resection of a posterior fossa epidermoid.
High signal on T2 images within the anterolateral medulla is not a specific imaging feature of HOD. It can be seen in a wide variety of lesions including demyelination, infection, inflammation and infarction. It can also be seen secondary to tumours such as astrocytoma, lymphoma and metastasis. However, signal changes confined to the olivary nucleus or nuclei (with or without enlargement of the ION), lack of contrast enhancement and the presence of an inciting lesion in the brain stem or cerebellum should point towards the diagnosis of HOD.
References
- 1.Guillain G, Mollaret P. Deux cas de myoclonies synchones et rhythms velopharyngo-laryngo-oculo-diaphragmatiques. Rev Neurol 1931:545–66 [Google Scholar]
- 2.Goto N, Kaneko M. Olivary enlargement: chronological and morphometric analyses. Acta Neuropathol 1981;54:275–82 [DOI] [PubMed] [Google Scholar]
- 3.Kitajima M, Korogi Y, Shimomura O, Sakamoto Y, Hirai T, Miyayama H, et al. Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology 1994;192:539–43 [DOI] [PubMed] [Google Scholar]
- 4.Birbamer G, Buchberger W, Felber S, Aichner F. MRI appearance of hypertrophic olivary degeneration: temporal relationships. AJNR Am J Neuroradiol 1992;13:1501–3 [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal M, Versnick E, Tuite P, Saint Cyr J, Kucharczyk W, Montanera W, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of the MRI findings. AJNR Am J Neuroradiol 2000;21:1073–7 [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce JM. Palatal myoclonus (palatal tremor). Eur Neurol 2008;60:312–15 Epub 3 Oct 2008 [DOI] [PubMed] [Google Scholar]
- 7.Salamon-Murayama N, Russel E, Rabin B. Case 17: Hypertrophic olivary degeneration secondary to pontine haemorrhage. Radiology 1999;213:814–17 [DOI] [PubMed] [Google Scholar]
- 8.Krings T, Foltys H, Meister IG, Reul J. Hypertrophic olivary degeneration following pontine haemorrhage: hypertensive crisis or cavernous haemangioma bleeding? J Neurol Neurosurg Psychiatry 2003;74:797–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerace C, Fele MR, Luna R, Piazza G. Neurological picture. Bilateral hypertrophic olivary degeneration. J Neurol Neurosurg Psychiatry 2006;77:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora V, Nijjar IS, Sandhu PS, Singh J, Abrol R, Roop A. Hypertrophic olivary degeneration following trauma — a case report. Ind J Radiol Imag 2006 16;4:701–4 [Google Scholar]
- 11.Hornyak M, Osborn AG, Couldwell WT. Hypertrophic olivary degeneration after surgical removal of cavernous malformations of the brainstem: report of four cases and review of the literature. Acta Neurochir (Wien). 2008;150:149–56 [DOI] [PubMed] [Google Scholar]
- 12.Akar S, Drappatz J, Hsu L, Blinder RA, Black PM, Kesari S. Hypertrophic olivary degeneration after resection of a cerebellar tumour. J Neuroncol 2008;87:341–5 [DOI] [PubMed] [Google Scholar]