Abstract
The purpose of this study was to identify the clinical and thin-section CT findings in patients with acute Klebsiella pneumoniae pneumonia (KPP) alone and with concurrent infection. We retrospectively identified 160 patients with acute KPP who underwent chest thin-section CT examinations between August 1998 and August 2008 at our institution. The study group comprised 80 patients (54 male, 26 female; age range 18–97 years, mean age 61.5) with acute KPP alone, 55 (43 male, 12 female; age range 46–92 years, mean age 76.0) with KPP combined with methicillin-resistant Staphylococcus aureus (MRSA) and 25 (23 male, 2 female; age range 56–91 years, mean age 72.7) with KPP combined with Pseudomonas aeruginosa (PA). Underlying diseases in patients with each type of pneumonia were assessed. Parenchymal abnormalities were evaluated along with enlarged lymph nodes and pleural effusion. In patients with concurrent pneumonia, underlying conditions such as cardiac diseases, diabetes mellitus and malignancy were significantly more frequent than in patients with KPP alone. The mortality rate in patients with KPP combined with MRSA or PA was significantly higher than in those with KPP alone. In concurrent KPP, CT findings of centrilobular nodules, bronchial wall thickening, cavity, bronchiectasis, nodules and pleural effusion were significantly more frequent with concurrent pneumonia than in those with KPP alone.
Klebsiella pneumoniae is among the most common Gram-negative bacteria encountered by physicians worldwide, and accounts for 0.5–5.0% of all cases of pneumonia. It is a clinically important type of pneumonia because of its severity, high incidence of complications and increased mortality [1–3]. The mortality rate in alcoholics with Klebsiella pneumoniae pneumonia (KPP) has been reported to be as high as 50–60% [4, 5].
The characteristics of KPP on plain radiography have been described previously [1, 3, 6–9]. Felson et al [8] have studied 14 patients with acute KPP and reported that the presence of certain radiological features supports a diagnosis of acute KPP. These features are bulging fissures, sharp margins of the advancing border of the pneumonic infiltrate and early abscess formation.
Recently, we have reported that in 764 of 962 patients (79.4%) with acute KPP, 1 or more additional pathogens, predominantly methicillin-resistant Staphylococcus aureus (MRSA) (36.7%) and Pseudomonas aeruginosa (PA) (23.3%), were found [10].
To the best of our knowledge, no studies have compared the pulmonary CT findings in patients with acute and concurrent KPP. Therefore, the present study compared the clinical and pulmonary thin-section CT findings of patients with acute KPP alone and concurrent KPP with MRSA or PA.
Methods and materials
Patients
Our institutional review board approved this retrospective study and waived informed consent.
We retrospectively identified 160 patients with acute KPP who underwent chest thin-section CT examinations between August 1998 and August 2008 at our institution. The study group comprised 80 patients (54 male, 26 female; age range 18–97 years, mean age 61.5) with acute KPP, 55 (43 male, 12 female; age range 46–92 years, mean age 76.0) with KPP combined with MRSA and 25 (23 male, 2 female; age range 56–91 years, mean age 72.7) with KPP combined with PA.
The diagnosis was established by isolation of K. pneumoniae from sputum in 115 patients, bronchoalveolar lavage fluid in 43 and blood specimens in 2; concurrent infections were diagnosed by isolation of MRSA (32, 23 and 0, respectively) and PA (9, 16 and 0, respectively).
A patient was considered to have community-acquired pneumonia (CAP) if, at the time of hospital admission, he/she presented with a cough, with or without sputum, fever, leucocytosis or leucopenia, and had pulmonary infiltrates on chest radiographs. None of the patients had been admitted to or treated in a hospital 2 weeks before admission. Of the 80 patients with KPP, 21 had CAP and 59 had nosocomial infections. Of the 55 patients with MRSA, 5 had CAP and 50 had nosocomial infections, and of the 25 with concurrent PA, 9 had CAP and 16 had nosocomial infections.
The patients with acute KPP alone or in combination with MRSA or PA included 57 with post-operative malignancy (n = 20, 23 and 14, respectively). Patients with cardiac disease (n = 9, 36 and 18, respectively), pulmonary emphysema (n = 18, 10 and 2, respectively) and diabetes mellitus (n = 10, 20 and 11, respectively) were included in the study (Table 1).
Table 1. Patient characteristics and underlying conditions.
| K. pneumoniae (n = 80) | K. pneumoniae with MRSA (n = 55) | K. pneumoniae with P. aeruginosa (n = 25) | K. pneumoniae vs K. pneumoniae with MRSA, p-value | K. pneumoniae vs K. pneumoniae with P. aeruginosa, p-value | |
| Sex, male/female | 54/26 | 43/12 | 23/2 | NS | NS |
| Age (year) | |||||
| Range | 18–97 | 46–92 | 56–91 | ||
| Mean | 61.5 | 76 | 72.7 | NS | NS |
| Community-acquired infection | 21 | 5 | 9 | NS | NS |
| Nosocomial infection | 59 | 50 | 16 | NS | NS |
| Underlying conditions | |||||
| Alcoholism | 52 (65.0) | 34 (61.8) | 11 (44.0) | NS | NS |
| Smoking habit | 39 (48.8) | 32 (58.2) | 13 (52.0) | NS | NS |
| Cardiac disease | 9 (11.3) | 36 (65.5) | 18 (72.0) | <0.001 | <0.001 |
| Pulmonary emphysema | 18 (22.5) | 10 (18.2) | 2 (8.0) | NS | NS |
| Diabetes mellitus | 10 (12.5) | 20 (36.4) | 11 (44.0) | <0.005 | <0.001 |
| Collagen disease | 6 (7.5) | 11 (20.0) | 5 (20.0) | <0.05 | NS |
| Malignancy | 20 (25.0) | 23 (41.8) | 14 (56.0) | <0.05 | <0.005 |
| Mortality | 12 (15.0) | 21 (38.2) | 11 (44.0) | <0.005 | <0.005 |
Data in parentheses are percentages. MRSA, methicillin-resistant Staphylococcus aureus; NS, not significant.
An alcoholic was defined as an individual with a daily consumption of alcohol ≥80 g during the past 2 years [11], and a patient was considered to be a heavy smoker if he/she had smoked more than 10 pack-years. Of the patients with acute KPP alone or concurrent with MRSA or PA, 97 were alcoholics (n = 52, 34 and 11, respectively) and 84 were chronic smokers (n = 39, 32 and 13, respectively) (Table 1).
CT examinations
Thin-section CT examinations were performed with 1-mm collimation at 10-mm intervals from the apex of the lung to the diaphragm (n = 45), or volumetrically with a multidetector CT system with 1-mm reconstruction (n = 115). CT examinations were performed with the patient in the supine position at full inspiration and were reconstructed using a high-spatial-frequency algorithm. Images were captured at window settings that allowed viewing of the lung parenchyma (window level, −600 to −700 HU; window width, 1200–1500 HU) and the mediastinum (window level, 20–40 HU; window width, 400 HU). The pulmonary CT examination was performed within 1–6 days (mean 3.79 days) after the onset of respiratory symptoms. Intravenously administered contrast material was used for 41 examinations.
Image interpretation
Two chest radiologists (with 21 and 13 years of experience in chest CT image interpretation) who were unaware of the underlying diagnoses retrospectively and independently interpreted the CT images. Conclusions were reached by consensus. An average of 2 sessions per week were reserved for review of the CT findings, with a total of approximately 80 sessions.
CT images were assessed for the following radiological patterns: ground-glass attenuation (GGA), consolidation, nodules, centrilobular nodules, bronchial wall thickening, interlobular septal thickening, intralobular reticular opacity, bronchiectasis, enlarged hilar/mediastinal lymph node(s) (>1 cm diameter short axis), cavities and pleural effusion. Areas of GGA were defined as areas showing hazy increases in attenuation without obscuring vascular markings [12, 13]. Areas of consolidation were defined as areas of increased attenuation that obscured the normal lung markings [12, 13]. Centrilobular nodules were defined as those present around the peripheral pulmonary arterial branches or 3–5 mm from the pleura, interlobular septa or pulmonary veins. Interlobular septal thickening was defined as abnormal widening of the interlobular septa [13]. Intralobular reticular opacity was considered present when interlacing line shadows were separated by a few millimetres [12, 13].
The distribution of parenchymal disease was also noted. Whether the abnormal findings were located unilaterally or bilaterally was assessed. If the main lesion was predominantly located in the inner third of the lung, the disease was classified as having a central distribution. On the other hand, if the lesion was predominantly located in the outer third of the lung, the disease was classified as having a peripheral distribution. If the lesions showed no predominant distribution, the disease was classified as having a random distribution. In addition, zonal predominance was classified as upper, lower or random. Upper lung zone predominance meant that most abnormalities were seen at a level above the tracheal carina, whereas lower zone predominance referred to most abnormalities being below the upper zone. When abnormalities showed no definite zonal predominance, the lung disease was classified as having a random distribution.
Statistical analysis
Statistical analysis for the frequency of symptoms and CT findings were conducted using Fisher's exact test and the χ2 test. A mean age comparison was conducted using the Student's t-test.
Results
Clinical features
All of the patients had respiratory symptoms. The most frequent presenting symptoms were fever and cough (160 patients each, 100%), followed by sputum (133 patients, 83.1%), dyspnoea (43 patients, 26.9%) and chest pain (28 patients, 17.5%). Most of the patients showed rapid progression in their respiratory symptoms. There were no significant differences in presenting symptoms between the three groups. The underlying conditions are summarised in Table 1. The frequencies of underlying diseases such as cardiac disease (p<0.001 and p<0.001, respectively), diabetes mellitus (p<0.005 and p<0.001, respectively) and malignancy (p<0.05 and p<0.005, respectively) were significantly higher in patients with MRSA and PA concurrent pneumonia than in those with KPP alone.
CT patterns
Chest CT revealed abnormalities in all patients with acute KPP (Table 2). Of the 80 patients, GGA (n = 80, 100%) (Figure 1) was most frequent, followed by consolidation (n = 73, 91.3%) (Figure 1), intralobular reticular opacity (n = 68, 85.0%) (Figure 1) and bronchial wall thickening (n = 21, 26.3%). Interlobular septal thickening (n = 7, 8.8%) (Figure 1), centrilobular nodules (n = 5, 6.3%) and bronchiectasis (n = 5, 6.3%) were also observed. Cavitary lesions were found in only one patient (1.3%).
Table 2. Comparison of thin-section CT findings.
| Findings | K. pneumoniae (n = 80) | K. pneumoniae with MRSA (n = 55) | K. pneumoniae with P. aeruginosa (n = 25) | K. pneumoniae vs K. pneumoniae with MRSA, p-value | K. pneumoniae vs K. pneumoniae with P. aeruginosa, p-value |
| Ground-glass attenuation | 80 (100) | 55 (100) | 25 (100) | NS | NS |
| Consolidation | 73 (91.3) | 53 (96.4) | 22 (88.0) | NS | NS |
| Intralobular reticular opacity | 68 (85.0) | 39 (70.9) | 14 (56.0) | <0.05 | <0.005 |
| Bronchial wall thickening | 21 (26.3) | 46 (83.6) | 18 (72.0) | <0.001 | <0.001 |
| Interlobular septal thickening | 7 (8.8) | 15 (27.3) | 6 (24.0) | <0.005 | <0.05 |
| Centrilobular nodules | 5 (6.3) | 33 (60.0) | 11 (44.0) | <0.001 | <0.001 |
| Bronchiectasis | 5 (6.3) | 18 (32.7) | 7 (28.0) | <0.001 | <0.005 |
| Nodules | 2 (2.5) | 12 (21.8) | 5 (20.0) | <0.001 | <0.005 |
| Cavity | 1 (1.3) | 9 (16.4) | 6 (24.0) | <0.001 | <0.001 |
| Pleural effusion | 42 (52.5) | 47 (85.5) | 22 (88.0) | <0.001 | <0.001 |
| Lymph node enlargement | 4 (5.0) | 5 (9.1) | 5 (20.0) | NS | <0.05 |
Data in parentheses are percentages. NS, not significant.
Figure 1.
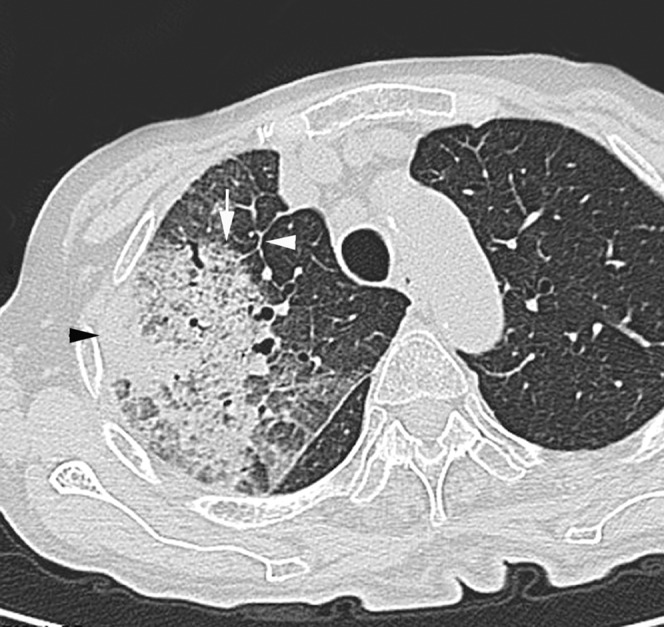
Acute Klebsiella pneumoniae pneumonia in an 82-year-old female alcoholic with a smoking habit at 2 days after onset of fever and cough with sputum. Transverse thin-section CT of right upper lobe shows consolidation (arrowhead), ground-glass attenuation and intralobular reticular opacity (arrow). Interlobular septal thickening (white arrowhead) is also present.
In comparison, of the 55 patients with concurrent MRSA or the 25 with concurrent PA, GGA (100% and 100%, respectively) (see Figures 3 and 4) was most frequent, followed by consolidation (96.4% and 88.0%, respectively) (Figures 2–4) and bronchial wall thickening (83.6% and 72.0%, respectively) (Figure 3). The frequency of intralobular reticular opacity was significantly higher in patients with KPP alone than in those with concurrent MRSA (p<0.05) or PA (p<0.005). In contrast, the frequencies of bronchial wall thickening (p<0.001 and p<0.001, respectively) (Figure 3), centrilobular nodules (both, p<0.001) (Figure 3) and bronchiectasis (p<0.001 and p<0.005, respectively) (Figure 2) were significantly higher in patients with concurrent infection than in those with KPP alone. Cavitary lesions were also more frequently observed in patients with concurrent MRSA (p<0.001) (Figures 2 and 3) or PA (p<0.001) (Figure 4) than in those with KPP alone.
Figure 3.
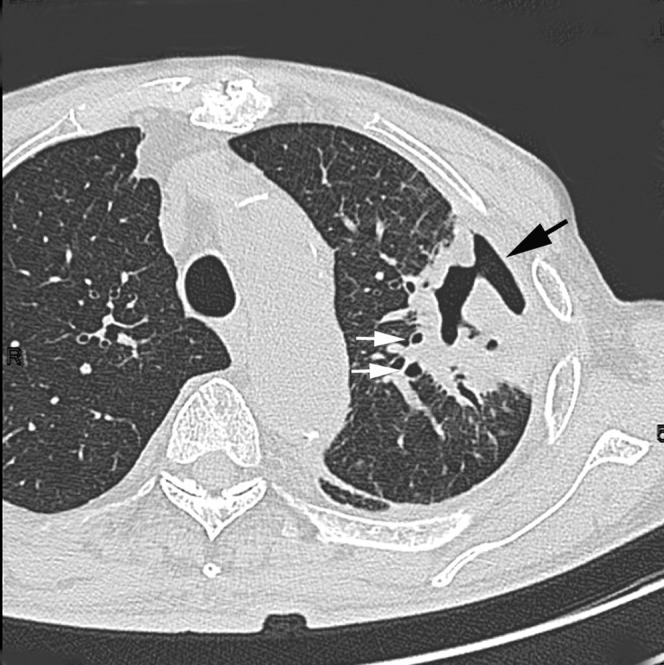
Acute concurrent Klebsiella pneumoniae pneumonia with MRSA in a 75-year-old male alcoholic with cardiac disease and diabetes mellitus at 4 days after onset of fever, cough with sputum and dyspnoea. Transverse thin-section CT 1 cm below the tracheal carina shows consolidation with cavity (arrows), centrilobular nodules (arrowheads) and bronchial wall thickening (white arrow) with peripheral distribution. Pleural effusion is also present.
Figure 4.
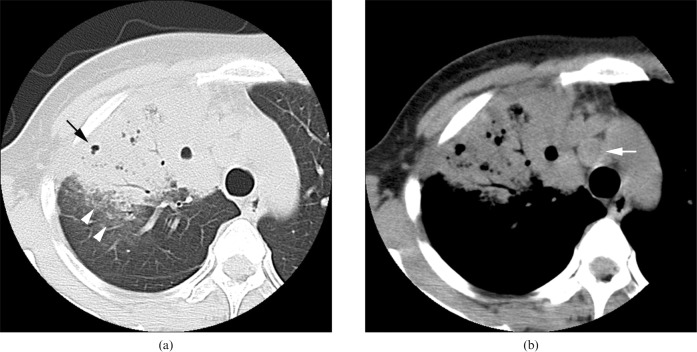
Acute concurrent Klebsiella pneumoniae pneumonia with Pseudomonas aeruginosa in a 35-year-old female alcoholic with a smoking habit at 3 days after onset of fever, cough with sputum and general weakness. (a) Transverse thin-section CT of the right upper lobe shows consolidation with cavity (arrow) and ground-glass opacity (white arrowheads). (b) Pre-tracheal lymph node enlargement is present (white arrow).
Figure 2.
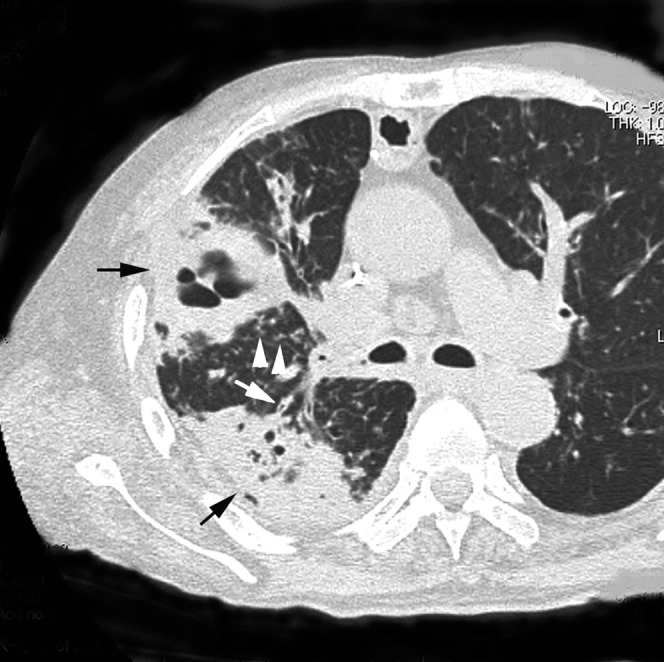
Acute concurrent Klebsiella pneumoniae pneumonia with methicillin-resistant Staphylococcus aureus in a 61-year-old male alcoholic with cardiac disease at 4 days after onset of fever, cough and chest pain. Transverse thin-section CT of left upper lobe shows consolidation with cavity (arrow) and bronchiectasis (white arrows).
In the 80 patients with KPP, the combination of consolidation and GGA (n = 73, 91.3%) was most frequent, followed by consolidation in combination with intralobular reticular opacity (n = 63, 78.8%) (Figure 1). These findings were similar to those in patients with concurrent MRSA or PA. However, in patients with KPP combined with either MRSA or PA, the combination of consolidation and bronchial wall thickening, centrilobular nodules or bronchiectasis was more frequently observed than in patients with KPP alone (p<0.001, each) (Figure 3).
In addition, there were no significant differences in CT findings between the patients with KPP in combination with MRSA vs those with KPP in combination with PA.
Disease distribution
Of the 160 patients with KPP and concurrent pneumonia, abnormal findings were found bilaterally in 123 patients (76.9%) and unilaterally in 37 patients (23.1%), and in the periphery (n = 154, 96.3%) (Figures 1–4). On the other hand, there were six patients with disease who showed a random distribution (3.8%), and no patients had a predominantly central distribution of abnormalities. The predominant zonal distribution was the upper zone in 20 patients (12.5%), and the lower zone in 110 patients (68.8%), and random distribution was observed in 30 patients (18.8%). There were no significant differences between the three groups in terms of disease distribution.
Effusion and lymph nodes
Pleural effusion was found in 42 (52.5%) patients with KPP alone, 47 (85.5%) with concurrent MRSA and 22 (88.0%) with concurrent PA. The frequency of pleural effusion was significantly higher in patients with KPP combined with MRSA and PA than in those with KPP alone (both p<0.001). Bilateral pleural effusion was also more frequently seen in patients with KPP combined with MRSA (n = 36, 65.5%) or PA (n = 16, 64.0%) than with KPP alone (n = 16, 20.0%) (both p<0.001).
Mediastinal and/or hilar lymph node enlargement was more frequent in patients with KPP combined with PA (n = 5, 20.0%) (Figure 4b) than in patients with KPP alone (n = 4, 5.0%) or combined with MRSA (n = 5, 9.1%) (p<0.05). Enlarged lymph nodes were found in the paratracheal, tracheobronchial and subcarinal regions.
Follow-up study
All 160 patients underwent antibiotic therapy. In 68 of 80 patients with KPP alone, the abnormal findings improved on follow-up CT examination or chest radiography. However, in the remaining 12 patients (15.0%), abnormal findings such as GGA, consolidation and intralobular reticular opacity were found to worsen on follow-up CT, and the patients died. In comparison, in 21 of the 55 patients with KPP in combination with MRSA (38.2%) and in 11 of the 25 patients with concurrent PA (44.0%), the abnormal findings worsened and the patients died. The mortality rate in patients with KPP combined with MRSA or PA was significantly higher than that in those with KPP alone (both p<0.005) (Table 1).
Discussion
K. pneumoniae, one of the most important Gram-negative bacterial pathogens, is of worldwide concern [14]. In addition, PA and MRSA are also important causes of nosocomial and community-acquired infection around the world. Initially limited to hospitals, in recent years MRSA has emerged as an increasingly important cause of community-acquired bacterial infection [15–17], often affecting healthy children and adults who have no apparent risk factors for infection [18]. Recently, we have reported that in about 80% of the patients with acute KPP, one or more additional pathogens, predominantly MRSA and PA, were present [10].
To the best of our knowledge, no comparisons of clinical and pulmonary CT findings in patients with acute K. pneumoniae pneumonia alone or combined with other pathogens have been published. We compared retrospectively the clinical and pulmonary thin-section CT findings in 80 patients with acute KPP alone and in 55 patients with concurrent MRSA or 25 with concurrent PA. All patients had several complaints, such as fever and cough. There were no significant differences in presenting symptoms between the three groups. KPP tends to affect people with underlying diseases, such as alcoholism, diabetes and chronic lung disease [19–21]. In the present study, alcoholism (60.6%) was the most commonly associated condition, followed by a smoking habit (52.5%), cardiac disease (39.4%) and malignant disease (35.6%). In the patients with concurrent pneumonia (MRSA or PA), underlying conditions such as cardiac diseases, diabetic mellitus and malignancy were significantly more frequent than in patients with KPP alone. As for mortality rate, that in patients with KPP combined with MRSA or PA was significantly higher than that in patients with KPP alone (both p<0.005). The mortality rate in patients with KPP alone was lower than that in previous studies, and the mortality rate in concurrent KPP was similar to that previously reported [2, 19, 22]. Therefore, the mortality rates reported previously might reflect that in concurrent KPP.
KPP has been classified by radiographic and clinical criteria [9, 22–24]. Carpenter [22] has divided Klebsiella pulmonary infections into acute pneumonia and complications of acute pneumonia. The complications consist of lung abscess, pulmonary gangrene and chronic KPP. The frequencies of abscess formation, gangrene and development of chronic pneumonia were 16–50%, 7–50% and 5–33%, respectively [22].
In the present study of 160 patients, GGA was most frequent, followed by consolidation and intralobular reticular opacity; however, there was no significant difference between the three groups. CT findings such as centrilobular nodules, bronchial wall thickening and bronchiectasis were significantly more frequent with concurrent pneumonia than those with KPP alone.
Shah et al [25] have reported CT findings in 28 patients with nosocomial PA pneumonia: 15 with PA and 13 with PA and other pathogens such as Staphylococcus aureus (n = 4) and Klebsiella species (n = 2). The CT findings consisted mainly of consolidation (100%) with bilateral involvement (64.3%), nodules (50.0%), necrosis (35.7%) and centrilobular nodules (32.1%). Bilateral pleural effusion was seen in 13 patients (46.4%). There was no significant difference in the distribution or frequency of abnormal findings between the two groups.
Recently, Nguyen et al [26] have reported the radiographic and CT findings in nine patients with MRSA pneumonia. The most common CT findings were bilateral consolidation (n = 8), followed by interlobular septal thickening (n = 7), cavitation (n = 6), multiple nodules (n = 5) and centrilobular nodules (n = 4). Pleural effusion was present in all patients. Enlarged lymph nodes were present in four patients.
Classified histologically as bronchopneumonia, nodular features would be expected upon CT evaluation of PA pneumonia. Pathologically, bronchopneumonia demonstrates inflammatory changes involving the bronchial and bronchiolar walls, with minimal exudation into adjacent alveoli [27]. Therefore, in concurrent KPP with PA or MRSA, CT findings of centrilobular nodules, bronchial wall thickening and bronchiectasis might be significantly more frequent than in those with KPP alone.
In the present study, a cavitary lesion was found in only one patient (1.3%) with KPP. The frequency was significantly lower than that in patients with concurrent KPP with PA or MRSA (both, p<0.001) and compared with previously reported rates [22]. However, this might be because most of the literature was published from the 1930s to the 1960s, which represents the pre-antibiotic era or a period of minimal antibiotic use. In addition, there have been no studies in which additional pathogens were evaluated in patients with KPP. We have recently reported that in 764 of 962 patients (79.4%) with acute KPP, one or more additional pathogens were found [10]. In the present study, the frequency of cavitary lesions in concurrent KPP was similar to that in previous studies; therefore, the frequencies reported previously might reflect those in concurrent KPP.
It should be noted that there were several limitations to our study. First, this was a retrospective study and CT image interpretation was performed by consensus. Second, our study lacked a pathological correlation with specific CT findings such as consolidation and intralobular reticular opacity. Third, the thin-section CT images were obtained at several institutions using different protocols. Fourth, CT images in the other concurrent pathogens other than PA and MRSA were not evaluated.
In summary, we investigated the clinical and thin-section CT findings of 80 patients with acute KPP alone, 55 with KPP combined with MRSA and 25 with KPP combined with P. aeruginosa. In the patients with concurrent pneumonia (MRSA or P. aeruginosa), underlying conditions such as cardiac diseases, diabetes mellitus and malignancy were significantly more frequent than in patients with KPP alone. Moreover, CT findings such as centrilobular nodules, bronchial wall thickening, cavity and pleural effusion were significantly more frequent in patients with concurrent pneumonia than those with KPP alone.
References
- 1.Holmes RB. Friedlander's pneumonia. Am J Roentgenol Radium Ther Nucl Med 1956;75:728–47 [PubMed] [Google Scholar]
- 2.Korvick AJ, Hackett AK, Yu LV, Muder RR. Klebsiella pneumonia in the modern era: clinicoradiographic correlations. South Med J 1991;84:200–4 [DOI] [PubMed] [Google Scholar]
- 3.Limson BM, Romansky MJ, Shea JG. An evaluation of 22 patients with acute and chronic pulmonary infection with Friedlander's bacillus. Ann Intern Med 1956;44:1070–81 [DOI] [PubMed] [Google Scholar]
- 4.Carden DL, Gibb KA. Pneumonia and lung abscess. Emerg Med Clin North Am 1983;1:345–70 [PubMed] [Google Scholar]
- 5.Jong GM, Hsiue TR, Chen CR, Change HY, Chen CW. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest 1995;107:214–17 [DOI] [PubMed] [Google Scholar]
- 6.Westermark NA. A case of Friedlander's pneumonia simulating tuberculosis with prolonged duration. Acta Radiol (Stockh) 1926;7:626–31 [Google Scholar]
- 7.Kornblum K. The roentgen-ray diagnosis of pulmonary infections with Friedlander's bacillus. Am J Roentgenol Radium Ther 1928;19:513–21 [Google Scholar]
- 8.Felson B, Rosenberg LS, Hmaburger M. Roentgen findings in acute Friedlander's pneumonia. Radiology 1949;53:559–65 [DOI] [PubMed] [Google Scholar]
- 9.Ritvo M, Martin F. The clinical and roentgen manifestations of pneumonia due to Bacillus mucosis capsulatus (primary Friedlander pneumonia). Am J Roentgenol Ther Nucl Med 1949;62:211–22 [PubMed] [Google Scholar]
- 10.Okada F, Ando Y, Honda K, Nakayama T, Kiyonaga M, Ono A, et al. Clinical and pulmonary thin-section CT findings in acute Klebsiella Pneumoniae pneumonia. Eur Radiol 2009;19:809–15 [DOI] [PubMed] [Google Scholar]
- 11.Tores A, Serra-Batlles J, Ferrer A, Jimenez P, Celis R, Cobo E, et al. Severe community-acquired pneumonia-epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312–18 [DOI] [PubMed] [Google Scholar]
- 12.Webb WR, Muller NL, Naidich DP. High-resolution computed tomography findings of lung disease. High-resolution CT of the lung, 3rd edn. Philadelphia: Lippincott Williams & Wilkins,2001;71–192 [Google Scholar]
- 13.Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327–31 [DOI] [PubMed] [Google Scholar]
- 14.Blot SI, Vandewoude KH, Colardyn FA. Clinical impact of nosocomial Klebsiella bacteremia in critically ill patients. Eur J Clin Microbiol Infect Dis 2002;21:471–3 [DOI] [PubMed] [Google Scholar]
- 15.Chambers HF. Community-associated MRSA: resistance and virulence converge. N Engl J Med 2005;352:1485–7 [DOI] [PubMed] [Google Scholar]
- 16.Crawford SE, Daum RS. Epidemic community-acquired methicillin-resistant Staphylococcus aureus: modern times for an ancient pathogen. Pediatr Infect Dis J 2005;24:459–60 [DOI] [PubMed] [Google Scholar]
- 17.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005;352:1436–44 [DOI] [PubMed] [Google Scholar]
- 18.Gorak EJ, Yamada SM, Brown JD. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis 1999;29:797–800 [DOI] [PubMed] [Google Scholar]
- 19.Kang CI, Kim SH, Bang JW, Kim HB, Kim NJ, Kim EC, et al. Community-acquired versus nosocomial Klebsiella pneumoniae bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. J Korean Med Sci 2006;21:816–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyde L, Hyde B. Primary Friedlander pneumonia. Am J Med Sci 1943;205:660–75 [Google Scholar]
- 21.Wylie RH, Kirschner PA. Friedlander's pneumonia. Am Rev Tuberc 1950;61:465–73 [Google Scholar]
- 22.Carpenter JL. Klebsiella pulmonary infections: outcome at one medical center and review. Rev Infect Dis 1990;12:672–82 [DOI] [PubMed] [Google Scholar]
- 23.Kornblum K. The roentgen-ray diagnosis of pulmonary infections with the Friedlander bacillus. Am J Roentgenol 1928;19:513–21 [Google Scholar]
- 24.Solomon S. Chronic Friedlander infections of the lung. JAMA 1940;115:1527–36 [Google Scholar]
- 25.Shah RM, Wechsler R, Salazar AM, Spirn PW. Spectrum of CT findings in nosocomial Pseudomonas aeruginosa pneumonia. J Thorac Imaging 2002;17:53–7 [DOI] [PubMed] [Google Scholar]
- 26.Nguyen ET, Kanne JP, Hoag LM, Reynold S, Dhingra V, Bryce E, et al. Community-acquired methicillin-resistant Staphylococcus aureus pneumonia: radiographic and computed tomography findings. J Thorac Imaging 2008;23:13–19 [DOI] [PubMed] [Google Scholar]
- 27.Groskin SA., Pneumonia and lung abscess In: Heitzman ER, editor. The lung: radiologic–pathologic correlations, 3rd edn. St Louis: Mosby, 1993 [Google Scholar]