Abstract
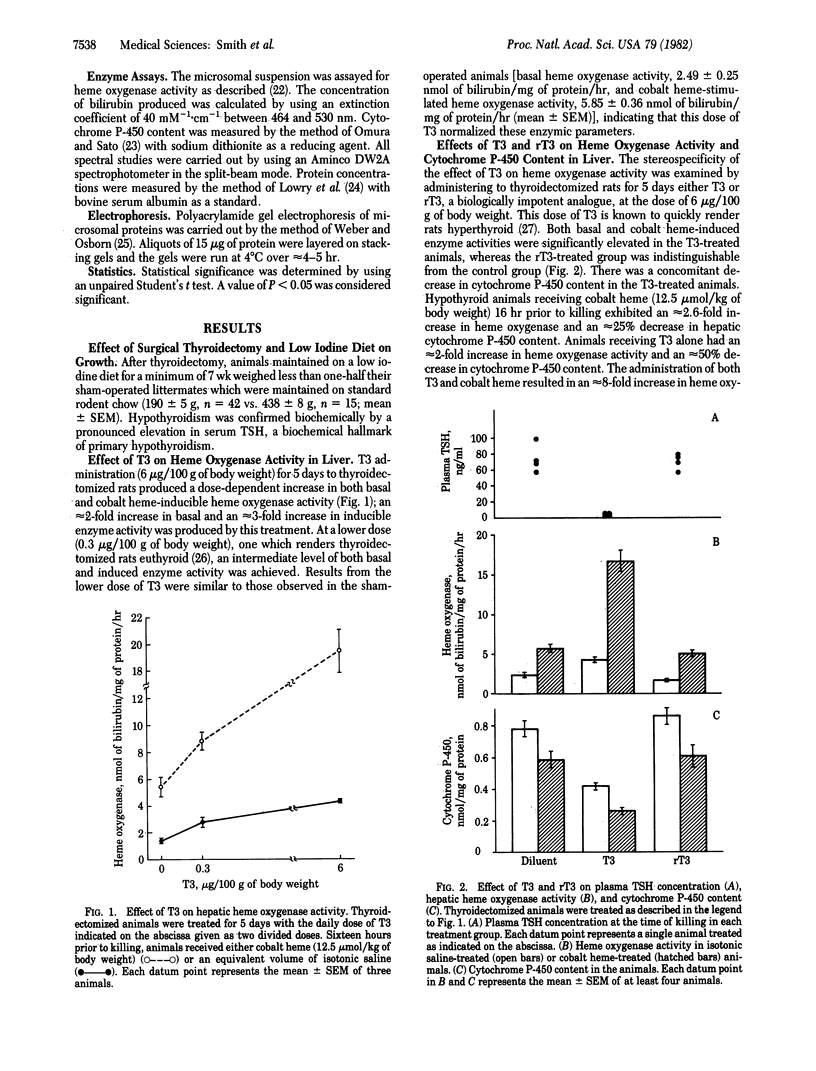
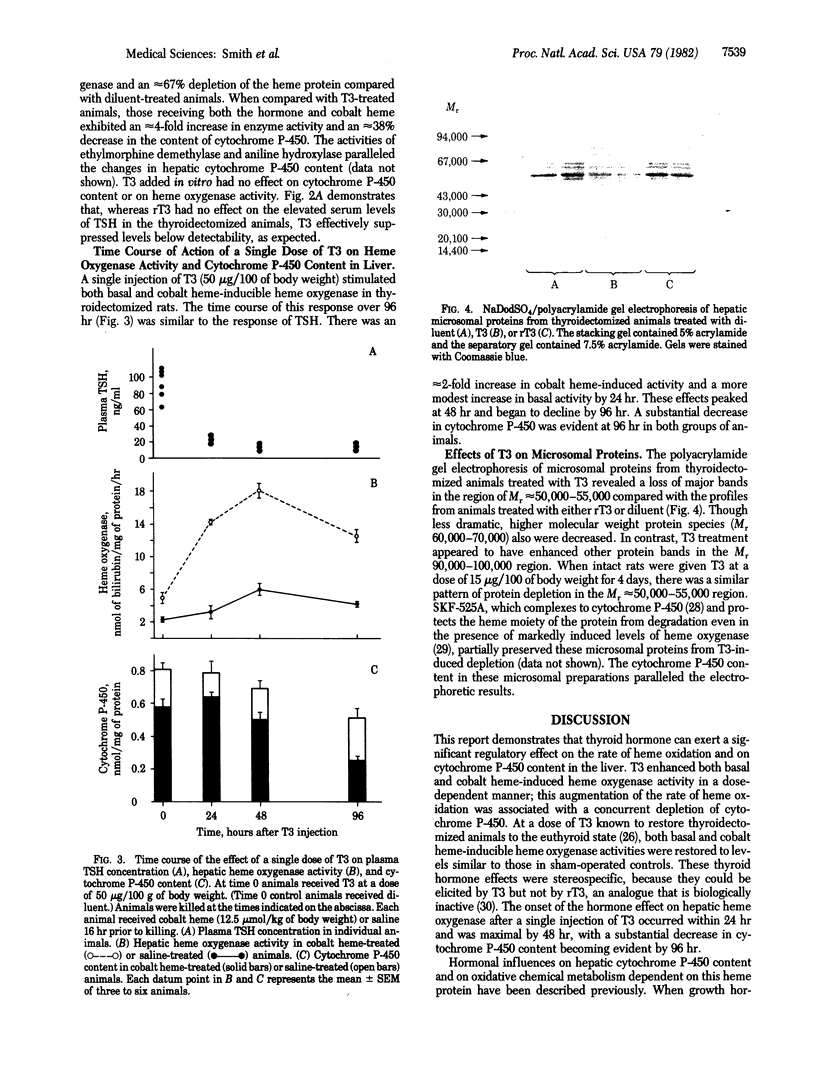
The effects of 3,5,3'-triiodothyronine (T3) on heme oxygenase (EC 1.14.99.3) activity and cytochrome P-450 content in liver were examined in thyroidectomized rats. T3, when administered for 5 days at a dose of 6 micrograms/100 g of body weight, stimulated basal heme oxygenase activity approximately equal to 2-fold compared to diluent-treated animals. The induction of heme oxygenase by cobalt heme also was enhanced approximately equal to 3-fold in T3-treated animals. T3 treatment lowered cytochrome P-450 content by approximately equal to 50% and potentiated the depletion of this heme protein after cobalt heme administration. Reverse T3 had no effect either on cytochrome P-450 content or on heme oxygenase activity in liver. The time course of response to a single dose of T3 (50 micrograms/100 g of body weight) revealed that both basal and cobalt heme-induced heme oxygenase activity peaked at 48 hr and that cytochrome P-450 content declined to approximately equal to 40% of controls at 96 hr. Examination of microsomal proteins by polyacrylamide gel electrophoresis after T3 treatment disclosed that major bands in the Mr approximately equal to 50,000-55,000 region were diminished. The administration of T3 together with SKF-525A, a compound known to complex with the heme prosthetic group of cytochrome P-450, resulted in partial preservation of these proteins. These data indicate that thyroid hormone can regulate heme oxygenase activity and concomitantly can lower cytochrome P-450 content in liver. The hormone also can act in a synergistic fashion to enhance the response of hepatic heme oxygenase to a chemical inducer of the enzyme. Thyroid status thus may be a potentially significant determinant of the rate of heme oxidation in the liver.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Freddara U., Kappas A. Induction of hepatic cytochrome P-450 by natural steroids: relationship to the induction of delta-aminolevulinate synthase and porphyrin accumulation in the avian embryo. Arch Biochem Biophys. 1982 Sep;217(2):597–608. doi: 10.1016/0003-9861(82)90542-2. [DOI] [PubMed] [Google Scholar]
- Bakken A. F., Thaler M. M., Schmid R. Metabolic regulation of heme catabolism and bilirubin production. I. Hormonal control of hepatic heme oxygenase activity. J Clin Invest. 1972 Mar;51(3):530–536. doi: 10.1172/JCI106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Healey J. F., Sinclair P. R., Sinclair J. F., Pomeroy J. S. Iron and the liver. Acute and long-term effects of iron-loading on hepatic haem metabolism. Biochem J. 1981 Apr 15;196(1):57–64. doi: 10.1042/bj1960057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buening M. K., Franklin M. R. SKF 525-A inhibition, induction, and 452-nm complex formation. Drug Metab Dispos. 1976 May-Jun;4(3):244–255. [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. The cytochrome P-450-depleted animal: an experimental model for in vivo studies in chemical biology. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2384–2388. doi: 10.1073/pnas.79.7.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Rosenberg D. W., Kappas A. Metal induction of haem oxygenase without concurrent degradation of cytochrome P-450. Protective effects of compound SKF 525A on the haem protein. Biochem J. 1982 Jan 15;202(1):59–66. doi: 10.1042/bj2020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSSON S. Influence of thyroid activity on excretion of bile acids and cholesterol in the rat. Proc Soc Exp Biol Med. 1957 Mar;94(3):582–584. doi: 10.3181/00379727-94-23019. [DOI] [PubMed] [Google Scholar]
- Edelman I. S., Ismail-Beigi F. Thyroid thermogenesis and active sodium transport. Recent Prog Horm Res. 1974;30(0):235–257. doi: 10.1016/b978-0-12-571130-2.50010-9. [DOI] [PubMed] [Google Scholar]
- Gemsa C., Woo C. H., Fudenberg H. H., Schmid R. Erythrocyte catabolism by macrophages in vitro. The effect of hydrocortisone on erythrophagocytosis and on the induction of heme oxygenase. J Clin Invest. 1973 Apr;52(4):812–822. doi: 10.1172/JCI107245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid induction of porphyrin synthesis in liver cell culture. I. Structural basis and possible physiological role in the control of heme formation. J Biol Chem. 1967 Oct 25;242(20):4587–4593. [PubMed] [Google Scholar]
- Ismail-Beigi F., Bissell D. M., Edelman I. S. Thyroid thermogenesis in adult rat hepatocytes in primary monolayer culture: direct action of thyroid hormone in vitro. J Gen Physiol. 1979 Mar;73(3):369–383. doi: 10.1085/jgp.73.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. Time-course of the effects of thyroid hormone on respiration and Na+ + K+-ATPase activity in rat liver. Proc Soc Exp Biol Med. 1974 Sep;146(4):983–988. doi: 10.3181/00379727-146-38232. [DOI] [PubMed] [Google Scholar]
- Kappas A., Song C. S., Levere R. D., Sachson R. A., Granick S. THE INDUCTION OF delta-AMINOLEVULINIC ACID SYNTHETASE in vivo IN CHICK EMBRYO LIVER BY NATURAL STEROIDS. Proc Natl Acad Sci U S A. 1968 Oct;61(2):509–513. doi: 10.1073/pnas.61.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R., Takahashi A., Omori Y. Effects of thyroxine and thyroidectomy on the hydroxylation of testosterone by liver microsomes from male and female rats. Biochim Biophys Acta. 1970 Apr 14;208(1):116–124. doi: 10.1016/0304-4165(70)90054-1. [DOI] [PubMed] [Google Scholar]
- Kato R., Takahashi A. Thyroid hormone and activities of drug-metabolizing enzymes and electron transport systems of rat liver microsomes. Mol Pharmacol. 1968 Mar;4(2):109–120. [PubMed] [Google Scholar]
- Kato R., Takanaka A., Takahashi A. Effect of thyroid hormone on the substrate interaction with P-450 in the oxidation of drugs by liver microsomes. J Biochem. 1970 Nov;68(5):613–623. doi: 10.1093/oxfordjournals.jbchem.a129395. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laker M. E., Mayes P. A. Effect of hyperthyroidism and hypothyroidism on lipid and carbohydrate metabolism of the perfused rat liver. Biochem J. 1981 Apr 15;196(1):247–255. doi: 10.1042/bj1960247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. The effect of thyroid hormone on bile salt-independent bile flow and Na+, K+ -ATPase activity in liver plasma membranes enriched in bile canaliculi. J Clin Invest. 1976 Apr;57(4):1009–1018. doi: 10.1172/JCI108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. S., Edelman I. S. Effect of triiodothyronine on the synthesis and degradation of renal cortical (Na+ + k+)-adenosine triphosphatase. J Biol Chem. 1976 Dec 25;251(24):7834–7840. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978 Apr 10;253(7):2321–2326. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Rajamanickam C., Rao M. R., Padmanaban G. On the sequence of reactions leading to cytochrome P-450 synthesis-effect of drugs. J Biol Chem. 1975 Mar 25;250(6):2305–2310. [PubMed] [Google Scholar]
- Ruegamer W. R., Newman G. H., Richert D. A., Westerfeld W. W. Specificity of the alpha-glycerophosphate dehydrogenase and malic enzyme response to thyroxine. Endocrinology. 1965 Oct;77(4):707–715. doi: 10.1210/endo-77-4-707. [DOI] [PubMed] [Google Scholar]
- Rumbaugh R. C., Kramer R. E., Colby H. D. Dose-dependent actions of thyroxine on hepatic drug metabolism in male and female rats. Biochem Pharmacol. 1978;27(16):2027–2031. doi: 10.1016/0006-2952(78)90062-x. [DOI] [PubMed] [Google Scholar]
- SELLINGER O. Z., LEE K. THE INDUCTION OF MITOCHONDRIAL ALPHA-GLYCEROPHOSPHATE DEHYDROGENASE BY THYROID HORMONE: EVIDENCE FOR ENZYME SYNTHESIS. Biochim Biophys Acta. 1964 Sep 11;91:183–186. doi: 10.1016/0926-6550(64)90189-6. [DOI] [PubMed] [Google Scholar]
- STASILLI N. R., KROC R. L., MELTZER R. I. Antigoitrogenic and calorigenic activities of thyroxine analogues in rats. Endocrinology. 1959 Jan;64(1):62–82. doi: 10.1210/endo-64-1-62. [DOI] [PubMed] [Google Scholar]
- Saenger P., Rifkind A. B., New M. I. Changes in drug metabolism in children with thyroid disorders. J Clin Endocrinol Metab. 1976 Jan;42(1):155–159. doi: 10.1210/jcem-42-1-155. [DOI] [PubMed] [Google Scholar]
- Sardana M. K., Sassa S., Kappas A. Adrenalectomy enhances the induction of heme oxygenase and the degradation of cytochrome P-450 in liver. J Biol Chem. 1980 Dec 10;255(23):11320–11323. [PubMed] [Google Scholar]
- Sardana M. K., Sassa S., Kappas A. Differential responses to inducers of delta-aminolaevulinate synthase and haem oxygenase during pregnancy. Biochem J. 1981 Aug 15;198(2):403–408. doi: 10.1042/bj1980403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Bradlow H. L., Kappas A. Steroid induction of delta-aminolevulinic acid synthase and porphyrins in liver. Structure-activity studies and the permissive effects of hormones on the induction process. J Biol Chem. 1979 Oct 25;254(20):10011–10020. [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Seelig S., Liaw C., Towle H. C., Oppenheimer J. H. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4733–4737. doi: 10.1073/pnas.78.8.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Edelman I. S. The role of sodium transport in thyroid thermogenesis. Fed Proc. 1979 Jul;38(8):2150–2153. [PubMed] [Google Scholar]
- Sterling K. Thyroid hormone action at the cell level (first of two parts). N Engl J Med. 1979 Jan 18;300(3):117–123. doi: 10.1056/NEJM197901183000304. [DOI] [PubMed] [Google Scholar]
- Sterling K. Thyroid hormone action at the cell level (second of two parts). N Engl J Med. 1979 Jan 25;300(4):173–177. doi: 10.1056/NEJM197901253000405. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Imai K., Ito A., Omura T., Sato R. Effects of thyroidectomy and triiodothyronine administration on oxidative enzymes in rat liver microsomes. J Biochem. 1967 Oct;62(4):447–455. doi: 10.1093/oxfordjournals.jbchem.a128688. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson J. T. Growth hormone modulation of liver drug metabolic enzyme activity in the rat. I. Effect of the hormone on the content and rate of reduction of microsomal cytochrome P-450. Biochem Pharmacol. 1973 Jul 15;22(14):1717–1728. doi: 10.1016/0006-2952(73)90385-7. [DOI] [PubMed] [Google Scholar]




