Abstract
In cervical cancer, the prognostic significance of bladder wall invasion on MRI without pathological evidence of mucosal invasion is not known. From 454 consecutive patients with cervical cancer who were treated with radiation, we reviewed images and analysed the outcome of 92 patients with the Federation of International Gynecology and Obstetrics (FIGO) stage IIIB–IVA. We analysed the patients in three groups, normal, wall (muscle and/or serosal) invasion and mucosal invasion, according to the findings on the MRI. Kaplan–Meier life table analysis and the log-rank test were used to assess the survival rates and differences according to prognostic factors. MRI detected abnormalities in the bladder wall in 42 patients (45.6%): wall invasion in 24 and mucosal invasion in 18. 5 of 18 patients, suspected on MRI to have mucosal invasion, showed no pathological evidence of mucosal invasion. Median follow-up period was 34 months. 3-year cause-specific survival (CSS) in the normal group compared with the wall invasion group was 76.2% vs 71.4% (p = 0.48). 3-year CSS for the wall invasion group compared with the mucosal invasion group was 71.4% vs 54.3% (p = 0.04). Mucosal invasion on MRI (p = 0.03) and concurrent chemoradiotherapy (p = 0.01) was significant for CSS. The prognosis for patients with cervical cancer with evidence of muscle and/or serosal invasion of the bladder on MRI may not differ from that for patients without abnormality on MRI. In patients with the MRI finding of bladder mucosal invasion, further studies should be conducted regarding the role of cystoscopy to determine the need for pathological confirmation.
According to the 2006 report by the Federation of International Gynecology and Obstetrics (FIGO) [1], the 5-year survival of patients with stage IVA cervical cancer is about half that of patients with stage IIIB cervical cancer (22.0% vs 41.5%). Reviewing the hazard ratios for patients with stages IIB, IIIB and IVA (2.7, 5.3 and 11.7, respectively), we noted a sharp increase in hazard ratio for stage IVA relative to stage IB. Because as the stage increases, the impact of lymph node involvement or tumour size on survival outcome decreases [1], mucosal involvement of the bladder and/or rectum may potentially have a strong influence on survival.
During the past two decades, there have been changing trends not only in the incidence of uterine cervical cancer [2] but also in the process of staging work-up. As MRI has become more applicable in planning the treatment of cervical cancer [3, 4], previously unnoticed invasion of the posterior wall of the urinary bladder without cystoscopic evidence of mucosal invasion appears frequently in advanced disease. However, there have been no published reports regarding the frequency of these findings or the prognosis for these patients with abnormal bladder wall findings on MRI without cystoscopic evidence of mucosal invasion.
Evidence suggests that MRI may predict the extent of disease more accurately than clinical staging [5]. With regard to bladder invasion, studies specifically tested the diagnostic accuracy of MRI against cystoscopic examination and/or surgical sampling as reference standards [6–10]. However, non-mucosal invasion cannot be diagnosed with cystoscopy, but can be confirmed only by exploration, which is not usually performed for locally advanced cervical cancer. For this reason, it is difficult to determine the diagnostic accuracy of MRI.
Following radiotherapy for advanced-stage tumours, MRI performance can be assessed only with clinical outcome. Few studies have reported on the use of MRI in cervical carcinoma treated with radiotherapy, and most have focused on the relationship between outcome and tumour diameter, tumour volume or lymph node status. We investigated the prognostic significance of abnormal bladder wall findings on MRI, with particular attention to those patients without cystoscopic evidence of mucosal invasion.
Methods and materials
Patients and pre-treatment evaluation
From 1997 to 2007, 454 patients with uterine cervical cancer were treated with curative radiation therapy, with or without chemotherapy, at the Samsung Medical Centre. We reviewed images and analysed the outcome of 97 patients with FIGO stage IIIB–IVA cervical cancer who had received pre-treatment pelvic MRI. After excluding 4 patients without follow-up data and 1 who was treated with adjuvant hysterectomy after radiotherapy, we retained the other 92 patients in our current study base. Our protocol for the investigation of cervical cancer included gynaecological examination with pathological confirmation of the cervical lesion, intravenous pyelography (IVP), endoscopic examination of the rectum and chest radiograph in all cases, according to the standard of practice of the FIGO staging criteria. Patients with suspected bladder invasion received a cystoscopic examination. Our protocol also included MRI and/or CT of the pelvis and abdomen for all patients. The pelvic or para-aortic lymph nodes that exceeded 1.5 cm in diameter on CT or MRI and/or that had significant 18F-fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) were considered positive for tumour involvement. MRI was performed using a 1.5 T unit (Sigma; General Electric Medical Systems, Milwaukee, WI). All subjects were examined using a pelvic or torso multicoil. Sequences included T1 weighted spin-echo images (time of repetition (TR) range/time of echo (TE), 450–700/10; matrix, 256 × 192; number of excitations (NEX), 2; echo-train length (ETL) with 1.5 T, 1; ETL with 3 T, 4) and axial, sagittal and coronal T2 weighted fast spin-echo images (TR range/effective TE range, 3000–6000/85–104; matrix, 512 × 256–512 × 192; NEX, 2; ETL with 1.5 T, 4; ETL with 3 T, 25). Contrast-enhanced axial and sagittal T1 weighted images were also obtained in all patients using a T1 weighted spin-echo sequence. A contrast scan was done at 15–30 s after injection of 7.5 ml gadolinium at a speed of 3 ml s−1, using an automatic power injector. The field of view was 22–26 cm and the section thickness was 5 mm with a 2 mm interscan gap.
Treatment
All patients were given a combination of external beam radiotherapy (EBRT) and intracavitary brachytherapy (ICR), which was initiated after an EBRT dose of 41.4–45 Gy (median dose 45 Gy). EBRT was performed with 15 MV photons to the entire pelvis with conventional simulation and the dose ranged from 41.4 Gy to 56.4 Gy (median dose 50.4 Gy). The extended pelvic field, including para-aortic lymph nodes, was used in 14 patients with para-aortic node involvement, with a dose up to 45 Gy.
Radiotherapy was administered with the patient in the prone position using a four-field box technique. In those patients with bladder invasion or para-aortic node involvement, parallel opposed anterior–posterior ports were used in the supine position. A customized small-bowel displacement system was used to reduce the volume of the irradiated small bowel. The radiation field encompassed a volume that included the primary tumour, uterus, the parametrial and uterosacral regions, as well as the pelvic lymph nodes.
ICR was begun 4–5 weeks after initiating the EBRT. Fletcher–Suit afterloading applicators were used for the high dose rate (HDR) brachytherapy with an iridium-192 source (Microselectron; Nucletron, Veenendaal, the Netherlands). Orthogonal films were taken to verify the placement of the applicators and to perform the dosimetric plan. The dose was prescribed at point A, and the rectal and bladder reference point doses were calculated according to the recommendations of the International Commission on Radiation Units and Measurements. The high dose rate (HDR) brachytherapy dose median was 24 Gy (range 8–24 Gy) at point A, with 4 Gy per fraction that was administered twice a week for 3 weeks.
Among the 92 patients, 49 patients were treated with concurrent chemoradiotherapy (CCRT) and 43 patients were treated with radiotherapy alone. The reasons for radiotherapy alone were as follows: age greater than 65 years and general condition insufficient to withstand CCRT in 12 patients, physician's preference in 18 cases, medical comorbidities in 9 cases, and patient's refusal of chemotherapy in 4 cases. The regimen for CCRT consisted of 3 cycles of 5-fluorouracil (1000 mg m−2 in a continuous infusion over 96 h) and cisplatin (60 mg m−2) at 3-week intervals in 32 patients, or 6 cycles of weekly cisplatin (30 mg m−2 or 40 mg m−2) alone in 17 patients.
Outcome analysis and follow-up
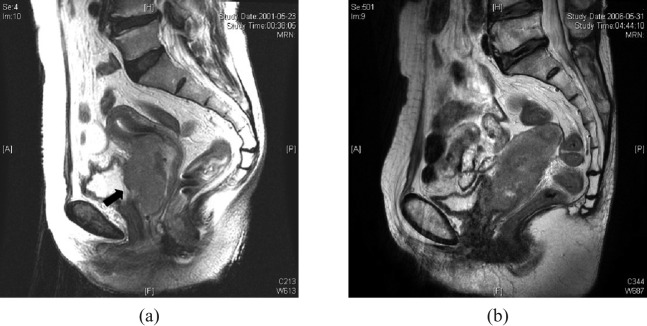
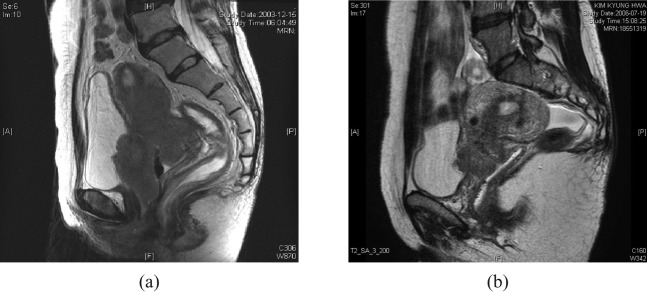
Based on the degree of posterior bladder wall invasion on MRI, patients were divided into three groups: normal, wall (muscle and/or serosal) invasion and mucosal invasion. MRI findings of wall irregularity with heterogeneous signal, enhancement with thickening and nodularity or loss of fat plane were interpreted as muscular and/or serosal invasion, and assigned to the wall invasion group (Figure 1). The MRI finding of a mass protruding into the bladder lumen was assigned to the mucosal invasion group (Figure 2). Patients without any of these abnormal findings were placed in the normal group.
Figure 1.
Sagittal T2 weighted images of patients assigned to the wall invasion group. (a) The tumour had established a large area of contact with the posterior bladder wall, with some interruption of the low signal intensity of the muscle layer (black arrow). Cystoscopy of this patient showed bullous oedema on the mucosal surface without tumour invasion. (b) The low-signal-intensity muscle layer was still preserved, but the tumour had established a large area of contact with the posterior bladder wall and some nodularity was noted. Cystoscopic biopsy showed no mucosal abnormality.
Figure 2.
Sagittal T2 weighted images of patients assigned to the mucosal invasion group. (a) A large tumour mass had entered the bladder lumen and disrupted the posterior bladder wall. Cystoscopic biopsy confirmed the mucosal invasion of the bladder. (b) A mass protruded into the bladder lumen. A cystoscopic examination, however, showed bullous oedema without mucosal invasion.
Locoregional failure was defined as the identification of recurrent or progressive tumours within the pelvis during the follow-up period. Efforts to obtain a pathological confirmation were made; however, image findings of recurrence were also used as a definition of treatment failure. The initial recurrence site was used to determine local recurrence and/or distant metastasis. The failure rates according to the variables studied were analysed using Fisher's exact test or the χ2 test. Kaplan–Meier life table analysis and the log-rank test were used to assess the survival rates and differences according to prognostic factors; p<0.05 was considered significant.
Results
MRI detected bladder abnormalities in 42 patients (42/92, 45.6%): wall invasion in 24 and mucosal invasion in 18. All patients received a cystoscopic examination. Pathological examinations confirmed mucosal invasion in 14 patients (14/92, 15.2%): 1 in the normal group and 13 in the mucosal invasion group. 5 of 18 patients suspected to have mucosal invasion from the MRI did not show pathological evidence of mucosal invasion. Patients with an overall treatment time (OTT) greater than 56 days and/or treatment with radiotherapy alone were more likely to be found in the normal group than in the wall invasion or mucosal invasion group (Table 1).
Table 1. Patient characteristics.
| Normal (n = 50) | pa | Wall invasion (n = 24) | pb | Mucosal invasion (n = 18) | ||
| Age, years | 28–77 (median 54) | 47–72 (median 63) | 32–77 (median 55) | |||
| ≤60 | 35 | 0.04 | 11 | 0.22 | 12 | |
| >60 | 15 | 13 | 6 | |||
| ECOG performance status | 0, 1 | 44 | 1 | 21 | 1 | 15 |
| 2 | 6 | 3 | 3 | |||
| Histology | Squamous | 47 | 0.66 | 22 | 0.49 | 18 |
| Adenocarcinoma | 3 | 2 | 0 | |||
| Cystoscopic finding | No invasion | 49 | 1 | 24 | <0.01 | 5 |
| Invasion | 1 | 0 | 13 | |||
| Sigmoidoscopic finding | No invasion | 50 | NA | 24 | 0.03 | 14 |
| Invasion | 0 | 0 | 4 | |||
| Pelvic node involvement | No | 20 | 0.21 | 6 | 1 | 5 |
| Yes | 30 | 18 | 13 | |||
| Para-aortic node involvement | No | 41 | 0.49 | 22 | 0.63 | 15 |
| Yes | 9 | 2 | 3 | |||
| Lymph node ≥3 cm | No | 40 | 0.31 | 22 | 1 | 16 |
| Yes | 10 | 2 | 2 | |||
| Tumour diameter, cm | 1.0–8.4 (median 5.0) | 2.5–10.6 (median 5.0) | 4–8.4 (median 5.4) | |||
| ≤5 cm | 26 | 0.86 | 13 | 0.75 | 8 | |
| >5 cm | 24 | 11 | 10 | |||
| Treatment modality | RT alone | 28 | 0.08 | 8 | 0.75 | 7 |
| CCRT | 22 | 16 | 11 | |||
| Overall treatment time, days | 46–98 (median 57) | 43–207 (median 53) | 49–85 (median 56) | |||
| ≤56 days | 25 | 0.01 | 20 | 0.46 | 13 | |
| >56 days | 25 | 4 | 5 | |||
CCRT, concurrent chemoradiotherapy; NA, not assessable; RT, radiotherapy.
aNormal group vs wall invasion group.
bWall invasion group vs mucosal invasion group.
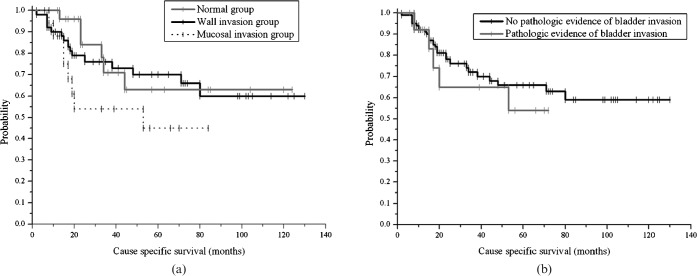
Median follow-up duration for the 92 patients was 34 months (range 2–130 months). During the follow-up period, four patients in the normal group and three patients in the wall invasion group died for reasons other than cervical cancer. Two died of lung cancer, one of colon cancer, one of cerebrovascular disease, one in a traffic accident, one of secondary sarcoma of the sacrum and one by suicide. 3-year cause-specific survival (CSS) in the normal group compared with the wall invasion group was 76.2% vs 71.4% (p = 0.48). 3-year CSS in the wall invasion group compared with the mucosal invasion group was 71.4% vs 54.3% (p = 0.04) (Figure 3). Overall survival (OS) and disease-free survival (DFS) did not differ between groups. Pathological evidence of mucosal invasion did not correlate with any type of survival (Figure 3).
Figure 3.
Cause-specific survival (CSS) of patients with FIGO stage IIIB–IVA cervical cancer, who were treated with radiation therapy, with or without chemotherapy. (a) 3-year CSS in the normal, wall invasion and mucosal invasion groups was 76.2%, 71.4% and 54.3%, respectively. (b) Pathological evidence of mucosal invasion did not correlate with CSS.
The most frequent type of locoregional failure was central recurrence. Central recurrence was found in 19 patients (19/23, 82.6%) and nodal failure without central recurrence was found in 4 patients. Among the seven patients with locoregional failure in the mucosal invasion group, the posterior wall of the bladder was the significant position of progression/recurrence in four patients. Of the five patients with locoregional failure in the wall invasion group, the cervix where it abuts the posterior bladder wall was the site of failure in two patients and the distal urethra was the site of failure in one patient. 3-year locoregional failure-free survival (LRFFS) in the normal group compared with the wall invasion group was 77.9% vs 75.0% (p = 0.90). 3-year LRFFS in the wall invasion group compared with the mucosal invasion group was 75.0% vs 59.0% (p = 0.17).
Mucosal invasion on MRI (p = 0.03, hazard ratio (HR) 5.15, 95% confidence interval (CI) 1.21–21.92) and CCRT (p = 0.01, HR 0.26, 95% CI 0.09–0.69) was a significant factor for CSS. Mucosal invasion on MRI was also a significant factor for LRFFS (p = 0.02, HR 6.47, 95% CI 1.29–32.41). Age, Eastern Cooperative Oncology Group (ECOG) performance score, histology, lymph node involvement, tumour size, pathological evidence of mucosal invasion and OTT showed no prognostic significance for any type of survival.
Discussion
The frequency of posterior bladder wall abnormalities on MRI in uterine cervical cancer has not been reported. We found such abnormalities in 45.6% of the 92 patients in our study with FIGO stage IIIB–IVA cervical cancer. These might well be expected results, because only a thin layer of fascia and loose areolar tissue separates the cervix from the posterior wall of the bladder. During hysterectomy, this median plane of vesicocervical and vesicovaginal space is dissected and the bladder is easily separated from the cervix [11]. Extension of disease to the vesicocervical space contraindicates radical hysterectomy because it precludes clear surgical margins. Radiotherapy is the treatment of choice for these patients, and the prognostic significance of the posterior bladder wall abnormality would only be known from analysis of the clinical outcome of the cancer.
The 1980 report by the Gynecologic Oncology Group (GOG), which reviewed data for 545 patients with surgically staged cervical cancer, found errors in the FIGO system in 53.4% of patients with stage IIB disease and in 49.2% of patients with stage IIIB disease [12]. As the American College of Radiology Imaging Network (ACRIN )6651/GOG 183 Intergroup study showed [5], current MRI technology delineates tumour boundaries accurately and is frequently used to guide both treatment development and application [3, 4]. In particular, MRI shows a high diagnostic accuracy for the exclusion of bladder involvement, with negative predictive values (NPV) of 96–100% [6–10]. This high NPV for bladder invasion suggests that the low pre-test probability of bladder involvement in early stage cervical cancer does not justify routine cystoscopy [7, 13].
However, for the positive predictive value (PPV) of MRI for bladder invasion, studies show highly discrepant values ranging from 7% to 100% [6–10]. Heterogeneity in the reference standard and differences between studies in MRI interpretation may largely explain this. For assignment to FIGO stage IVA, a carcinoma should have biopsy-proven involvement of bladder and/or rectum mucosa; a herald lesion such as bullous oedema does not suffice. As defined, stage IVA disease cannot be diagnosed with a usual surgical specimen without violating the bladder and/or rectal wall for a mucosal specimen. One study, which adopted the cystoscopic finding as a reference standard for “bladder invasion”, measured the performance of MRI using the MRI finding of not only mucosal invasion but also serosal and/or muscular invasion. These authors accepted a PPV of 7% to obtain an NPV of 100% [6]. To explain the low PPV in their study, they suggested two possibilities; namely, cystoscopic misdiagnosis and correct but undetectable serosal and/or muscular invasion. Hertel et al [10] compared laparoscopic findings with MRI and/or CT. In this study, which took the laparoscopic finding as a reference standard for “bladder wall invasion”, the authors obtained a PPV of 50% and an NPV of 96%. They did not describe the MRI finding for the positive interpretation. Kim and Han [9] evaluated bladder invasion in 300 consecutive patients who underwent MRI for pre-operative staging of uterine cervical carcinoma. These authors accepted either cystoscopic or surgical findings as the reference standard for “bladder invasion” and obtained an NPV of 99% and PPV of 100% for MRI. Findings on MRI that suggested bladder invasion was described as a mass protruding into the bladder lumen (n = 2), nodularity and irregularity of the bladder wall (n = 5) and high signal intensity of the anterior aspect of the posterior wall of the bladder (n = 3). Among these 10 MRI findings with true-positive results, 1 with nodularity and 3 with high-signal intensity of the anterior aspect of the posterior wall of the bladder were confirmed surgically, without evidence of mucosal invasion by cystoscopy.
We expected that involvement of the posterior bladder wall, as determined by MRI, could distinguish three distinct prognostic groups. We assumed that the prognosis for the wall invasion group would at least approach that of the mucosal invasion group more closely than that of the normal group. However, we found no significant difference in clinical outcome between the normal and wall invasion groups. Several factors might explain this result. First, the MRI may not gauge the degree of invasion with sufficient accuracy to exclude invasion of the bladder wall. Second, MRI detection of wall irregularity with heterogeneous signal and enhancement with thickening, nodularity or loss of fat plane may not necessarily mean serosal/muscular invasion. As we noted above, a direct comparison of the MRI finding of serosal/muscular invasion of the bladder wall with a surgical (not cystoscopic) analysis could resolve these two possibilities. Third, bladder muscle and/or serosal invasion may have occurred, but the retrospective nature and small number of patients in this study obscured the difference in prognosis between the normal and wall invasion groups. A significantly larger number of patients in the normal group received radiotherapy alone and experienced a longer overall treatment time than patients in the wall invasion group. According to the 2006 FIGO report [1], relative to the patients with stage IB, hazard ratios for stages IIIB and IVA were 3.0 and 7.3, respectively, in patients treated with radiotherapy alone, and 1.7 and 2.6, respectively, in patients treated with CCRT. Because of the retrospective nature of this study, the follow-up of our study population was dated back to 1997. Since 2000, we have used concurrent chemoradiotherapy as the standard of care for patients with cervical cancer. If chemotherapy had been uniformly given for the study population, we could draw more firm conclusions regarding the significance of non-mucosal invasion of the bladder wall.
In our study, patients with mucosal invasion on MRI, regardless of the cystoscopic finding, had significantly worse outcomes than patients in the wall invasion group. Pathological evidence of mucosal invasion did not correlate with any type of survival among patients with FIGO stage IIIB–IVA cervical cancer who were treated with radiotherapy. Efforts to enhance locoregional control are likely to increase survival in the mucosal invasion group, because the poor CSS for this group, as compared with the normal and wall invasion groups, was largely attributed to a worse LRFFS. Modern radiation therapy techniques such as image-guided radiation therapy would be helpful for adequate coverage of the bladder in consideration of filling status.
Conclusion
The prognosis for patients with findings of muscle and/or serosal invasion of the bladder wall on MRI may not differ from the prognosis for patients without bladder wall abnormalities on MRI. Mucosal invasion of the bladder wall on MRI may provide a more accurate prognosis than pathological evidence of bladder mucosal invasion. Considering the possible complications and sampling errors in the cystoscopic procedure, the finding of mucosal invasion on MRI could potentially replace cystoscopy for predicting the outcome of these patients. Further studies with larger patient populations may resolve the remaining questions.
References
- 1.Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, et al. Carcinoma of the cervix uteri. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95 Suppl 1:S43–103 [DOI] [PubMed] [Google Scholar]
- 2.Shin HR, Jung KW, Won YJ. 2002 annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Cancer Res Treat 2004;36:103–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACOG practicebulletin Diagnosis and treatment of cervical carcinomas. Number 35, May 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;78:79–91 [DOI] [PubMed] [Google Scholar]
- 4.Toita T, Kodaira T, Uno T, Shinoda A, Akino Y, Mitsumori M, et al. Patterns of pretreatment diagnostic assessment and staging for patients with cervical cancer (1999–2001): patterns of care study in Japan. Jpn J Clin Oncol 2008;38:26–30 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell DG, Snyder B, Coakley F, Reinhold C, Thomas G, Amendola M, et al. Early invasive cervical cancer: tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. J Clin Oncol 2006;24:5687–94 [DOI] [PubMed] [Google Scholar]
- 6.Rockall AG, Ghosh S, Alexander-Sefre F, Babar S, Younis MT, Naz S, et al. Can MRI rule out bladder and rectal invasion in cervical cancer to help select patients for limited EUA? Gynecol Oncol 2006;101:244–9 [DOI] [PubMed] [Google Scholar]
- 7.Chung H, Ahn HS, Kim YS, Lee EJ, Ryu HS, Chang KH, et al. The value of cystoscopy and intravenous urography after magnetic resonance imaging or computed tomography in the staging of cervical carcinoma. Yonsei Med J 2001;42:527–31 [DOI] [PubMed] [Google Scholar]
- 8.Hricak H, Lacey CG, Sandles LG, Chang YC, Winkler ML, Stern JL. Invasive cervical carcinoma: comparison of MR imaging and surgical findings. Radiology 1988;166:623–31 [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Han MC. Invasion of the urinary bladder by uterine cervical carcinoma: evaluation with MR imaging. AJR Am J Roentgenol 1997;168:393–7 [DOI] [PubMed] [Google Scholar]
- 10.Hertel H, Kohler C, Elhawary T, Michels W, Possover M, Schneider A. Laparoscopic staging compared with imaging techniques in the staging of advanced cervical cancer. Gynecol Oncol 2002;87:46–51 [DOI] [PubMed] [Google Scholar]
- 11.DeCherney AH, Nathan L, Goodwin TM, Laufer N. Current diagnosis & treatment obstetrics & gynecology (10th edn). New York: McGraw-Hill, 2007 [Google Scholar]
- 12.Lagasse LD, Creasman WT, Shingleton HM, Ford JH, Blessing JA. Results and complications of operative staging in cervical cancer: experience of the Gynecologic Oncology Group. Gynecol Oncol 1980;9:90–8 [DOI] [PubMed] [Google Scholar]
- 13.Hricak H, Powell CB, Yu KK, Washington E, Subak LL, Stern JL, et al. Invasive cervical carcinoma: role of MR imaging in pretreatment work-up: cost minimization and diagnostic efficacy analysis. Radiology 1996;198:403–9 [DOI] [PubMed] [Google Scholar]