Abstract
Objective
This prospective study compares MRI of atherosclerotic plaque in the abdominal aorta at 3 T with that at 1.5 T in patients suffering from hereditary hyperlipidaemia, a major risk factor for atherosclerosis.
Methods
MRI of the abdominal aorta at 1.5 and 3 T was performed in 21 patients (mean age 58 years). The study protocol consisted of proton density (PD), T1, T2 and fat-saturated T2 weighted black blood images of the abdominal aorta in corresponding orientation. Two independent radiologists performed image rating. First, image quality was rated on a five-point scale. Second, atherosclerotic plaques were scored according to the modified American Heart Association (AHA) classification and analysed for field strength-related differences. Weighted κ statistics were calculated to assess interobserver agreement.
Results
Interobserver agreement was substantial for nearly all categories. MRI at 3 T offered superior image quality in all contrast weightings, most significantly in T1 and T2 weighted techniques. Plaque burden in the study collective was unexpectedly moderate. The majority of plaques were classified as AHA III lesions; no lesions were classified above AHA V. There was no significant influence of the field strength regarding the AHA classification.
Conclusion
Abdominal aortal plaque screening is basically feasible at both field strengths, whereas the image quality is rated superior at 3 T. However, the role of the method in clinical practice remains uncertain, since substantial findings in the high-risk collective were scarce.
Atherosclerosis is a systemic disease of the vessel wall that mainly occurs in medium-sized and large arteries; its thrombotic or thromboembolic complications are the main cause of mortality and morbidity in industrialised countries [1,2]. It is characterised by a thickening of the vessel wall, especially the intima, and is histologically composed of a lipid core with an overlying fibrous cap. The main plaque components are fibrous elements (e.g. connective tissue, collagen, proteoglycans), lipids (e.g. cholesterol, phospholipids), smooth muscle cells and inflammatory cells (e.g. macrophages, T lymphocytes). The composition of the atherosclerotic plaque determines its vulnerability [3-6]. The so-called “vulnerable plaque” consists of a large lipid core and a thin fibrous cap, although the characteristics of the vulnerable plaques vary depending on the arterial region (i.e. coronaries, carotids, aorta) [6-8]. Rupture of atherosclerotic plaques as a result of an endothelial lesion is the most frequent cause of the unpredictable onset of acute thromboembolic vascular events such as myocardial infarction, ischaemic stroke or sudden cardiac death [9,10]. Therefore, it is necessary to characterise plaque components and determine them at an early stage to prevent cardiovascular events. Several invasive and non-invasive imaging modalities are used to study atherosclerotic vessel lesions: conventional (B-mode) ultrasound, intravascular ultrasound (IVUS), conventional angiography, CT, angioscopy and MRI [5,10]. Most of them identify luminal diameter or stenosis, wall thickness and plaque volume, but are not able to determine plaque components [5,11]. Several recent studies showed that in vivo and ex vivo MRI as a non-invasive method can characterise the composition of atherosclerotic plaques such as fibrous tissue, lipid core, calcification, haemorrhage and thrombus [5,10,12-18]. The aim of this characterisation is the determination of the risk of plaque rupture.
Most of the previous publications regarding plaque imaging in the human aorta are of studies performed at 1.5 T. Newer publications show the advantages of high field strength, such as faster imaging with parallel imaging techniques and higher signal-to-noise ratio (SNR) [19,20].
The purpose of this study was to compare in vivo multimodality MR plaque imaging of the human aorta at 1.5 T and 3 T in a patient collective at high risk for atherosclerosis, in which atherosclerotic wall alterations can be expected. The evaluation focused on image quality and on the analysis of atherosclerotic plaque components according to the American Heart Association (AHA) classification [3,21].
In the context of the prospective study, plaque imaging was always performed in addition to whole-body MR angiography (WBMRA) and is therefore part of a whole-body screening approach.
Material and methods
Patients
After approval of the study by the local ethics committee and written informed consent were obtained, 26 consecutive patients were included in the prospective study within a period of 12 months. Patients had been evaluated for lipid disorders in the Lipid Clinic of our university hospital. Both MRI studies were completed in 21 patients (7 females, 14 males), and clinical data have been given for these patients. The remaining five subjects refused the second scan. Mean age was 58 years (range 40–69 years). Type 2 diabetes was documented in 1 patient, and the mean body mass index (BMI) was 27 ± 3.9 kg m–2. Arterial hypertension was present in only four patients and cigarette smoking in five patients. The inclusion criteria for the patients participating in the study were Type III hyperlipoproteinaemia according to the Fredrickson classification (apolipoprotein (Apo) E 2/2, n = 9) or familial defective Apo B-100 hyperlipidaemia (Apo B 3500 mutation, n = 10). Genotyping of Apo E and Apo B 3500 was carried out by standard methods. The lipid phenotypes are given in Table 1. Type III hyperlipoproteinaemia with elevation of lipoprotein remnants and familial defective Apo B-100 hyperlipidaemia with elevation of low-density lipoprotein (LDL) are rare diseases and known to be associated with premature atherosclerosis.
Table 1. Lipid phenotypes of the study collective (n = 21).
| Apo E type | Cholesterola | Triglyceridesa | LDL chola | HDL chola | |
| HLP Type III patients | 2/2 | 524 | 443 | NA | 32 |
| 2/2 | 764 | 774 | NA | 89 | |
| 2/2 | 571 | 666 | NA | 33 | |
| 2/2 | 556 | 498 | NA | 82 | |
| 2/2 | 350 | 816 | NA | 42 | |
| 2/2 | 263 | 322 | NA | 43 | |
| 2/2 | 404 | 753 | NA | 34 | |
| 2/2 | 302 | 620 | NA | 74 | |
| 2/2 | 132 | 69 | NA | 24 | |
| Mean (±SD) | 430 (±192) | 551 (±244) | 50 (±24) | ||
| Familial defective Apo B-100 HLP patients | 3500 heb | 309 | 78 | 222 | 71 |
| 3500 he | 288 | 145 | 216 | 43 | |
| 3500 he | 315 | 241 | 209 | 58 | |
| 3500 he | 316 | 52 | 261 | 45 | |
| 3500 he | 292 | 136 | 168 | 47 | |
| 3500 he | 278 | 56 | 202 | 65 | |
| 3500 he | 369 | 102 | 287 | 52 | |
| 3500 he | 171 | 161 | 96 | 43 | |
| 3500 he | 347 | 102 | 282 | 45 | |
| 3500 he | 259 | 104 | 192 | 46 | |
| Mean (±SD) | 294 (±51) | 118 (±56) | 214 (±57) | 51 (±10) | |
| Add. pat. | 3/3 | 164 | 152 | 79 | 55 |
| 3/4 | 294 | 232 | 214 | 34 |
HDL, high-density lipoprotein; HPL, hyperlipoproteinaemia; LDL, low-density lipoprotein.
aCholesterol and triglycerides in mg dl–1.
bApo B 3500 heterozygotes.
Exclusion criteria for the study were secondary hyperlipidaemia, heart failure New York Heart Association III or IV, unstable coronary heart disease, claustrophobia and common MRI exclusion criteria such as metallic implants or implanted electronic devices.
MRI
All MR studies were performed on a 1.5 and 3 T MR scanner (Intera/Achieva, Philips, Medical Systems, Best, the Netherlands) within an interval of a few days. The contrast-enhanced MRA examinations mentioned above were not always performed during the same session; if so, the plaque protocol was performed first in order to avoid primary contrast enhancement. The study protocol included transverse cross-sectional images of the abdominal aorta located 2–3 cm proximal to the aortic bifurcation; for reproducibility of the positioning a coronal and a sagittal fast gradient-echo vessel scout were always performed at the beginning of the protocol. In order to achieve best possible consistency between the systems, the examinations were always performed with particular regard to the slice positioning by the responsible radiologist.
The MR images were acquired in supine position using a clinical cardiac coil capable of sensitivity encoding (SENSE) parallel imaging modalities. All sequences were optimised to the same effective voxel size (0.4 × 0.4 × 5 mm) regardless of the field strength.
The key sequence parameters at 3 T were the following: proton density (PD)-weighted turbo spin echo (TSE) sequence (repetition time (TR) 2000 ms, time to echo (TE) 25 ms), T2 weighted TSE sequence (TR 2000 ms, TE 98 ms), T2 weighted TSE sequence with fat suppression (spectral selection attenuated inversion recovery (SPAIR), TR 2000 ms TE 98 ms) and a T1 weighted turbo field echo (TFE) sequence (TR 6 ms, TE 3.2 ms, flip angle 35°).
The key sequence parameters at 1.5 T were the following: PD-weighted TSE sequence (TR 1558 ms, TE 20 ms), T2 weighted TSE sequence (TR 1558 ms, TE 80 ms,), T2 weighted TSE sequence with fat suppression (SPAIR, TR 1558 ms, TE 80 ms) and a T1 weighted TFE sequence (TR 6 ms, TE 3.1 ms).
All sequences were triggered by breath and electrocardiography. With each sequence, three slices with an interslice gap of 5 mm were acquired. At each scanner the examination took 30 min on average and was well tolerated by all patients.
MR image evaluation
The images were independently reviewed by two radiologists firstly regarding image quality on the basis of a five-point scale (5, best image quality, excellent depiction of the vessel wall and all plaque components; 4, good delineation of the vessel wall structures; 3, partial insufficiency in delineation of vessel wall structures in less than 50% of the circumference; 2, partial insufficiency in delineation of vessel wall structures in more than 50% of the circumference; 1, worst image quality, insufficiency of delineating the vessel wall itself). The responsible radiologist who performed the examinations was not one of the raters.
Second, the images were evaluated regarding vessel wall alterations and atherosclerotic plaques according to the modified AHA classification (Table 2) [3,4,21,22]. In this classification scheme, Types I and II of the conventional AHA classification have been summarised into Type I–II because a differentiation between discrete foam cells (Type I) and multiple foam cell layers of fatty streaks (Type II) has not been possible so far by MRI [21]. Accordingly Type IV and V have been combined into Type IV–V since proteoglycan composition of the Type IV cap can currently not be distinguished from the dense collagen of the Type V cap [21].
Table 2. Conventional and modified American Heart Association (AHA) classification of atherosclerotic plaque [21].
| Conventional AHA classification | Modified AHA classification for MRI |
| I: initial lesion with foam cells | I–II: near normal wall thickness, no calcification |
| II: fatty streak with multiple cell layers | |
| III: pre-atheroma with extracellular lipid pools | III: diffuse intimal thickening or small eccentric plaque with no calcification |
| IV: atheroma with a confluent extracellular lipid core | IV–V: plaque with a lipid or necrotic core surrounded by fibrous tissue with possible calcification |
| V: fibroatheroma | |
| VI: complex plaque with possible surface defect, haemorrhage or thrombus | VI: complex plaque with possible surface defect, haemorrhage or thrombus |
| VII: calcified plaque | VII: calcified plaque |
| VIII: fibrotic plaque without lipid core | VIII: fibrotic plaque without lipid core and without lipid core and with possible small calcifications |
Statistical analysis
Cohen's linear-weighted κ was calculated in order to assess interobserver variabilities regarding the image quality and the AHA ratings. The conventions are κ<0.20, “poor agreement”; 0.21<κ<0.40, “fair agreement”; 0.41<κ<0.60, “moderate agreement”; 0.61<κ<0.80, “substantial agreement”; 0.81<κ<1.00, “very good agreement” [23,24].
To test for significant differences due to the field strength the general linear model with sequence (PD, T1, T2, T2 SPAIR) as the intrasubject factor (repeated measurements/paired test) and modality ( = field strength) as the intersubject factor was applied.
Results
Of the 26 patients primarily enrolled, plaque imaging completely according to the study protocol at both field strengths was completed in 19 patients. T1 weighted sequences were not acquired for two patients at 1.5 T. These patients had complete examinations at 3 T and were additionally included in the data evaluation (n = 21).
Image quality
The rater agreement was at least “substantial” for all sequences at 3 T, moderate agreement was observed for PD- and T1 weighted sequences at 1.5 T (Figure 1). Calculating the agreement independently from the field strength yielded at least substantial agreement for all sequences. According to this result mean ratings were put into the general linear model statistics.
Figure 1.
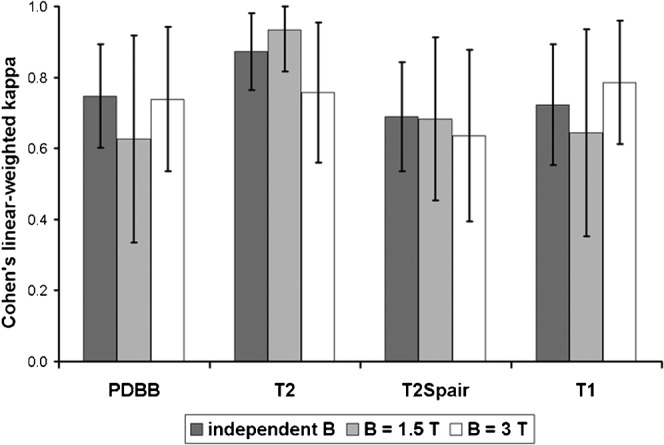
Rater agreement regarding the image quality. The bars display Cohen's linear-weighted κ; the error bars represent 95% confidence interval. There is no significant influence of the field strength (term B) on the level of agreement. PDBB, proton density black blood.
The results of the image quality ratings are displayed in Figure 2. Image quality was rated significantly superior at 3 T in all contrast weightings (highly significant intersubject effect: F = 12.030, df = 1, p = 0.001). The T2 weighted images were rated best at both scanners followed by the PD-weighted images.
Figure 2.
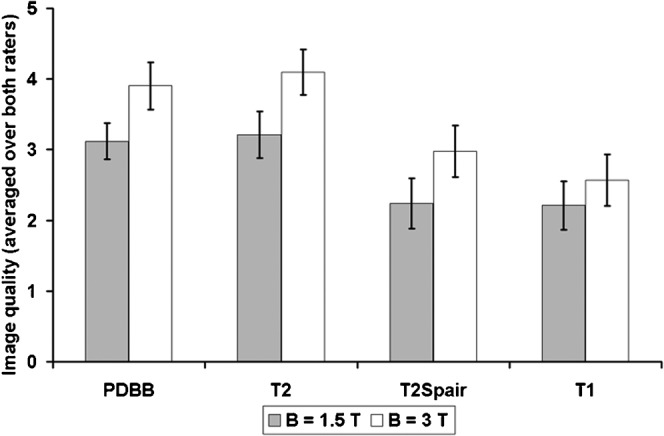
Ratings of image quality. Bars display mean ratings of image quality averaged over the two raters; error bars represent 2 times the standard error of the mean (≈95% confidence interval). All 3 T sequences were rated superior over 1.5 T. PD- and T2 weighted sequences achieved best ratings in both modalities. PDBB, proton density black blood.
Plaque evaluation
The results of the evaluation of the atherosclerotic plaques according to the modified AHA classification are displayed in Table 3 [21]. In four patients no vessel wall changes were depicted. Slight differences between the raters occurred, all independently from the field strength: Rater 2 classified 6 patients as AHA IV–V, whereas 3 of these were classified as AHA III by Rater 1 (the other three were rated concordantly). The majority of patients were rated as AHA III (Table 3). No patients were classified higher than AHA IV–V (Figure 3).
Table 3. American Heart Association scores of Rater 1 vs Rater 2, independent of field strength.
| Rater 2 |
Total | ||||||
| 0 | I–II | III | IV–V | ≥ VI | |||
| Rater 1 | 0 | 8 | 0 | 0 | 0 | 0 | 8 |
| I–II | 0 | 7 | 2 | 0 | 0 | 9 | |
| III | 0 | 0 | 13 | 6 | 0 | 19 | |
| IV–V | 0 | 0 | 0 | 6 | 0 | 6 | |
| ≥VI | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 8 | 7 | 15 | 12 | 0 | 42 | |
Figure 3.
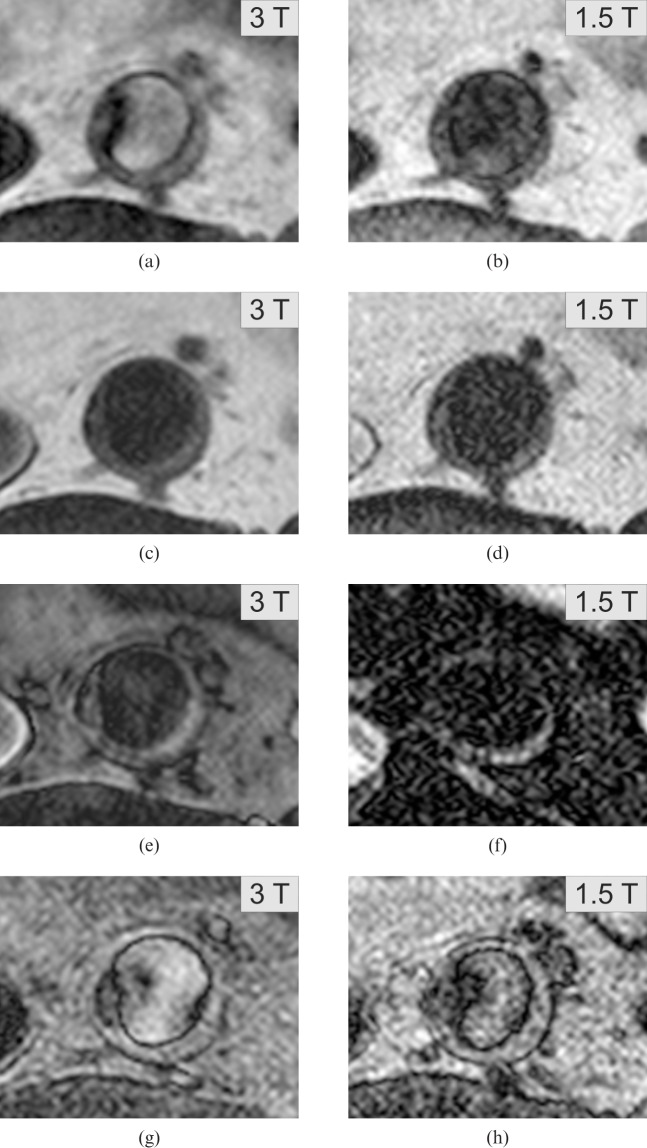
Example of a large plaque with necrotic core, rated as American Heart Association IV–V. Findings like these occurred more rarely than expected owing to the genetic risk. Presented are the PD-weighted (a, 3 T; b, 1.5 T), T2 weighted (c, 3 T; d, 1.5 T), T2 SPAIR (e, 3 T; f, 1.5 T) and T1 weighted sequences (g, 3 T; h, 1.5 T).
The agreement of the two raters regarding the AHA score was very good, and this result was independent of the field strength. Cohen's linear weighted κ was 0.842 (0.691–0.993) at 1.5 T (n = 21), 0.844 (0.739–0.949) at 3.0 T (n = 21) and 0.845 (0.699–0.992) independent of field strength (n = 42). The further statistical analysis, therefore, was restricted to Rater 1. AHA scores were highly concordant between 1.5 T and 3 T. Cohen's linear weighted κ for Rater 1 was 0.958 (0.874–1.000).
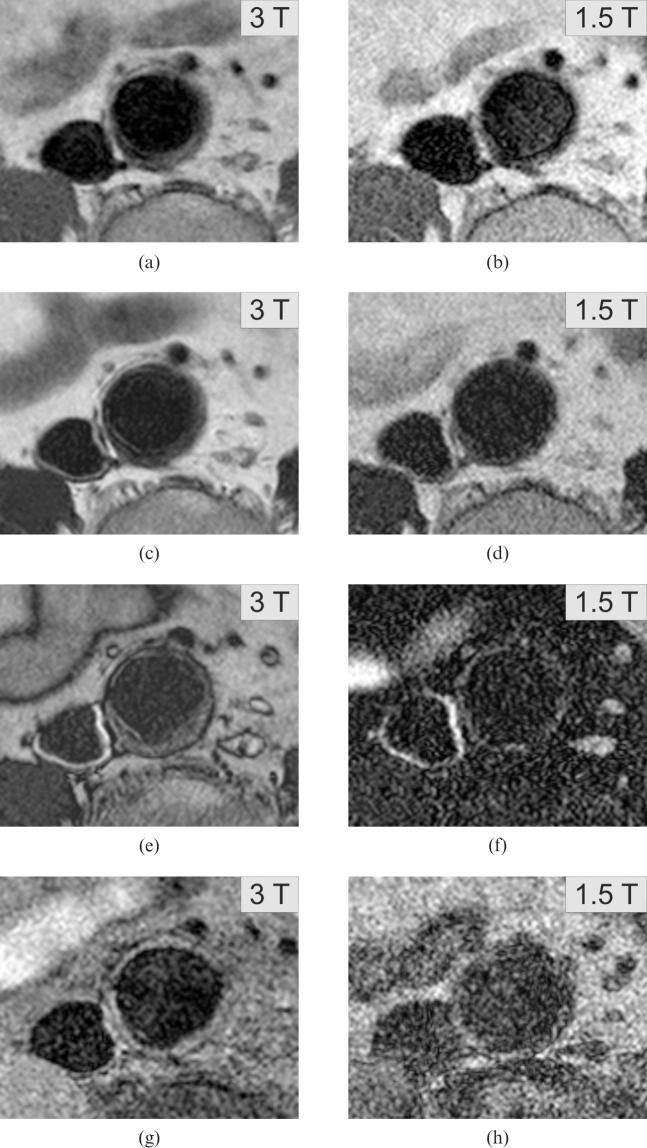
Regarding the influence of the field strength on the plaque classification there was a different score in only 1 of the 21 cases (Table 4): a plaque, which was rated as I–II at 1.5 T, was upgraded to III at 3 T (both raters). The images are displayed in Figure 4.
Table 4. American Heart Association score at 1.5 T vs 3 T for all 21 aorta probes (rater 1).
| 3 T |
Total | ||||||
| 0 | I–II | III | IV–V | ≥VI | |||
| 1.5 T | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
| I–II | 0 | 4 | 1 | 0 | 0 | 5 | |
| III | 0 | 0 | 9 | 0 | 0 | 9 | |
| IV–V | 0 | 0 | 0 | 3 | 0 | 3 | |
| ≥VI | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 4 | 4 | 10 | 3 | 0 | 21 | |
Figure 4.
In this particular case the American Heart Association scores were upgraded from I–II at 1.5 T to III at 3 T (consensus). Presented are the PD-weighted (a, 3 T; b, 1.5 T), T2 weighted (c, 3 T; d, 1.5 T), T2 SPAIR (e, 3 T; f, 1.5 T) and T1 weighted sequences (g, 3 T; h, 1.5 T). The eccentric plaque at the dorsal vessel wall is better depicted in all 3 T sequences.
Discussion
Applying MRI for screening for atherosclerotic plaque in a patient collective at high risk for suffering from atherosclerosis is an approach increasingly used [5,20]. Newer MRI data from patients with heterozygous hypercholesterolaemia provide evidence for elevated plaque burden despite long-term lipid-lowering therapy, which enhances the importance of having a reliable, non-invasive screening method [19]. The comparability of the MRI-modified AHA classification with the histological AHA classification has been shown in previous work [6,21,22].
Whereas most previous studies were performed at 1.5 T, our study provides intra-individual and therefore best comparable vessel wall image data from 1.5 T as well as 3 T. We have to admit that both the amount of plaque and the severity of plaque burden were expected to be much more pronounced in the collective we examined. It is usually very likely that these patients will develop severe atherosclerotic disease. All patients had pathological lipid phenotypes (Table 1) in addition to other metabolic risk factors, e.g. elevated BMI. No patients in our study were diagnosed with complex or even calcified plaque (AHA VI or higher) (Tables 2 and 3).
Regarding the image quality, there was a significant benefit in applying 3 T. Since the voxel size was the same at both field strengths, this benefit is partly explained by the higher SNR at 3 T. The results of the AHA scorings revealed more (but still few) differences between the two raters than according to the field strength. The anatomic correlation between both examinations was not a scored item but is of course an issue regarding the comparability of the studies. One plaque that was scored higher at 3 T (Figure 3) could hypothetically be explained by insufficient anatomical correlation, although the slice positioning based on a bidirectional survey scan was performed by the responsible radiologist himself (with particular regard to the examination performed first). However, the poor image quality at 1.5 T should in this particular case be taken into consideration.
The upcoming issue of molecular contrast agents specific for thrombotic material or reorganising markers was not addressed in this work, although it would have been an interesting add-on with regard to the relatively moderate plaque burden diagnosed in our collective [5,6,25,26]. Since the modified AHA classification seems to be applicable at 1.5 as well as 3 T with comparable results, and plaque load is sometimes small, there may be an increasing need for such additional tissue information in order to assess a patient's risk properly.
One major limitation of our study was that our study population consisted only of an in vivo patient group so that no histological correlation could be given to assess the correctness of the plaque ratings. However, the lack of a gold standard does not affect the analysis of inter-rater agreement, which was encouragingly good.
A “healthy” control group did not exist either. A correlation with an age- and sex-matched control group would have been desirable, especially regarding the plaque findings (but also regarding image quality). Since the plaque burden was much lower than expected in our collective, neither the method nor the distal abdominal aorta as an anatomical region qualify as a suitable parameter for screening for atherosclerosis in patients with hereditary lipid disorders. As a consequence, based on our limited data, plaque MRI of the abdominal aorta cannot be recommended as a first-line method for screening purposes. However, it still has its place in research regarding aortic aneurysm development or monitoring known plaques for instability or during lipid-lowering medication [27-30].
In addition, the method is susceptible to incompliant patients and, which is a relevant matter in healthcare politics today, is quite expensive and time-consuming, especially when combined with WBMRA.
The results of the evaluation of the WBMRA scans performed in the context of the study will discussed in another article.
Altogether, sufficient classification of atherosclerotic plaque can be performed at 1.5 T. Regarding image quality, MRI at 3 T was superior in our collective. We can conclude that 3 T is preferable if available; however, we advise careful consideration of the indications because the clinical consequences are not definite.
Conclusion
An evaluation of atherosclerotic plaque according to the modified AHA classification is possible at 1.5 and 3 T with good correlation between the reading radiologists; image quality at 3 T was rated superior.
However, the further role of aortic plaque MRI in clinical practice and particularly in screening for atherosclerosis remains uncertain owing to a substantially low plaque burden in our collective.
Footnotes
The study was supported by Deutsche Forschungsgemeinschaft (project number AD 125/4-1).
References
- 1.Sirol M, Fuster V, Fayad ZA. Plaque imaging and characterization using magnetic resonance imaging: towards molecular assessment. Curr Mol Med 2006;6:541–8 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization World health statistics. Geneva, Switzerland: WHO, 2008 [Google Scholar]
- 3.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 2000;20:1177–8 [DOI] [PubMed] [Google Scholar]
- 4.Larose E, Yeghiazarians Y, Libby P, Yucel EK, Aikawa M, Kacher DF, et al. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation 2005;112:2324–31 [DOI] [PubMed] [Google Scholar]
- 5.Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, et al. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology 2007;244:64–77 [DOI] [PubMed] [Google Scholar]
- 6.Briley-Saebo KC, Mulder WJ, Mani V, Hyafil F, Amirbekian V, Aguinaldo JG, et al. Magnetic resonance imaging of vulnerable atherosclerotic plaques: current imaging strategies and molecular imaging probes. J Magn Reson Imaging 2007;26:460–79 [DOI] [PubMed] [Google Scholar]
- 7.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13–18 [DOI] [PubMed] [Google Scholar]
- 8.Cury RC, Houser SL, Furie KL, Stone JR, Ogilvy CS, Sherwood JB, et al. Vulnerable plaque detection by 3.0 tesla magnetic resonance imaging. Invest Radiol 2006;41:112–15 [DOI] [PubMed] [Google Scholar]
- 9.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005;46:937–54 [DOI] [PubMed] [Google Scholar]
- 10.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part II: approaches by noninvasive computed tomographic/magnetic resonance imaging. J Am Coll Cardiol 2005;46:1209–18 [DOI] [PubMed] [Google Scholar]
- 11.Halliburton SS, Paschal CB. Atherosclerotic plaque components in human aortas contrasted by ex vivo imaging using fast spin-echo magnetic resonance imaging and spiral computed tomography. Invest Radiol 1996;31:724–8 [DOI] [PubMed] [Google Scholar]
- 12.Skinner MP, Yuan C, Mitsumori L, Hayes CE, Raines EW, Nelson JA, et al. Serial magnetic resonance imaging of experimental atherosclerosis detects lesion fine structure, progression and complications in vivo. Nat Med 1995;1:69–73 [DOI] [PubMed] [Google Scholar]
- 13.Toussaint JF, LaMuraglia GM, Southern JF, Fuster V, Kantor HL. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 1996;94:932–8 [DOI] [PubMed] [Google Scholar]
- 14.von Ingersleben G, Schmiedl UP, Hatsukami TS, Nelson JA, Subramaniam DS, Ferguson MS, et al. Characterization of atherosclerotic plaques at the carotid bifurcation: correlation of high-resolution MR imaging with histologic analysis—preliminary study. Radiographics 1997;17:1417–23 [DOI] [PubMed] [Google Scholar]
- 15.Shinnar M, Fallon JT, Wehrli S, Levin M, Dalmacy D, Fayad ZA, et al. The diagnostic accuracy of ex vivo MRI for human atherosclerotic plaque characterization. Arterioscler Thromb Vasc Biol 1999;19:2756–61 [DOI] [PubMed] [Google Scholar]
- 16.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959–64 [DOI] [PubMed] [Google Scholar]
- 17.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051–6 [DOI] [PubMed] [Google Scholar]
- 18.Coombs BD, Rapp JH, Ursell PC, Reilly LM, Saloner D. Structure of plaque at carotid bifurcation: high-resolution MRI with histological correlation. Stroke 2001;32:2516–21 [DOI] [PubMed] [Google Scholar]
- 19.Schmitz SA, O'Regan DP, Fitzpatrick J, Neuwirth C, Potter E, Tosi I, et al. Quantitative 3-T MR imaging of the descending thoracic aorta: patients with familial hypercholesterolemia have an increased aortic plaque burden despite long-term lipid-lowering therapy. J Vasc Interv Radiol 2008;19:1403–8 [DOI] [PubMed] [Google Scholar]
- 20.Maroules CD, McColl R, Khera A, Peshock RM. Assessment and reproducibility of aortic atherosclerosis magnetic resonance imaging: impact of 3-Tesla field strength and parallel imaging. Invest Radiol 2008;43:656–62 [DOI] [PubMed] [Google Scholar]
- 21.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368–73 [DOI] [PubMed] [Google Scholar]
- 22.Koops A, Ittrich H, Petri S, Priest A, Stork A, Lockemann U, et al. Multicontrast-weighted magnetic resonance imaging of atherosclerotic plaques at 3.0 and 1.5 Tesla: ex-vivo comparison with histopathologic correlation. Eur Radiol 2007;17:279–86 [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull 1968;70:213–20 [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 [PubMed] [Google Scholar]
- 25.Burtea C, Laurent S, Murariu O, Rattat D, Toubeau G, Verbruggen A, et al. Molecular imaging of alpha v beta3 integrin expression in atherosclerotic plaques with a mimetic of RGD peptide grafted to Gd-DTPA. Cardiovasc Res 2008;78:148–57 [DOI] [PubMed] [Google Scholar]
- 26.Bitar R, Moody AR, Leung G, Kiss A, Gladstone D, Sahlas DJ, et al. In vivo identification of complicated upper thoracic aorta and arch vessel plaque by MR direct thrombus imaging in patients investigated for cerebrovascular disease. AJR Am J Roentgenol 2006;187:228–34 [DOI] [PubMed] [Google Scholar]
- 27.Helft G, Worthley SG, Fuster V, Fayad ZA, Zaman AG, Corti R, et al. Progression and regression of atherosclerotic lesions: monitoring with serial noninvasive magnetic resonance imaging. Circulation 2002;105:993–8 [DOI] [PubMed] [Google Scholar]
- 28.Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Smith D, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation 2002;106:2884–7 [DOI] [PubMed] [Google Scholar]
- 29.Kramer CM, Cerilli LA, Hagspiel K, DiMaria JM, Epstein FH, Kern JA. Magnetic resonance imaging identifies the fibrous cap in atherosclerotic abdominal aortic aneurysm. Circulation 2004;109:1016–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonemura A, Momiyama Y, Fayad ZA, Ayaori M, Ohmori R, Kihara T, et al. Effect of lipid-lowering therapy with atorvastatin on atherosclerotic aortic plaques: a 2 year follow-up by noninvasive MRI. Eur J Cardiovasc Prev Rehabil 2009;16:222–8 [DOI] [PubMed] [Google Scholar]