Abstract
Objectives
To investigate the role of diffusion-weighted MRI (DWI) in the diagnosis of urinary bladder (UB) tumours by means of measuring apparent diffusion coefficient (ADC) values.
Methods
A total of 83 people aged between 18 and 86 years were included in the study: 63 patients with UB pathology (46 malignant, 17 benign) constituted the case group; 20 individuals without any UB pathology constituted the control group. DWI was applied to all individuals. The ADC values were measured based on the tissue of the UB mass entities and normal UB wall in the control group.
Results
The mean ADC value in the UB carcinoma group was significantly lower than that in the control group: 1.0684 ± 0.26 × 10−3 mm2 s–1 and 2.010 ± 0.11 × 10−3 mm2 s–1, respectively (p<0.01). There was a significant difference among the mean ADC values of different grades of malignant tumours, corresponding to 0.9185 ± 0.20 mm2 s–1 and 1.281 ± 0.18 mm2 s–1 in high-grade and low-grade malignant UB carcinomas, respectively (p<0.01). The ADC value in the carcinoma group was significantly lower than that in the benign lesion group: 1.0684 ± 0.26 × 10−3 mm2 s–1 and 1.803 ± 0.19 × 10−3 mm2 s–1, respectively (p<0.01). All 46 malignant lesions displayed a restriction in diffusion; 4 of the 17 benign lesions displayed a mild restriction in diffusion. The sensitivity, specificity and accuracy of DWI in the diagnosis of malignant UB lesions was 100%, 76.5% and 93.65%, respectively.
Conclusion
DWI can be beneficial in the differentiation of benign and malignant UB lesions, as well as of high-grade and low-grade UB carcinomas, using quantitative ADC measurements.
In recent years, a number of different MRI methods together with conventional ones have been used as part of a routine radiodiagnostic application. Diffusion-weighted MRI (DWI) is considered to be such an imaging method, evaluated within the context of functional MRI and based on the measurement of the accelerated or decelerated microscopic diffusion movements in the protons of the tissues' water molecules. Images can be obtained in short-period snapshots and do not require any contrast [1-4].
The use of DWI had previously been limited to brain examinations because it is sensitive to cardiac, respiratory and peristaltic movements; however, it has been adopted for a much broader use, i.e. for other parts of the body, with the development of fast MRI sequences such as echoplanar imaging (EPI). In early studies, the diffusion-weighted images and the apparent diffusion coefficient (ADC) of tissues and lesions were measured, and the different values obtained were shown to be useful in the differential diagnosis. In later studies, it was reported that ADC vales were related to the cellular intensity of a tumour, and a significant reduction in ADC indicated a malignant tumour [1-4].
In DWI studies conducted on patients with head and neck lesions, the mean ADC level was significantly lower in malignant lymphomas, moderately low in patients with carcinomas, moderately high in those with a benign solid masses and markedly higher in those with benign cystic lesions [5].
In recent years, DWI has been adopted for widespread use in abdominal examinations. In DWI studies conducted on liver masses, patients with metastatic lesions and hepatocellular carcinoma showed significantly lower ADC levels, those with cavernous haemangiomas showed moderately higher ADC levels, and those with cysts showed markedly higher ADC levels [6].
There are also studies reporting that benign renal tumours have much higher ADC values than malignant tumours, and that cystic renal lesions have much higher ADC values than benign solid renal tumours [7].
Various researchers in different studies have pointed out that DWI can be an important diagnostic tool in the detection and characterisation of tumours in different regions such as breast, prostate, bladder, cervix, colon, ovary, pancreas and liver. In these studies, it was also indicated that malignant tumours showed much more diffusion restriction and much lower ADC levels than benign tumours owing to their cellularity [8,9].
Although DWI studies conducted on bladder masses have not been reported on a broader scale, there has been an increasing tendency to conduct such studies recently. In these studies, the mean ADC levels in bladder tumours have been compared with the surrounding soft tissues and statistically significant results have been obtained. ADC levels in bladder carcinomas were found to be significantly lower than those of the surrounding soft tissues. DWI was also found to have a high sensitivity and specificity in the detection of bladder tumours [10,11].
In this study, the mean ADC values obtained from malignant and benign urinary bladder wall pathologies in patients referred to our clinic with a prediagnosis of bladder tumour were compared with the mean ADC values of normal bladder wall in the control group. Also, the ADC levels of the histopathological subgroups of patients with urinary bladder carcinomas were compared among each other. Our aim was to investigate whether DWI could be used in the differentiation of malignant and benign urinary bladder lesions, and whether ADC values found in malignancies could provide any information about the nature of the malignant masses, and thus to research the diagnostic contribution of DWI in standard treatment protocols.
Materials and methods
This prospective study was carried out between January 2008 and July 2009 at our institution. A total of 88 individuals were recruited for evaluation. However, five of them had poor general health and could not tolerate MRI examination; as a result, they were excluded from the study. Therefore, 83 individuals were included in the study. 63 patients (53 men, 10 women; mean age 62.84 ± 11.23 years) found to have bladder wall pathology (mass, wall thickening) on conventional MRI constituted the case group. Cystoscopic biopsy was performed in all of the patients in the case group. For the control group, 20 healthy individuals (13 males, 7 females; mean age 42 ± 18.17 years) who had no bladder pathology were enrolled in the study.
Ethics committee approval was obtained, and the patients were informed about the study before the procedure and the consent form was signed. During the MRI examination, appropriate communications were made with patients through the earphone system. To improve evaluation of the bladder wall, bladder distension was encouraged before the procedure.
Imaging
Pelvic examinations were obtained using a 1.5 T MRI system (Magnetom Symphony, Siemens Medical Solutions, Erlangen, Germany), with a phase-array body coil. The gradient power of the superconductive (niobium–titanium) magnet was 30 mT m–1, and the field of view (FOV) width was 350 cm.
Before the DWI examination was performed with SS-EPI, a T2 weighted true fast imaging with steady state precession (FISP) sequence with a chemical shift fat suppression technique in the axial and coronal planes (repetition time (TR), 4.3 s; echo time (TE), 2.15 s; average, 1; flip angle, 75°; matrix, 256 × 256; slice number, 23; slice thickness, 5 mm; FOV, 350 cm; slice gap, 15%) was applied. The protocol used for echoplanar diffusion in our clinic was defined as 0 mm2 s–1/500 mm2 s–1/1000 mm2 s–1 ADC, or trace diffusion for short.
Image analysis
The images were transferred to a work station (Leonardo Syngo 2002B, Siemens Medical Solutions) in order to process the data and ADC maps in DWI. Measurements were conducted through a circular region of interest (ROI) on lesions. ADC levels were measured by using the ROI from the most hypointense region of the mass lesions on the bladder wall or the pathological wall thickening, where the ROI areas were recorded in the range of 15–180 mm2. In the control group, measurements were made from the region limited to 3–5 pixels (6–9 mm2) from the normal bladder wall with a freehand ROI. The signal intensity changes in the lesions were visually determined according to b = 1000 diffusion-weighted trace images and the signal intensities on ADC images. In our study, the diagnostic relevance in patients diagnosed with the DWI technique were compared with the histopathological results of the cystoscopic biopsy.
Statistical analysis
Statistical values are expressed as the mean and standard deviation. One-way analysis of variance (ANOVA) was carried out to determine whether there was any difference between the patient and control groups in terms of ADC levels. The significance level in the measurements was taken as 5%, and the measurements were carried out using SPSS 13.0 (Chicago, IL) software. In our study, sensitivity, specificity, positive predictive value, negative predictive value and accuracy rate of DWI were obtained for detecting bladder carcinomas.
Results
Of the 63 patients in the case group, 46 (37 male, 9 female; mean age 61.54 ± 12.29 years) had urothelial carcinoma and 17 (1 female, 16 male; mean age 66.35 ± 10.04 years) had benign wall pathology. 27 of the 46 patients with a malignant mass had high-grade and 19 had low-grade carcinoma. In the 17 patients with a benign lesion, 12 had benign wall thickening secondary to benign prostatic hyperplasia (BPH), 3 had eosinophilic cystitis and 2 had polypoid cystitis (uroepithelial papilloma).
The histopathology of the 46 cases was consistent with carcinoma, and a restriction in diffusion (hyperintense appearance on DWI) was observed in all of the malignant masses (Figure 1 and 2). Of the 17 patients in the benign group, 4 (2 with eosinophilic cystitis and 2 with polypoid cystitis) showed a slight restriction in diffusion (Figure 3). In the remaining 13 benign cases (12 with wall thickening secondary to BPH and 1 with eosinophilic cystitis), no restriction in diffusion was observed (Figure 4). Two of the three patients with eosinophilic cystitis and both of the patients with polypoid cystitis (uroepithelial papilloma) showed diffusion restriction. In the detection of malignant lesions based on diffusion restriction, the sensitivity of DWI was 100%, specificity 76.5%, positive predictive value 92%, negative predictive value 100% and the accuracy was 93.65% (Table 1).
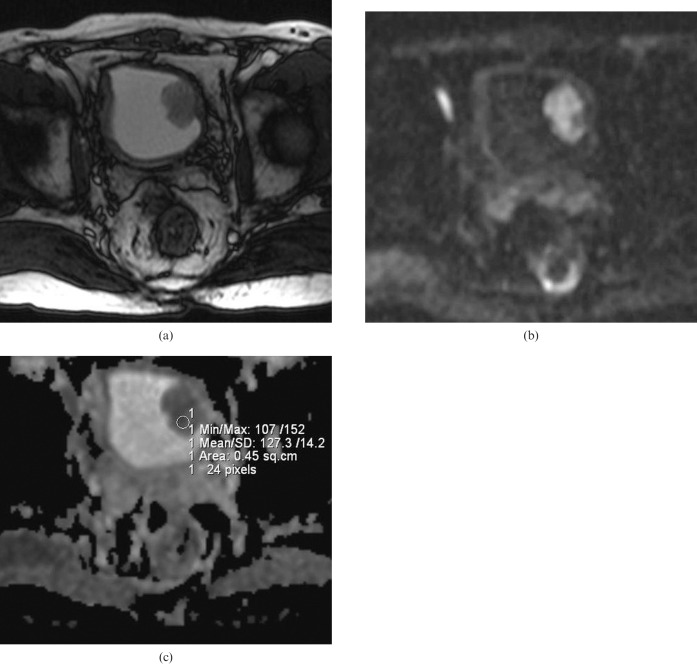
Figure 1.
(a) Axial T2 weighted MR image in a 64-year-old male depicting a 4 cm polypoid solid mass with a lobulated contour on the left lateral wall of the bladder. (b) On b = 1000 diffusion-weighted imaging, the mass shows a hyperintense signal corresponding to a restriction in diffusion. (c) Apparent diffusion coefficient (ADC) map of the same lesion. The ADC value of the lesion was measured as 1.27 × 10−3 mm2 s–1. Histopathologically, the lesion was reported as low-grade non-invasive urothelial carcinoma.
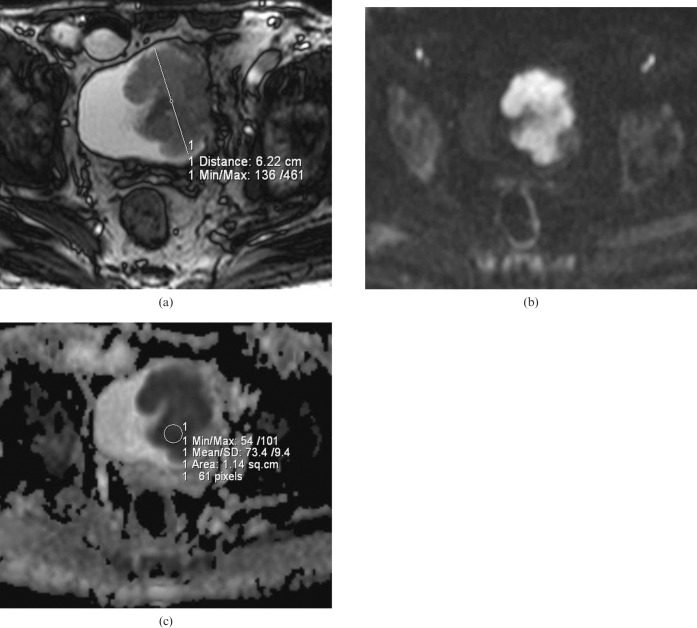
Figure 2.
(a) Axial T2 weighted MR image of a 76-year-old man depicting a 7 cm polypoid solid mass with a lobulated contour on the left wall of the bladder. (b) On b = 1000 diffusion-weighted imaging, the mass shows a hyperintense signal corresponding to a restriction in diffusion. (c) Apparent diffusion coefficient (ADC) map of the same lesion. The ADC value of the lesion was measured as 0.73 × 10−3 mm2 s–1. Histopathologically, the lesion was reported as high-grade invasive urothelial carcinoma.
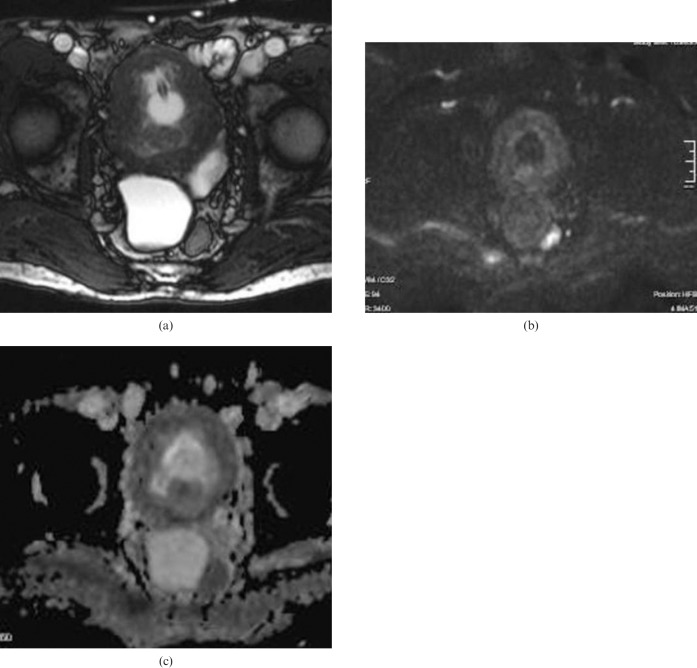
Figure 3.
(a) Axial T2 weighted MR image in a 37-year-old female depicting a small polypoid solid mass with a lobulated contour on the left posterior wall of the bladder. (b) On b = 1000 diffusion-weighted imaging, the mass lesion on the left bladder wall does not show any restriction in diffusion. The apparent diffusion coefficient of the lesion was measured as 2.01 × 10−3 mm2 s–1. The histopathological result was polypoid cystitis.
Figure 4.
(a) Axial T2 weighted MR image in a 53-year-old male shows diffuse thickening of the bladder wall. (b) On b = 1000 diffusion-weighted imaging, the thickened bladder wall does not show any restriction in diffusion. (c) The apparent diffusion coefficient of the thickened bladder wall was measured as 1.64 × 10−3 mm2 s–1. The histopathological result was benign wall thickening.
Table 1. Statistical results of diffusion-weighted imaging in the detection of malignant bladder lesions.
| Sensitivity | 46/46 (100%) |
| Specificity | 13/17 (76.5%) |
| Negative predictive value | 13/13 (100%) |
| Positive predictive value | 46/50 (92%) |
| Accuracy | (13 + 46)/63 (93.65%) |
In malignant masses, lower ADC levels and diffusion restriction were observed. Definitive statistics and a comparison of the results according to the patient and control groups in terms of ADC values are given in Table 2.
Table 2. Mean apparent diffusion coefficient (ADC) values in the malignant group, benign group, and control group.
| n | Mean ADC (× 10−3 mm2 s–1) | SD | |
| Urinary bladder carcinoma | 46 | 1.0684 | 0.26 |
| Benign pathology | 17 | 1.8030 | 0.19 |
| Control group | 20 | 2.0105 | 0.11 |
SD, standard deviation.
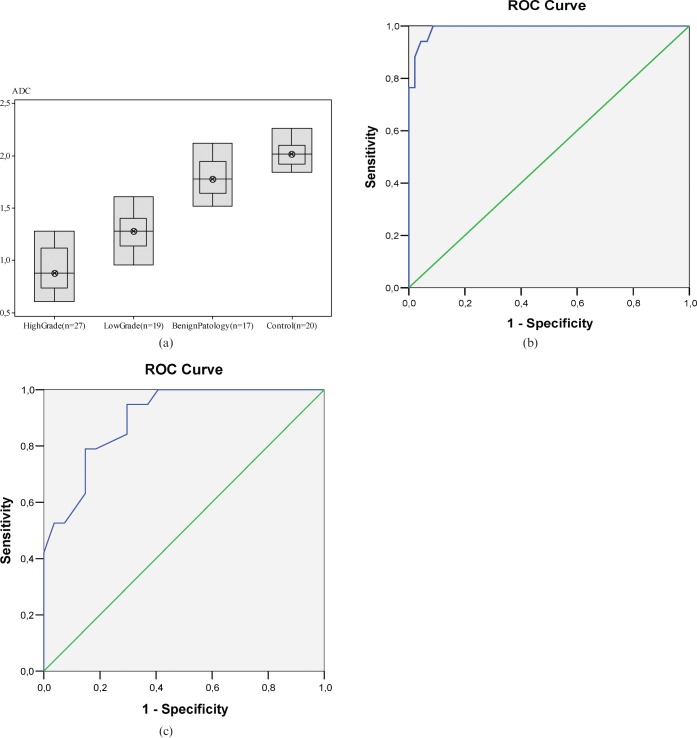
The mean ADC in the malignant group (1.068 ± 0.26 × 10−3 mm2 s–1) was significantly lower than that of benign wall pathologies (1.803 ± 0.19 × 10−3 mm2 s–1). It was also markedly lower than that of the bladder wall in the control group (2.010 ± 0.11 × 10−3 mm2 s–1). The difference was statistically significant (p<0.001). According to the histopathological subgroups, the mean ADC of high-grade malignant masses (0.918 ± 0.20 × 10−3 mm2 s–1) was found to be significantly lower than that of low-grade malignant masses (1.28 ± 0.18 × 10−3 mm2 s–1) (p<0.01), as shown in Table 3. The ADC distribution of the groups is shown in Figure 5a.
Table 3. Apparent diffusion coefficient (ADC) values based on histopathological subgroups of malignant lesions.
| n | Mean ADC (× 10−3 mm2 s–1) | SD | |
| High-grade carcinoma | 27 | 0.9185 | 0.20 |
| Low-grade carcinoma | 19 | 1.2815 | 0.18 |
SD, standard deviation.
Figure 5.
(a) Apparent diffusion coefficient (ADC) distribution of the groups as a box plot. (b) Receiver operating characteristic (ROC) curve analysis. The cut-off value in the differentiation of malignant and benign bladder wall pathologies according to ADC values based on this curve is 1.545 × 10−3 mm2 s–1, with a sensitivity of 94.1% and specificity of 95.7%. (c) Cut-off value in the differentiation of high-grade and low-grade bladder carcinomas according to ADC values based on the ROC curve is 1.135 × 10−3 mm2 s–1, with a sensitivity of 78.9% and specificity of 85.2%.
The cut-off value in the receiver operating characteristic (ROC) curve for differentiating malignant and benign bladder wall pathologies according to ADC values was found to be 1.545 × 10−3 mm2 s–1. Based on this value, the sensitivity was 94.1% and the specificity was 95.7%. In addition, the cut-off value in the ROC curve for differentiating high- and low-grade malignant masses according to ADC levels was found to be 1.135 × 10−3 mm2 s–1. Based on this value, the sensitivity was 78.9% and the specificity was 85.2% (Figure 5b,c).
Discussion
In patients with haematuria who have normal upper urinary system examinations, bladder tumour is considered among the foremost possible diagnoses. More than half of urinary system tumours are located in the bladder. More than 90% of bladder tumours, 80–90% of which are macroscopically of polypoid origin, are considered to be carcinomas with mutating epithelial cells [12].
Cystoscopy is accepted as the most reliable examination method in the detection of bladder tumours and is considered to be the gold standard. However, radiological examinations are also needed in order to look for tumours in the follow-up process and in the staging of the disease. There is no accepted algorithm for radiological imaging of bladder tumours. Imaging techniques such as intravenous pyelography, ultrasonography, CT and MRI are used either alone or together [12]. DWI, which was formerly used in neuroradiology, has recently been used in clinical applications as a new functional imaging method and as the preferred method in abdominal examinations with the development of ultrafast sequences such as EPI. Although there have previously been a few DWI studies conducted on bladder masses, a spate of new research is emerging. Most of the investigations conducted on abdomen diffusion have been carried out with an SS-EPI sequence [13].
With the SS-EPI technique, images can be taken in less 1 s and physiological movements are frozen [13]. Using breath-holding with the EPI technique, respiratory artefacts are removed and ADC measurements on the abdomen can be made [14]. Although previous studies using DWI were conducted using breath-holding, Thoeny et al [15] carried out a study during normal respiration and observed no remarkable movement artefact.
Also, in our study, the SS-EPI technique was used without breath-holding during DWI. Thus, an appropriate examination could be made in patients who were not able to hold their breath for a long period of time. Also, since the bladder is located in the lower abdomen, respiration movement has little effect upon it. There are studies in the literature reporting that DWI and mean ADC levels are useful tools in the differentiation of malignant and benign lesions and in the characterisation of solid renal tumours, liver lesions and gastric tumours. These studies also reported that there were statistically significant differences between ADC levels of the lesions [9,16-21].
DWI studies related to bladder masses have been more intensely conducted in recent years. In a study by Matsuki et al [22], 17 tumour masses in 15 patients with bladder cancer were examined and the ADC levels in the tumour tissue were found to be significantly lower than those in the surrounding tissue. Therefore, these researchers stressed that further studies should be carried out.
Kılıçkesmez et al [23], in a study which included 14 bladder carcinomas and 9 prostate carcinomas, found that the mean ADC level of tumours was significantly lower than that in the control group.
Takeuchi et al [24] investigated a total of 52 bladder tumours in 40 patients and found that DWI contributed to T staging, and that ADC levels in the tumours of higher grade were found to be significantly lower than those in tumours of lower grade.
In a study carried out by Abou-El-Ghar et al [10], 130 (106 with bladder carcinoma) patients with gross haematuria underwent T2 weighted MRI, DWI and, after 48 h, cystoscopy. The sensitivity of DWI in detecting the tumour mass by itself was found to be 98.5% and the positive predictive value was 100%.
In a study by El-Assmy et al [25], DWI and T2 weighted MRI were compared for accuracy of tumour staging in a total of 106 patients with 106 bladder tumours. DWI alone was found to be more accurate than T2 weighted MRI for tumour staging. In another study carried out by El-Assmy et al [11] on 43 patients with bladder tumours, the ADC was found to be significantly lower in bladder carcinomas than in the surrounding tissues.
In our study, the ADC levels of 63 patients who were referred to our clinic with a prediagnosis of bladder tumour were measured. These levels were compared with bladder wall ADC levels of 20 healthy individuals in the control group. Of the 63 patients, 46 showed carcinomas on histopathology. In all of the 46 mass lesions in the malignant group, diffusion restriction was observed. Of the 17 cases in the benign group, 4 manifested diffusion restriction and their pathology showed accordance with cystitis (2 patients had polypoid cystitis and 2 had eosinophilic cystitis). In the 13 benign cases (12 patients with wall thickening secondary to BPH and 1 with eosinophilic cystitis) diffusion restriction was not observed. In the two patients with eosinophilic cystitis and the two with polypoid cystitis, diffusion restriction was apparent. Based on these results, in the detection of malignant lesions, the sensitivity of DWI was found to be 100%, specificity 76.5%, positive predictive value 92%, negative predictive value 100% and the accuracy rate was 93.65% (Table 1).
In our study, the mean ADC levels of 46 patients with malignant tumours (1.068 ± 0.26 × 10−3 mm2 s–1) were found to be significantly lower than those of normal bladder wall in the control group (2.010 ± 0.11 × 10−3 mm2 s–1) (p<0.001). These results support the results of other studies in the literature. The mean ADC levels of 17 patients found to have benign wall pathology were found to be 1.803 ± 0.19 × 10−3 mm2 s–1, which was significantly higher than the mean ADC levels of the malignant urinary bladder tumours (p<0.01). In terms of the subgroups, the mean ADC of the high-grade uroepithelial carcinoma cases (0.918 ± 0.20 × 10−3 mm2 s–1) was found to be significantly lower than that of the low-grade urothelial carcinoma cases (1.28 ± 0.18 × 10−3 mm2 s–1) (p<0.01).
The cut-off value in the ROC curve for the differentiation of malignant and benign wall pathologies according to ADC level was 1.545 × 10−3 mm2 s–1. Based on this value, the sensitivity was 94.1% and the specificity was 95.7%. In addition, the cut-off value in the ROC curve for the differentiation of high- and low-grade malignant masses according to ADC levels was 1.135 × 10−3 mm2 s–1. According to these levels, the sensitivity was 78.96% and the specificity was 85.2%.
In our study, in the detection of malignant lesions based on diffusion restriction, the sensitivity of DWI was 100%, specificity was 76.5%, positive predictive value was 92%, negative predictive value was 100% and the accuracy rate was 93.65%.
Conclusion
Before histopathological sampling, more accurate predictions can be made about the malignant potential of urinary bladder lesions by means of ADC quantification. Because it is non-invasive, fast and does not require contrast material administration or ionising radiation, DWI is a beneficial and safe method for the differentiation of malignant and benign urinary bladder lesions and for providing information about the grade of urinary bladder carcinomas.
References
- 1.Naganawa S, Kawai H, Fukatsu H, Sakurai Y, Aoki I, Miura S, et al. Diffusion weighted imaging of the liver: technical challenges and prospects for the future. Magn Reson Med Sci 2005;4:175–86 [DOI] [PubMed] [Google Scholar]
- 2.Woodhams R, Matsunaga K, Kan S, Hata H, Ozaki M, Iwabuchi K, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 2005;4:35–42 [DOI] [PubMed] [Google Scholar]
- 3.Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 2006;239:632–49 [DOI] [PubMed] [Google Scholar]
- 4.Unal O, Koparan HI, Avcu S, Kalender AM, Kisli E. The diagnostic value of diffusion weighted magnetic resonance imaging in soft tissue abscesses. Eur J Radiol 2009; doi:10.1016/j.ejrad.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echoplanar MR imaging. Radiology 2001;220:621–30 [DOI] [PubMed] [Google Scholar]
- 6.Sun XJ, Quan XY, Liang W, Wen ZB, Zeng S, Huang FH, et al. Quantitative study of diffusion weighted imaging on magnetic resonance imaging in focal hepatic lesions less than 3cm. Zhonghua Zhong Liu Za Zhi 2004;26:165–7 [PubMed] [Google Scholar]
- 7.Squillaci E, Manenti G, Di Stefano F, Miano R, Strigari L, Simonetti G. Diffusion weighted MR imaging in the evaluation of renal tumours. J Exp Clin Cancer Res 2004;23:39–45 [PubMed] [Google Scholar]
- 8.Kinoshita T, Yashiro N, Ihara N, Funatu H, Fukuma E, Narita M. Diffusion-weighted half-Fourier single-shot turbo spin echo imaging in breast tumors: differentiation of invasive ductal carcinoma from fibroadenoma. J Comput Assist Tomogr 2002;26:1042–6 [DOI] [PubMed] [Google Scholar]
- 9.Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol 2005;15:71–8 [DOI] [PubMed] [Google Scholar]
- 10.Abou-El-Ghar ME, El-Assmy A, Refaie HF, El-Diasty T. Bladder cancer: diagnosis with diffusion-weighted MR imaging in patients with gross hematuria. Radiology 2009;251:415–21 [DOI] [PubMed] [Google Scholar]
- 11.El-Assmy A, Abou-El-Ghar ME, Refaie HF, El-Diasty T. Diffusion-weighted MR imaging in diagnosis of superficial and invasive urinary bladder carcinoma: a preliminary prospective study. Sci World J 2008;8:364–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song JH, Francis IR, Platt JF, Cohan RH, Mohsin J, Kielb SJ, et al. Bladder tumor detection at virtual cystoscopy. Radiology 2001;218:95–100 [DOI] [PubMed] [Google Scholar]
- 13.Chow LC, Bammer R, Moseley ME, Sommer FG. Single breath-hold diffusion-weighted imaging of the abdomen. J Magn Reson Imaging 2003;18:377–82 [DOI] [PubMed] [Google Scholar]
- 14.Müller MF, Prasad PV, Bimmler D, Kaiser A, Edelman RR. Functional imaging of the kidney by means of measurement of the apparent diffusion coefficient. Radiology 1994;193:711–15 [DOI] [PubMed] [Google Scholar]
- 15.Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 2005;235:911–17 [DOI] [PubMed] [Google Scholar]
- 16.Cova M, Squillaci E, Stacul F, Manenti G, Gava S, Simonetti G, et al. Diffusion-weighted MRI in the evaluation of renal lesions: preliminary results. Br J Radiol 2004;77:851–7 [DOI] [PubMed] [Google Scholar]
- 17.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 1999;210:617–23 [DOI] [PubMed] [Google Scholar]
- 18.Namimoto T, Yamashita Y, Sumi S, Tang Y, Takahashi M. Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology 1997;204:739–44 [DOI] [PubMed] [Google Scholar]
- 19.Quan XY, Sun XJ, Yu ZJ, Tang M. Evaluation of diffusion weighted imaging of magnetic resonance imaging in small focal hepatic lesions: a quantitative study in 56 cases. Hepatobiliary Pancreat Dis Int 2005;4:406–9 [PubMed] [Google Scholar]
- 20.Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The usefulness of diffusion-weighted imaging (DWI) for the detection of gastric cancer. Hepatogastroenterology 2007;54:1378–81 [PubMed] [Google Scholar]
- 21.Avcu S, Arslan H, Unal O, Kotan C, Izmirli M. The role of DWI and ADC values in gastric cancer. Proceedings of the ESMRMB Congress 2009; 2009 October 1–3; Antalya, Turkey [Google Scholar]
- 22.Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol 2007;17:201–4 [DOI] [PubMed] [Google Scholar]
- 23.Kılıçkesmez O, Cimilli T, Inci E, Kayhan A, Bayramoğlu S, Taşdelen N, et al. Diffusion-weighted MRI of urinary bladder and prostate cancers. Diagn Interv Radiol 2009;15:104–10 [PubMed] [Google Scholar]
- 24.Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging—accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009;251:112–21 [DOI] [PubMed] [Google Scholar]
- 25.El-Assmy A, Abou-El-Ghar ME, Mosbah A, El-Nahas AR, Refaie HF, Hekal IA, et al. Bladder tumour staging: comparison of diffusion- and T2-weighted MR imaging. Eur Radiol 2009;19:1575–81 [DOI] [PubMed] [Google Scholar]