Abstract
Objectives
Percutaneous radiofrequency ablation (PRFA) of liver tumours performed under local anaesthesia and intravenous sedation can cause severe pain to patients. This prospective study evaluated the efficacy of a right thoracic paravertebral block (TPVB) for anaesthesia and analgesia during PRFA of liver tumours.
Methods
20 patients, aged 44–74 years, with liver malignancies received a multiple injection TPVB at the T6–10 levels 30 min before the PRFA. An intravenous infusion of propofol (3–5 mg kg–1 h–1) was administered to patients who requested to be sedated and intravenous fentanyl (25 µg bolus) was administered as rescue analgesia. Pain during the TPVB and PRFA was assessed using a numerical rating scale (NRS; 0, no pain; 10, worst imaginable pain). Patients were also assessed for residual pain and analgesic consumption during the 24 h after the intervention.
Results
The TPVB was well tolerated and produced ipsilateral sensory anaesthesia with satisfactory spread (median (range); 8 (6–11) dermatomes). The PRFA procedure caused mild pain (mean (standard deviation, SD); NRS 1.4 (1.9)) during the insertion of the ablation needle and the peak pain intensity during the therapeutic burn was moderate (mean (SD); NRS 5.0 (3.3)) in severity. During the 24 h after the PRFA, patients reported minimal pain and consumed very few analgesics. The mean (SD) satisfaction score (0, totally dissatisfied; 10, very satisfied) of the patients was 8.9 (1.1) and that of the radiologists was 8.8 (1.4).
Conclusion
A right TPVB is safe and effective for anaesthesia and analgesia during PRFA of malignant liver tumours.
Percutaneous radiofrequency ablation (PRFA) is currently the mainstay of management, at most centres, for patients with primary and secondary malignant liver tumours [1-3]. Anaesthesia for PRFA of liver tumours usually involves local anaesthesia and intravenous sedation [1,4]. However, intraoperative and early post-operative pain is frequently reported by the majority of patients undergoing such procedures [5-8].
Thoracic paravertebral block (TPVB) is the technique of injecting local anaesthetic adjacent to the thoracic vertebra close to where the spinal nerves exit the intervertebral foramina [9]. This produces ipsilateral, segmental, somatic and sympathetic nerve blockade without causing major haemodynamic changes [9]. TPVB is effective in managing liver capsule pain after blunt trauma and for analgesia following radiofrequency ablation of a liver mass [10,11]. It has been used extensively at our centre for chest and upper abdominal surgery, including hepatectomy [12]. However, there are no data on the safety and efficacy of TPVB as the sole anaesthetic technique for PRFA of liver tumours, which this study was designed to investigate.
Methods and materials
This study took the form of a prospective case series. After institutional ethics committee approval and written informed consent, 20 consecutive adult patients scheduled for PRFA of primary and secondary malignant liver tumours (solitary nodule ≤5 cm or 2 nodules ≤3 cm) were recruited. Patients were excluded if they had a history of psychiatric illness, chronic pain, regular analgesic usage or there were contraindications to performing a TPVB (namely chest wall deformity, severe coagulopathy, local or systemic infection and allergy to local anaesthetic drugs). All patients were instructed on the use of a numeric rating scale (NRS; 0, no pain; 10, worst imaginable pain) for pain assessment during the pre-operative visit and they were fasted for at least 6 h before the procedure. No pre-medication was prescribed. The TPVB was performed in the interventional radiology suite (IRS) by the principal investigator, a specialist anaesthetist, under standard aseptic precautions, 30 min before the scheduled PRFA, using a technique previously described [9]. Intravenous access and standard monitoring, which included electrocardiography (ECG), pulse oximetry and non-invasive blood pressure monitoring, was established before the TPVB. Patients were then positioned comfortably in the sitting position and the spinous processes of the 6th, 7th, 8th, 9th and 10th thoracic vertebrae (T6–10) were located. A point was then marked on the skin, 2.5 cm lateral to the tip of these spinous processes, on the right side of the back with a skin-marking pen. Under aseptic precautions, the skin and subcutaneous tissue at the marked levels were anaesthetised with 1–2 ml of 1% lidocaine. Starting at the T6 level, a 22G Tuohy needle (8 cm; B Braun Medical Inc., Bethlehem, PA) was then inserted perpendicular to the skin in all planes and advanced until it contacted the transverse process. The needle was then “walked” above the transverse process and slowly advanced until a subtle loss of resistance to the injection of air from a 5 ml glass syringe was felt. After negative aspiration for blood or cerebrospinal fluid (CSF), 3–4 ml of 0.75% ropivacaine with freshly added adrenaline (1:200 000) was slowly injected in aliquots into the paravertebral space. The same process was repeated at the T7–10 levels. On completing the paravertebral injections, which took around 20 min, the patients were returned to the supine position and were left undisturbed; their haemodynamic parameters were closely monitored. Any complication during the TPVB procedure was recorded and treated appropriately. Loss of sensation to cold (ice) was assessed over the targeted dermatomes at 10 min after the TPVB, after which the patients were transferred to the CT fluoroscopy room in the IRS.
After confirming adequate anaesthesia over the skin entry site, patients who requested to remain sedated during the procedure were administered an intravenous infusion of propofol (3–5 mg kg–1 h–1) and the dose was titrated to maintain a Ramsay Sedation Scale (RSS) score between 2 and 3 (RSS 2, co-operative, orientated and tranquil; RSS 3, responds to commands) [13]. The interventional radiologist then inserted a multitined 14-gauge electrode (LeVeen CoAccess Electrode System; Boston Scientific, Watertown, MA) through the right upper quadrant of the abdomen, under ultrasound guidance, into the tumour nodule (Figure 1). The NRS score for pain experienced by the patients during this process was assessed and recorded. Correct positioning of the electrode within the liver tumour usually required further fine adjustments under CT fluoroscopy guidance. Therapeutic burn of the tumour was then achieved by maintaining the power output of the radiofrequency system (RF 3000 Radiofrequency Ablation System; Boston Scientific) according to its standard protocol under impedance control. The end point of the therapeutic burn using this system was the roll-off when impedance increased rapidly following tumour cell death. Total time taken for the therapeutic burn in each patient was recorded. The character and severity of pain or discomfort experienced by the patient during the therapeutic burn was also assessed and recorded at 2–3 min intervals. Fentanyl (25 µg bolus) was administered intravenously as rescue analgesia if the patient reported an NRS score >6 (we considered NRS 1–3 as mild pain, 4–6 as moderate pain and 7–10 as severe pain) and repeated this every 3–5 min if necessary. Hypotension, defined as a decrease in systolic blood pressure >30% of the patient's baseline, was treated with intravenous fluid and ephedrine (3 mg bolus). Oxygen (4–6 l min–1) was administered using a Hudson face mask to maintain an Sao2 ≥96% or when propofol was used for sedation. The end-tidal CO2 waveform, as an indicator of respiration, was also monitored via the Hudson face mask in patients who were sedated. At the end of the PRFA procedure, the interventional radiologist's satisfaction with the anaesthetic technique used was assessed (satisfaction score; 0, totally dissatisfied; 10, very satisfied).
Figure 1.
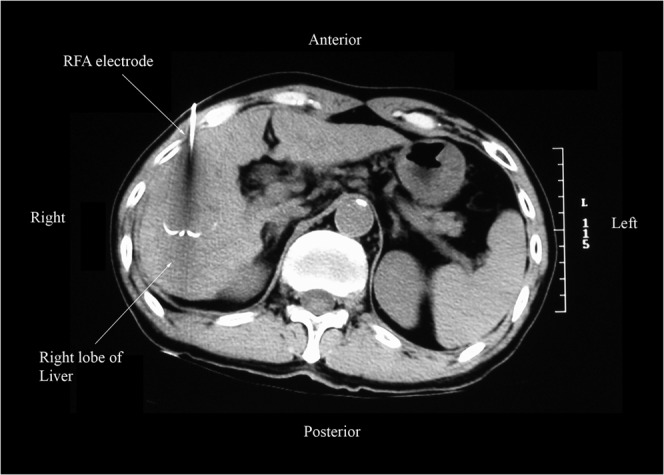
A non-contrast CT fluoroscopic image of the abdomen (axial view) showing a 14-gauge LeVeen radiofrequency ablation (RFA) electrode with its hooks deployed around the target lesion in the right lobe of the liver. The RFA electrode was positioned under real-time ultrasound guidance and its final position was confirmed by CT fluoroscopy.
After completion of the PRFA, sedation was discontinued in those receiving propofol and the patients were transferred to the post-anaesthetic care unit (PACU) where their vital signs were monitored every 5 min for 30 min. Wound pain experienced by the patient was also recorded on arrival and before discharge from the PACU. Patients were administered morphine (1.5 mg intravenous bolus) for analgesia if the NRS during the PACU stay was >6 and the dose was repeated if necessary every 7–10 min to a maximum of 3 doses. Before discharge from the PACU, patients' satisfaction with the anaesthetic technique used was also assessed (satisfaction score, 0–10). On return to the surgical ward, patients were allowed to eat and drink as tolerated. Oral Doloxene (dextropropoxyphene) 32 mg was prescribed for breakthrough pain and administered on a pro re nata basis to a maximum of 4 doses per day. The principal investigator visited the patients 24 h after the PRFA and recorded wound pain score, patients' satisfaction towards the anaesthesia technique and any complications.
The data were processed using the Statistical Package for Social Science v.14.0 (SPSS 14.0, SPSS Inc., Chicago, IL) for Windows. Data that were normally distributed are presented as mean (SD) (range) and data that were not normally distributed are presented as median (range).
Results
15 men and 5 women aged 61.5 (8.5) (44–77) years were recruited for this study. Their mean body weight was 62.8 (13.0) (40–87) kg and the body mass index (BMI) was 23.0 (4.7) (15.7–34.1) kg m–2. In our cohort, 5 patients belonged to American Society of Anesthesiologists (ASA) class I, 6 patients to ASA class II and 9 to ASA class III, and 11 out of the 20 patients were suffering from liver cirrhosis (10 had Child's class A and 1 had Child's class B cirrhosis). 12 patients had hepatocellular carcinoma and the rest had liver metastases from colorectal malignancies. 16 out of the 20 patients had a single tumour nodule whereas the rest had 2 nodules. The mean diameter of the first tumour nodule was 2.4 (0.9) (0.8–4.2) cm whereas that of the second nodule was 1.8 (0.7) (0.7–2.4) cm. The tumour nodule was located in the right lobe of the liver in 14 patients, in the left lobe of the liver in 4 and in both lobes of the liver in 2 patients.
The multiple injection TPVB that was performed at the T6–10 levels was well tolerated by our patients (mean (SD) NRS, 3.4 (2.1)) and it produced ipsilateral sensory blockade over 8 (6–11) dermatomes. 7 patients (35%) developed contralateral segmental sensory blockade over 9 (7–11) dermatomes. 10 patients requested to be sedated during the procedure and the mean total dose of propofol infused in these patients was 111 (143) mg. Patients reported mild pain (mean (SD) NRS, 1.4 (1.9)) during the insertion of the ablation electrode into the liver tumour. The total duration of the therapeutic burn during the PRFA was 25.1 (12.6) (10–57) min and the maximum pain or discomfort experienced by the patients during the therapeutic burn was judged as moderate in intensity (mean (SD) NRS, 5.0 (3.3)). The nature of pain or discomfort experienced by the patients during the PRFA was variable. 7 (35%) patients reported a visceral type of pain, which was described as a vague discomfort with a poorly localised hotness, bloating and nauseating sensation. 5 (25%) patients had referred pain to the right shoulder and 1 (5%) patient reported a mixture of visceral and referred pain. Only 2 out of the 20 patients (10%) required a single dose of the fentanyl (25 µg) for rescue analgesia.
The majority of patients reported minimal pain after the PRFA (mean (SD) NRS on arrival on the PACU was 2.0 (2.5), on discharge from the PACU was 1.7 (2.1) and at the 24 h ward visit was 1.3 (1.6)). Only 1 patient required morphine (total dose 4.5 mg) for analgesia in the PACU and during the 24 h after the PRFA; 4 patients required oral Doloxene (1 dose, 32 mg) and 3 patients were administered pethidine (1 dose, 50 mg) intramuscularly, as they were still fasted, for analgesia. Overall both the patients (satisfaction score, 8.9 (1.1)) and the interventional radiologist (satisfaction score, 8.8 (1.4)) were satisfied with the anaesthetic technique used.
There were no major complications following the TPVB. Only 1 (5%) patient developed a vasovagal attack after the TPVB, presenting with bradycardia and hypotension which was treated with intravenous fluid bolus and ephedrine (6 mg). During the therapeutic burn, 4 patients (20%) developed nausea and vomiting which was treated with intravenous metoclopramide (10 mg).
Discussion
In this study we have demonstrated the effectiveness of TPVB in providing perioperative anaesthesia and analgesia during PRFA of primary and secondary malignant liver tumours. Our results are consistent with a recently published case report, which described complete relief of acute post-PRFA pain using a single-injection TPVB [11]. Although previous studies on paravertebral block suggest that it usually takes 15–30 min for surgical anaesthesia to occur [14,15], all patients in our study had evidence of sensory blockade within 10 min after the TPVB, which reflects the rapid onset and high success rate of the multiple injection technique of TPVB [9]. The incidence of contralateral segmental sensory blockade (35%) in our cohort was higher than that published in the literature [16,17]. Karmakar et al [16] report an incidence of 20% after a single-injection TPVB that was used for pain management in patients with multiple fractured ribs, while Lönnqvist et al [17] report a much lower incidence (1.1%) in 367 paravertebral blocks, both thoracic and lumbar, which they evaluated for complications. Currently there are no data showing that a multiple injection TPVB produces a higher incidence of contralateral segmental anaesthesia than a single-injection TPVB, but our results do suggest that this may be the case and future research should evaluate this aspect of TPVB. Moreover, the exact mechanism by which spread occurs to the contralateral paravertebral space is not clear, but may be due to epidural [18] or prevertebral spread [19,20] (Figure 2). It was also interesting to find that the contralateral sensory blockade was wider (9 (7–11) dermatomes) in 7 patients than the overall ipsilateral sensory blockade (8 (6–11) dermatomes) in 20 patients, and the mechanism for this phenomenon is not clear.
Figure 2.
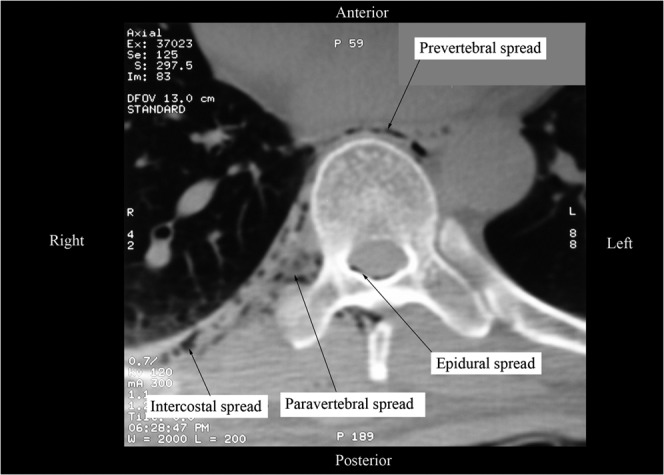
A non-contrast CT fluoroscopic image of the thorax (magnified axial view) at the level of the T9 vertebra showing spread of the injectate from the right paravertebral space to the epidural, intercostal and prevertebral spaces. The air that was introduced into the paravertebral space while eliciting the “loss of resistance”, now seen as tiny air bubbles, served as a “contrast” to delineate the spread of the local anaesthetic from the paravertebral space.
Side effects from the TPVB, such as vasovagal attack, were infrequent in our patients. Vasovagal attack during the TPVB could be related to the upright sitting position during the performance of TPVB, stress, epidural spread with bilateral sympathetic blockade or activation of the Bezold–Jarisch reflex resulting in parasympathetic activation and sympathetic withdrawal [21]. However, vasovagal episodes related to the TPVB are not common [15]. In our study, the single episode of vasovagal attack was easily treated by laying the patient supine and administering intravenous atropine and ephedrine.
According to most standard anatomical textbooks, the liver and its capsule are innervated by sympathetic and parasympathetic nerves via the hepatic plexus. The sympathetic fibres arise from the thoracic sympathetic chain from the T5 (or T6) to T11 levels and reach the liver via the greater and lesser splanchnic nerves and the coeliac plexus. Visceral pain experienced by 8 out of the 20 patients may be attributed to the heat from the therapeutic burn, which was sensed by nociceptive afferent sympathetic fibres from the unblocked left side and the parasympathetic fibres in the vagus nerve, which may not be blocked by a right TPVB. Indeed, failure to achieve total visceral anaesthesia as a result of the inability to block the parasympathetic as well as contralateral sympathetic fibres may be considered a major drawback of a unilateral TPVB. However, the higher incidence of contralateral sensory blockade (35%) in our cohort may be considered an unexpected beneficial effect in this regard. Referred shoulder pain in the six patients could be caused by the therapeutic burn to a peripheral liver tumour adjacent to the diaphragm, which is innervated by the phrenic nerve and not blocked by the TPVB. A physical insulation method such as instilling 5% dextrose water around the liver to decrease pain associated with the PRFA of peripheral liver tumours has previously been described [22]. Such a method may be used to supplement the TPVB to reduce the incidence of visceral or referred shoulder pain during the PRFA. Nevertheless, it is noteworthy that the referred shoulder pain can alert the operator to a potential diaphragmatic injury, which is a known complication of PRFA of liver tumours [23]. During the PACU stay, one patient required intravenous morphine for relief of the shoulder pain, which was expected as diaphragm irritation after the therapeutic burn of a nearby liver tumour can last for some time after the PRFA [23].
We acknowledge that a single-injection TPVB may be considered a less invasive and possibly less time-consuming technique than the multiple injection TPVB used in this study [9,11,15]. Nevertheless, multiple injection TPVB appears to be safer than a single-injection TPVB, which uses a larger bolus dose of local anaesthetic [24]. In addition, we considered multiple injection TPVB to be more reliable and appropriate for intraoperative anaesthesia as it might provide a more reliable and denser block [25]. Furthermore, alternative anaesthetic techniques to TPVB may also be considered for the PRFA of liver tumours. These include local anaesthesia with intravenous sedation, thoracic epidural anaesthesia and general anaesthesia. As mentioned earlier, local anaesthesia with intravenous sedation often fails to provide successful intraoperative and post-operative analgesia despite it being widely used for PRFA of liver tumours, including at our institution prior to the conduction of the present study. This may be the result of a lack of anaesthetic service in most IRSs. Radiologists often struggle with balancing complex interventions while trying to simultaneously direct sedation in these patients, with inadequate analgesia perhaps being preferred to hypotension and respiratory depression [26]. In view of such a need to provide improved analgesia to patients undergoing interventional radiological procedures such as PRFA, in a recent editorial [26] the radiology community has requested more interspecialty collaboration to help solve the problem [26]. This was also the motivation behind this study, as the interventional radiologists at our institution also expressed the same problem while performing PRFA under local anaesthesia and sedation, i.e. intolerable pain, and they were impressed by the difference provided by the TPVB during the study. Thoracic epidural anaesthesia has the potential to achieve the same anaesthetic and analgesic efficacy as a TPVB. However, it carries with it a higher risk of hypotension, owing to the bilateral sympathectomy, urinary retention and shivering, as well as a higher potential risk of spinal cord injury and epidural haematoma formation. In contrast, TPVB carries a much lower risk of spinal haematoma and subsequent cord compression in the presence of moderate haemostatic deficiency and coagulopathy is considered to be only a relative contraindication for it [15,27]. Finally, general anaesthesia would have to be used for patients with contraindications for regional anaesthesia techniques such as TPVB or for patients who cannot tolerate the PRFA under local anaesthesia and intravenous sedation. However, one should bear in mind that general anaesthesia lacks the ability to provide prolonged post-operative analgesia [10]. It usually requires airway instrumentation, anaesthetic assistance and monitoring equipment that is less readily available in the radiology suites, which are also peripherally located.
In this study we were unable to compare TPVB with an alternative anaesthetic technique for overall analgesia efficacy and complication rates. A double-blind randomised controlled trial would have been a better study design. However, this would have involved performing a sham TPVB in patients with malignant liver tumours, which is ethically less acceptable for a pilot study investigating the safety and efficacy of TPVB for anaesthesia during PRFA.
Conclusion
A right TPVB is an effective technique for anaesthesia during PRFA of primary and secondary malignant liver tumours. The block was well tolerated by the patients and was well received by the interventional radiologists. It also produced prolonged post-operative analgesia after the PRFA. Future studies should compare the safety and efficacy of TPVB with alternative anaesthetic techniques for PRFA.
Acknowledgments
The authors would like to thank Dr Paul Lee Sing Fun (interventional radiologist), Dr Simon Ho Sze Ming (interventional radiologist) and Dr John Wong (hepatobiliary surgeon) for their co-operation during this study. They are also very grateful to the entire staff of the interventional radiology suite at the Prince of Wales Hospital, Hong Kong, for their assistance.
Financial support: This work was locally funded by the Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Shatin, Hong Kong.
References
- 1.Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol 2007;10:38–46 [DOI] [PubMed] [Google Scholar]
- 2.Ng KK, Poon RT. Radiofrequency ablation for malignant liver tumor. Surg Oncol 2005;14:41–52 [DOI] [PubMed] [Google Scholar]
- 3.Crocetti L, Lencioni R. Thermal ablation of hepatocellular carcinoma. Cancer Imaging 2008;27:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabo B, Dodd GD, 3rd, Halff GA, Naples JJ. Anesthetic considerations in patients undergoing percutaneous radiofrequency interstitial tissue ablation. AANA J 1999;67:467–8 [PubMed] [Google Scholar]
- 5.Buscarini E, Buscarini L. Radiofrequency thermal ablation with expandable needle of focal liver malignancies: complication report. Eur Radiol 2004;14:31–7 [DOI] [PubMed] [Google Scholar]
- 6.Bonny C, Abergel A, Gayard P, Chouzet S, Ughetto S, Slim K, et al. Radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis. Gastroenterol Clin Biol 2002;26:735–41 [PubMed] [Google Scholar]
- 7.Giorgio A, Tarantino L, Stefano G, Coppola C, Ferraioli G. Complications after percutaneous saline-enhanced radiofrequency ablation of liver tumors: 3-year experience with 336 patients at a single center. AJR Am J Roentgenol 2005;184:207–11 [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Nahum GS. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441–51 [DOI] [PubMed] [Google Scholar]
- 9.Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95:771–80 [DOI] [PubMed] [Google Scholar]
- 10.Hall H, Leach A. Paravertebral block in the management of liver capsule pain after blunt trauma. Br J Anaesth 1999;83:819–21 [DOI] [PubMed] [Google Scholar]
- 11.Culp WC, Jr, Payne MN, Montgomery ML. Thoracic paravertebral block for analgesia following liver mass radiofrequency ablation. Br J Radiol 2008;81:e23–5 [DOI] [PubMed] [Google Scholar]
- 12.Ho AMH, Karmaker MK, Cheung M, Lam GCS. Right thoracic paravertebral analgesia for hepatectomy. Br J Anaesth 2004;93:458–61 [DOI] [PubMed] [Google Scholar]
- 13.Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974;2:656–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hura G, Knapik P, Misiołek H, Krakus A, Karpe J. Sensory blockade after thoracic paravertebral injection of ropivacaine or bupivacaine. Eur J Anaesthesiol 2006;23:658–64 [DOI] [PubMed] [Google Scholar]
- 15.Klein SM, Greengrass RA, Weltz C, Warner DS. Paravertebral somatic nerve block for outpatient inguinal herniorrhaphy: an expanded case report of 22 patients. Reg Anesth Pain Med 1998;23:306–10 [DOI] [PubMed] [Google Scholar]
- 16.Karmakar MK, Critchley LAH, Ho AMH, Gin T, Lee TW, Yim APC. Continuous thoracic paravertebral infusion of bupivacaine for pain management in patients with multiple fractured ribs. Chest 2003;123:424–31 [DOI] [PubMed] [Google Scholar]
- 17.Lönnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade: failure rate and complications. Anaesthesia 1995;50:813–15 [DOI] [PubMed] [Google Scholar]
- 18.Purcell-Jones G, Pither CE, Justins DM. Paravertebral somatic nerve block: a clinical, radiographic, and computed tomographic study in chronic pain patients. Anesth Analg 1989;68:32–9 [PubMed] [Google Scholar]
- 19.Karmakar MK, Kwok WH, Kew J. Thoracic paravertebral block: radiological evidence of contralateral spread anterior to the vertebral bodies. Br J Anaesth 2000;84:263–5 [DOI] [PubMed] [Google Scholar]
- 20.Karmakar MK, Chui PT, Joynt GM, Ho AM. Thoracic paravertebral block for management of pain associated with multiple fractured ribs in patients with concomitant lumbar spinal trauma. Reg Anesth Pain Med 2001;26:169–73 [DOI] [PubMed] [Google Scholar]
- 21.Watkins EJ, Dresner M, Calow CE. Severe vasovagal attack during regional anaesthesia for caesarean section. Br J Anaesth 2000;84:118–20 [DOI] [PubMed] [Google Scholar]
- 22.Hinshaw JL, Laeseke PF, Winter TC, Kliewer MA, Fine JP, Lee FT. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol 2006;186:S306–10 [DOI] [PubMed] [Google Scholar]
- 23.Head HW, Dodd GD, 3rd, Dalrymple NC, Prasad SR, El-Merhi FM, Freckleton MW, et al. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology 2007;243:877–84 [DOI] [PubMed] [Google Scholar]
- 24.Baumgarten RK. Thoracic paravertebral block: is single injection really safer? Reg Anesth Pain Med 2006;31:584. [DOI] [PubMed] [Google Scholar]
- 25.Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tayara K, Younes F, et al. Thoracic paravertebral block: influence of the number of injections. Reg Anesth Pain Med 2006;31:196–201 [DOI] [PubMed] [Google Scholar]
- 26.Watkinson AF, Francis IS, Torrie P, Platts AD. The role of anaesthesia in interventional radiology. Br J Radiol 2002;75:105–6 [DOI] [PubMed] [Google Scholar]
- 27.Richardson J, Lönnqvist PA. Thoracic paravertebral block. Br J Anaesth 1998;81:230–8 [DOI] [PubMed] [Google Scholar]


