Abstract
Secondary involvement of the urinary bladder in non-Hodgkin's lymphoma is relatively common; however, primary malignant lymphoma of this organ is extremely rare. The most common type of primary bladder lymphoma is a low-grade B-cell mucosa-associated lymphoid tissue (MALT) lymphoma. We report here on the imaging findings of a primary bladder lymphoma with bone marrow infiltration.
Non-Hodgkin's lymphoma usually presents as localised or generalised lymphadenopathy, rarely it may present as a primary in extranodal sites where lymphoid tissue is present. Among the common extranodal sites, stomach, connective tissues and skin are known to be the most commonly involved. Primary malignant non-Hodgkin's lymphoma of the bladder was first described by Eve in 1885 and it remains a rare entity, representing less than 1% of bladder tumours and 0.2% of extranodal lymphomas. Several pathological and clinical studies of Maltoma of the bladder have been reported. However, to the best of our knowledge, there has only been one study which has focused on the imaging findings. We report a case of primary bladder lymphoma with bone marrow infiltration in a 65-year-old female patient who, despite response to chemotherapy, presented in relapse and died from septicaemia several months after concluding the treatment.
Case report
A 65-year-old female presented with a 2 week history of nausea and feeling unwell associated with one episode of vomiting, reduced oral intake and dizziness. She also had a history of a suprapubic mass, which had gradually increased in size over the past 20 years. She had underlying medical conditions of diabetes mellitus, chronic anaemia and renal impairment.
On examination she was pale and sallow. She was also very lethargic and unable to mobilise. Her Glasgow coma scale (GCS) was 14/15 and she had a persistent horizontal nystagmus on right gaze. Central nervous system examination did not elicit any focal neurological deficit. There was a palpable suprapubic mass. The rest of the systemic examination was unremarkable. Biochemical evaluation of renal function revealed a raised creatinine value of 137 mmol l−1 and urea level of 9.1 mmol l−1. Haemoglobin was low at 8.9 gm dl−1, white blood cell count was raised at 20.6 × 109 l, and platelet level was normal at 450 × 109 l.
MRI brain was normal and ultrasound of the kidneys and urinary bladder was performed in view of the increased creatinine level to rule out obstructive uropathy. The ultrasound examination revealed bilateral hydronephrosis with a diffusely thickened urinary bladder wall, and a large well-defined hypoechoic soft-tissue mass was seen arising from the trigone of the bladder (Figure 1).
Figure 1.
Ultrasound of the urinary bladder, which demonstrates a hypoechoic mass within the urinary bladder (white arrows) in (a) longitudinal and (b) transverse sections. Note the thickened urinary bladder wall (black arrowheads).
Subsequently, a contrast enhanced CT scan of the abdomen and pelvis (Figure 2) was performed, which revealed a large homogeneously enhancing solitary mass measuring 11.2 cm × 9.0 cm × 10.6 cm arising from the trigone of the urinary bladder and had diffusely thickened walls. Vessels were seen traversing this mass. There was no paravesical fat infiltration seen and no pelvic or abdominal lymph node enlargement. However, there was associated bilateral moderate hydronephrosis and hydroureters as well as mediastinal lymphadenopathy.
Figure 2.
Post-contrast (CT) examination of the abdomen and pelvis demonstrates a large bladder mass (white arrows) and thickened urinary bladder wall (white arrowheads) in (a) axial section (b) sagittal section and (c) coronal section with left hydronephrosis (black arrow).
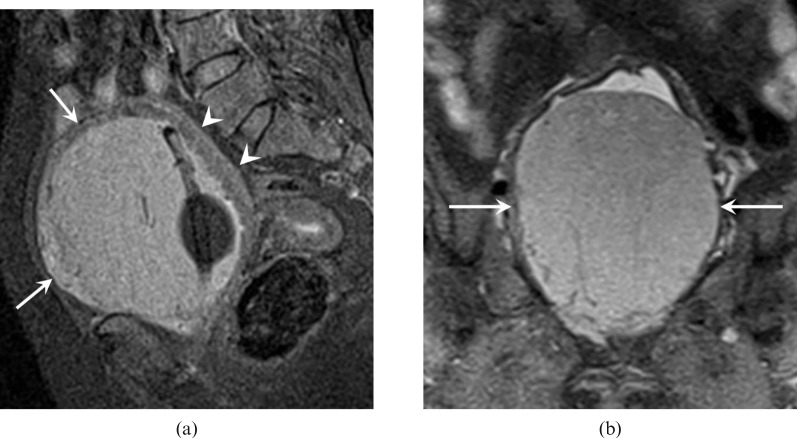
MRI of the pelvis with gadolinium (Figures 3 and 4) was subsequently performed and demonstrated this tumour as hypointense on T1 weighted (T1W), intermediate intensity on T2 weighted (T2W), hyperintense on short tau inversion-recovery (STIR) and showed a well-defined homogeneously enhancing mass occupying the entire urinary bladder inkeeping with a urinary bladder tumour. There was a clear fat plane between the urinary bladder and uterus posteriorly. Radiologically, it was thought to be a bladder tumour and most likely a transitional cell carcinoma (TCC).
Figure 3.
MRI pelvis demonstrated this tumour (white arrows) as (a) hypointense on axial T1 weighted; (b) intermediate intensity on sagittal T2 weighted; and (c) hyperintense on coronal short tau inversion-recovery occupying the entire urinary bladder. There is a clear fat plane between the urinary bladder and the uterus posteriorly (white arrow head).
Figure 4.
Post-gadolinium enhanced T1 weighted fat saturated MRI demonstrating the homogeneously enhancing bladder tumour (white arrows) and thickened urinary bladder wall (white arrowheads) in (a) sagittal section and (b) coronal section.
Bone marrow aspirate was suggestive of a B-cell lymphoma. The patient subsequently presented with frank haematuria approximately 1 month later. Rigid cystoscopy revealed a highly suspicious bladder tumour arising from the left lateral wall of the bladder and a transurethral resection of bladder tumour (TURBT) was performed. The tumour was incompletely resected by cystoscopy. The histopathology features were those of a non-Hodgkin's lymphoma, compatible with an extra-nodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) or MALToma (Figure 5). The patient was treated successfully with chemotherapy (R-CHOP), which was completed by December 2008.
Figure 5.
(a–d) Histopathology sections showed a relatively monotonous proliferation of small- to medium-sized lymphoid cells, which diffusely infiltrated the fibromuscular bladder stroma (a). These neoplastic cells exhibited hyperchromatic irregular nuclei and scanty cytoplasm. Mitotic figures and apoptotic bodies were noted. Occasional reactive lymphoid follicles were seen (b). Closer examination showed presence of interspersed larger cells with vesicular nuclei and pale cytoplasm, as well as scattered plasma cells. Most of the mucosa has been ulcerated, but residual overlying transitional epithelium showed focal squamous metaplasia. Immunohistochemistry showed that the neoplastic lymphoid cells diffusely expressed CD20 (c), a pan-B cell marker. BCL2 was also expressed (d). The Ki67 proliferation marker was low, i.e. 5–10%. Immunostains for CD3, CD5, CD10, Cyclin D1, CD21, CD23, CD15 and CD30 were all negative.
In early February 2009, the patient had a relapse associated with acute renal failure and right-sided deep vein thrombosis (DVT). A contrasted CT scan of the abdomen and pelvis revealed that the bladder mass had reduced in size; however, there was evidence of a left perinephric abscess. The patient died 3 weeks later owing to septicaemic shock secondary to a bladder abscess, which was a result of repeated instrumentation.
Discussion
The urinary bladder has secondary involvement in 10–20% of terminal non-Hodgkin's lymphoma cases, while primary lymphoma is very rare, accounting for less than 0.2% of extranodal lymphomas [1]. It affects women 6.5 times more than men and is typically seen between the ages of 20 and 85 years (median age 64 years) [1,2]. Low-grade lymphomas including the B-cell derived non-Hodgkin's lymphomas of the MALT type are among the common primary terminal lymphomas of the bladder and have a good prognosis. High-grade primary lymphomas are less common, constituting of approximately 20% of cases, and are mostly of the diffuse large B-cell type [1].
Primary malignant lymphoma of the bladder usually exhibits a benign clinical course with a favourable prognosis, which is associated with the tumour grade and stage at presentation. If pathological examination reveals high-grade lymphoma of the bladder, the possibility of systemic lymphoma should be excluded [3]. In our patient, the bone marrow aspiration findings revealed a systemic lymphoma by infiltration of the bone marrow.
The most common presenting symptom is haematuria followed by urinary frequency, dysuria or nocturia [4]. As there is no naturally occurring lymphoid tissue in the bladder, the aetiology of bladder MALToma remains unclear; however, a history of chronic cystitis has been a preceding feature in approximately 22% of patients [5].
In view of the fact that previous cases have been reported in the absence of cystitis, as noted in our case, it has been postulated that the bladder's embryonic origin from the cloacae may be the other possible origin of MALT [1,4].
In the present case, based on the initial imaging findings of a well-defined enhancing mass within the urinary bladder arising from the trigone with bilateral hydronephrosis, we concluded that the most likely diagnosis would be a TCC because it is the most common bladder tumour. However, our patient also presented with mediastinal lymph nodes. Based on these features and the fact that the bone marrow aspirate was inkeeping with a lymphoma, we were prompted to investigate further to exclude the possibility of a primary bladder lymphoma. A cystoscopy and TURBT was performed and the diagnosis was finally made by histopathological examination. A primary bladder lymphoma is a very rare tumour and is usually misdiagnosed as a TCC, as in our case.
The radiological findings between a bladder lymphoma and the more common TCC cannot be differentiated. However, the presence of a large mass without extravesical spread should raise the possibility of lymphoma or rhabdomyosarcoma, rather than assuming it is TCC; the diagnosis therefore must be made by histology [6]. Ultrasound is usually the initial investigation of choice; however, a contrasted CT is necessary to provide information of the local extent of the tumour. It has been reported that MRI shows no signal difference between a lymphoma and carcinoma [7].
Based on previous reports, it has been suggested that MALTomas are frequently large sessile solitary tumours (66%), less frequently multiple (14%) and occasionally polypoidal (10%). They are commonly seen in the lateral wall of the bladder (40% of cases), 22% in the inferior wall and 10% in the posterior wall with approximately 2% in each of the superior and anterior walls. Diffuse thickening of the bladder wall has only been demonstrated in 10% of cases [7]. Primary bladder lymphoma does not typically involve the entire bladder wall or the urethral orifices, therefore hydronephrosis is an uncommon feature. On the other hand, TCCs are multiple, involving any part, or all, of the collecting system and are classified into papillary and non-papillary types. Hydronephrosis is commonly seen in TCC [6].
Our patient had a large sessile solitary subserosal mass arising from the inferior wall of the urinary bladder with evidence of bladder wall thickening. The bilateral hydronephrosis seen in our patient could be attributed to the fact that the tumour was arising from the base of the bladder and was most likely due to an outlet obstruction rather than a tumour infiltration.
Treatment for primary bladder lymphoma is the same as nodal lymphoma where chemotherapy, radiotherapy and surgery, or a combination of these therapies, are among the treatment options available. Most patients respond well to a combination of chemotherapy and radiotherapy, which is the treatment of choice [3,7]. If the tumour is of low-grade type and small in size, it can be treated with radiotherapy. Radiotherapy can be used for local control of the disease after surgical intervention [1]. The role of surgery is controversial; surgical extraction of the bladder appears unnecessary because a relapse may be possible in other organs and it can reduce the quality of life [2-4]. For the favourable prognosis group, conventional chemotherapy with a CHOP (cyclophosphamide/doxorubicin/vincristine/prednisolone) regimen is used most frequently. However, for the poor prognosis group, where fewer than 50% of patients are cured, the combination of a newer drug, rituximab, with CHOP demonstrated a much better survival rate [3].
In our case, owing to the involvement of bone marrow, the disease was Stage IV, according to the Ann Arbor staging system. So far there has only one such case reported in previous literature, by Oh et al (2003) [3]. Therefore, owing to systemic involvement we administered six cycles of systemic chemotherapy using the R-CHOP regimen. Upon completion of treatment, the lesion in the bladder regressed in size and the bilateral hydronephrosis resolved. However, the patient developed a relapse and had acute renal failure owing to bladder outlet obstruction secondary to the tumour, which was located at the inferior wall of the bladder. The patient eventually developed a bladder abscess as a result of repeated instrumentation and died as a result of septicaemic shock.
Conclusion
We have reported a case of a primary high-grade malignant lymphoma of the urinary bladder with mediastinal lymph nodes and bone marrow involvement. The patient also had bilateral hydronephrosis and hydroureters that were most likely due to an outlet obstruction in view of the position of the tumour at the trigone of the bladder. Based on the CT findings, which showed the presence of mediastinal lymph nodes along with the bone marrow aspirate confirming the diagnosis of lymphoma, a differential diagnosis of primary bladder lymphoma should be considered. MRI findings were non-specific and in our case the diagnosis was finally made by histopathology. By definition, the prognosis of low-grade lymphoma of the bladder is favourable as they are confined to a single organ at the time of diagnosis. Although there is a relatively high incidence of secondary bladder involvement in patients with systemic lymphoma, it is exceptionally rare for disseminated lymphoma to present with primary bladder involvement, as in our case, and it indicates a very poor prognosis.
References
- 1.Horasanli K, Kadihasanoglu M, Aksakal OT, Ozagari A, Miroglu C. A case of primary lymphoma of the bladder managed with multimodal therapy. Nat Clin Pract Urol 2008;5:167–70 [DOI] [PubMed] [Google Scholar]
- 2.Hayashi A, Miyakawa Y, Bokuda K, Kimura T, Nakashima E, Irie R, et al. Primary diffuse large B-cell lymphoma of the bladder. Intern Med 2009;48:1403–6 [DOI] [PubMed] [Google Scholar]
- 3.Oh KC, Zang DY. Primary non-Hodgkin's lymphoma of the bladder with bone marrow involvement. Korean J Intern Med 2003;18:40–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadway P, Riaz AA, Lotzof KL, Gelister JS. Renal colic: an unusual presentation of non-Hodgkin's lymphoma of the urinary bladder. e-MED Ltd 2004;4:7–9 [Google Scholar]
- 5.Bates AW, Norton AJ, Baithun SI. Malignant lymphoma of the urinary bladder: a clinicopathological study of 11 cases. J Clin Pathol 2000;53:458–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeoman LJ, Mason MD, Olliff JF. Non-Hodgkin's lymphoma of the bladder—CT and MRI appearances. Clin Radiol 1991;44:389–92 [DOI] [PubMed] [Google Scholar]
- 7.Tasu JP, Geffroy D, Rocher L, Eschwege P, Strohl D, Benoit G, et al. Primary malignant lymphoma of the urinary bladder: report of three cases and review of the literature. European Radiology 2000;10:1261–4 [DOI] [PubMed] [Google Scholar]