Abstract
We present the case of a metastatic adrenal tumour from hepatocellular carcinoma (HCC) showing the uptake of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) on MRI. To our knowledge, this is the first case of metastatic HCC in which Gd-EOB-DTPA uptake was shown on MRI and this finding facilitated the accurate pre-operative diagnosis of a metastatic adrenal tumour.
Recently, gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA), a contrast agent with perfusion and hepatoselective properties, has been used for the diagnosis of hepatocellular carcinoma (HCC) on MRI [1-3]. It allows combined dynamic imaging and hepatocyte-specific imaging in one examination. In the hepatobiliary phase, hepatic lesions like HCC lacking normally functioning hepatocytes are imaged as a defect of hepatocyte-selective enhancement compared with normal parenchyma. Some HCCs may show the paradoxical uptake of Gd-EOB-DTPA and are recognised as iso- or hyperintense lesions in the hepatobiliary phase compared with normal parenchyma [3-7]. However, to date there have been no published reports describing the uptake of Gd-EOB-DTPA in a metastatic lesion from HCC. We describe a patient with adrenal metastasis from HCC, showing Gd-EOB-DTPA uptake on MRI.
Case report
A 73-year-old male with a history of hepatitis C was referred from a district hospital for further HCC treatment and suspected left adrenal metastasis. He also had a history of colon cancer, which had been curatively treated 19 years earlier without recurrence, and cholelithiasis, which had been treated with cholecystectomy 7 years previously.
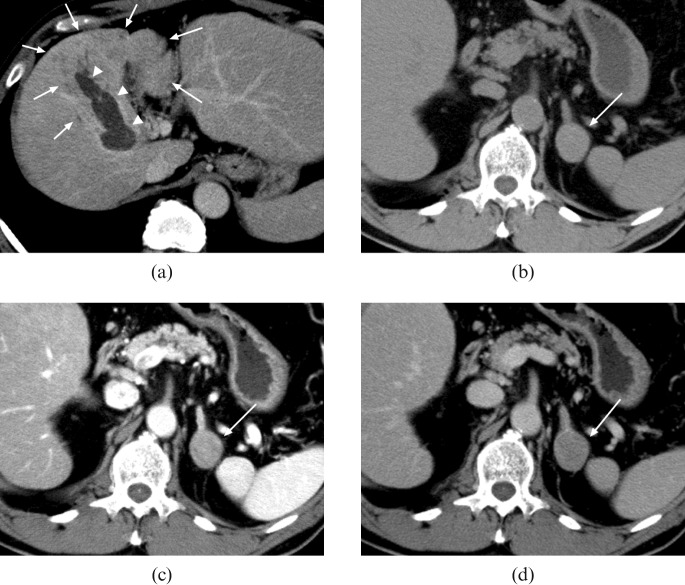
A CT scan with contrast enhancement was performed, which revealed an ill-defined low density area in the segment 4/8 region of the liver accompanied by a filling defect in the enlarged intrahepatic portal vein (Figure 1a). A 28 mm round-shaped left adrenal nodule was also noted; it showed soft-tissue attenuation (>10 HU) on unenhanced CT, homogeneous enhancement in the early phase after the injection of contrast material and washout in the equilibrium phase (Figure 1b–d).
Figure 1.
(a) Contrast-enhanced CT shows an ill-defined low density area in the segment 4/8 region of the liver (arrows), accompanied with filling defect in the enlarged intrahepatic portal vein which represents tumour thrombosis (arrowheads). At a more caudal level, a 28 mm round-shaped well-demarcated nodule can be noted; (b) soft-tissue attenuation (>10 HU) on plain CT (arrow), (c) homogeneous enhancement in the early phase after the injection of contrast material (arrow) and (d) washout in the equilibrium phase (arrow).
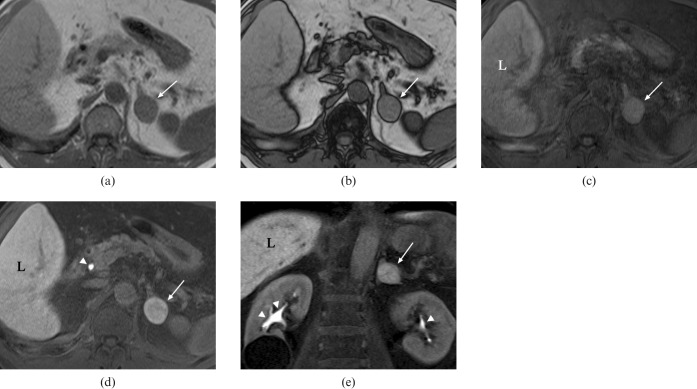
On MRI with Gd-EOB-DTPA injection, the hepatic lesion was depicted as a heterogeneously enhanced ill-defined area during the arterial phase and a heterogeneous low-intensity area during portal and equilibrium phases. It was more clearly depicted as a low-intensity area in the hepatobiliary phase 20 min after contrast medium injection. The adrenal lesion showed low intensity on T2 weighted fast-spin echo images and no signal drop was demonstrated on in- and opposed-phase T1 weighted gradient recalled echo images (Figure 2a,b), which suggests a poor fatty component within the lesion. After Gd-EOB-DTPA administration, the lesion showed enhancement in the arterial phase compared with the unenhanced fat-suppressed T1 weighted image and a washout pattern on portal and equilibrium phase images. On hepatobiliary phase images, the nodule and hepatic parenchyma appeared homogeneously hyperintense compared with a pre-contrast image (Figure 2c–e), which suggests uptake of Gd-EOB-DTPA within the left adrenal lesion.
Figure 2.
On (a) in- and (b) opposed-phase T1 weighted gradient recalled echo MRI, no signal drop of the left adrenal nodule is demonstrated (arrows in a,b), which suggests a poor fatty component within the lesion. Compared with a (c) pre-contrast fat-suppressed T1 weighted image, (d,e) the lesion is depicted as a homogeneously hyperintense nodule as well as hepatic parenchyma in the hepatobiliary phase 20 min after Gd-EOB-DTPA administration (arrows in c–e), which suggests the uptake of Gd-EOB-DTPA within the tumour (L, liver). Note high signal intensity in the common bile duct (arrowhead in d) and bilateral renal pelves (arrowheads in e), which represents contrast material excreted in the bile and urine, respectively.
The serum alpha-fetoprotein (AFP) concentration was 5.1 ng ml−1 (normal range (NR) <15 ng ml−1) and the protein level induced by vitamin K antagonist-2 (PIVKA-2) was 2050 mAU ml−1 (NR <40 mAU ml−1). The results of other biochemical tests were as follows: total protein 8.8 mg dl−1 (NR 6.3–8.1 g dl−1); albumin 3.0 mg dl−1 (NR 3.9–5.1 g dl−1); aspartate aminotransferase 66 IU/l (NR 13–33 IU l–1); alanine aminotransferase 52 IU l–1 (NR 8–42 IU l–1); alkaline phosphatase 990 IU l–1 (NR 115–359 IU l–1); γ-glutamyl transpeptidase 300 IU l–1 (NR 9–54 IU l–1); lactate dehydrogenase 216 IU l–1 (NR 129–241 IU l–1); and total bilirubin 0.4 mg dl–1 (NR 0.3–1.3 mg dl–1).
From the patient’s history of hepatitis C, the laboratory data (especially elevated AFP and PIVKA-2) and the imaging findings noted above, a diagnosis of diffuse HCC in the liver with portal vein tumour thrombosis and a metastatic left adrenal tumour from HCC was made.
The continuous administration of 5-fluorouracil (250 mg body−1 per day, days 1–5) and cisplatin (10 mg body−1 per day, days 1–5) was performed by hepatic arterial infusion through a subcutaneous injection port implanted into the right inguinal region. After 3 weeks, the PIVKA-2 level in serum decreased to 324 mAU ml−1 and reduction of the tumour thrombus in the intrahepatic portal vein was confirmed on a follow-up CT. The left adrenal tumour did not change in size and features. Consecutive CT, MRI and PET using 18-fluoro-2-deoxy-d-glucose revealed neither intrahepatic nor distant metastasis other than left adrenal metastasis, and so it was planned for the patient to undergo hepatectomy and left adrenalectomy. After receiving percutaneous transhepatic portal embolisation of the right posterior intrahepatic portal vein to induce hypertrophy of the left lateral lobe, he underwent an extended right lobectomy and left adrenalectomy.
On macroscopic findings, the resected hepatic lesion was a solid pale-yellow tumour and the left adrenal lesion was a solid pale-green tumour. Based on microscopic histopathological analysis, the liver tumour was moderately differentiated HCC, post-chemotherapy, with intrahepatic portal vein tumour thrombosis. The left adrenal tumour showed a highly-to-moderately differentiated metastatic carcinoma, consistent with hepatic origin.
Discussion
Recently, Gd-EOB-DTPA has been used for the diagnosis of HCC on MRI. In the hepatobiliary phase, HCCs are usually imaged as a defect of hepatocyte-selective enhancement compared with normal parenchyma. It is known, however, that some HCCs show the paradoxical uptake of Gd-EOB-DTPA with an incidence of 9–27% [3-7] and are recognised as iso- or hyperintense lesions in the hepatobiliary phase compared with the normal parenchyma. To our knowledge, there has been no report describing the uptake of Gd-EOB-DTPA in metastatic lesions from HCC and our report is the first to describe metastatic HCC in which the uptake of Gd-EOB-DTPA was shown on MRI.
An early report indicated that 50% of well-differentiated HCCs exhibited iso- or hyperintensity to the surrounding liver parenchyma and that moderately or poorly differentiated HCCs exhibited no uptake of Gd-EOB-DTPA [5]. According to more recent reports, however, no relationship between uptake of Gd-EOB-DTPA and tumour grade has been reported [3,4,6,7]. The mechanism of uptake of Gd-EOB-DTPA in HCCs and the characteristics of Gd-EOB-DTPA-positive HCCs have also been gradually clarified. Narita et al [6] showed that the expression of OATP1B3, which is one of the sodium-independent organic anion transporters and is expressed in the human liver in the basolateral membrane of hepatocytes, determines the uptake of Gd-EOB-DTPA in the hepatobiliary phase in HCCs. Tsuboyama et al [7] reported that hepatocyte-selective enhancement is induced by expression patterns of transporters including OATP1B3, OATP1B1 and MRP2. Unfortunately, the expression of these transporters was not investigated in our case, either in the hepatic nor adrenal tumour.
The adrenal gland is a common target organ of haematogenous metastasis from HCC, with an incidence of 11–16.9% in clinical practice [8-10]. There are a few reports describing the CT findings of metastatic adrenal tumours from HCC. Nakamura et al [11] described large (>8 cm) adrenal metastases with a central low density area owing to necrosis and rather smaller ones homogeneously enhanced. Katyal et al [8] reported that many of the adrenal metastases from HCC demonstrated enhancement during the hepatic arterial phase, consistent with our case. The findings on MRI are not well described.
The presence of an enlarged adrenal mass does not always imply malignancy because statistically adrenal adenomas are a more common cause of an enlarged adrenal gland, even in patients with known extra-adrenal primary tumours [12]. If the presence of fat within adrenal tumours is confirmed based on the density on unenhanced CT (<10 HU) or a dropped signal on an opposed phase T1 weighted MRI, a diagnosis of adenoma can be made [13,14]. However, lipid-poor adenoma cannot be diagnosed by this procedure and both adenoma and metastatic HCC often show similar findings of early enhancement and washout. 99Tcm pyridoxyl–5-methyl tryptophan hepatobiliary scintigraphy is useful for specifically diagnosing metastatic HCC but only when the tumour cells produce bile [15-17]. We estimate that the uptake of Gd-EOB-DTPA in any masses outside the liver is also a specific finding of metastatic lesions from HCC and that this finding is useful for diagnosing metastatic tumours from HCC, although its sensitivity is presumably low.
Conclusion
We have presented a case of a metastatic adrenal tumour from HCC showing the uptake of Gd-EOB-DTPA on MRI. This MR finding enabled us to make the correct pre-operative diagnosis of a metastatic adrenal tumour.
References
- 1.Reimer P, Rummeny EJ, Daldrup HE, Hesse T, Balzer T, Tombach B, et al. Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur Radiol 1997;7:275–80 [DOI] [PubMed] [Google Scholar]
- 2.Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 2004;230:266–75 [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, et al. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol 2009;192:1675–81 [DOI] [PubMed] [Google Scholar]
- 4.Saito K, Kotake F, Ito N, Ozuki T, Mikami R, Abe K, et al. Gd-EOB-DTPA enhanced MRI for hepatocellular carcinoma: quantitative evaluation of tumor enhancement in hepatobiliary phase. Magn Reson Med Sci 2005;4:1–9 [DOI] [PubMed] [Google Scholar]
- 5.Huppertz A, Haraida S, Kraus A, Zech CJ, Scheidler J, Breuer J, et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT—initial observations. Radiology 2005;234:468–78 [DOI] [PubMed] [Google Scholar]
- 6.Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 2009;44:793–8 [DOI] [PubMed] [Google Scholar]
- 7.Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology 2010;255:824–33 [DOI] [PubMed] [Google Scholar]
- 8.Katyal S, Oliver JH, 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000;216:698–703 [DOI] [PubMed] [Google Scholar]
- 9.Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 2005;20:1781–7 [DOI] [PubMed] [Google Scholar]
- 10.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol 2007;13:414–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura K, Sato Y, Nakata H. Computed tomography of adrenal metastases in hepatocellular carcinoma. Report of four cases. Acta Radiol 1989;30:550–2 [PubMed] [Google Scholar]
- 12.Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology 2002;222:629–33 [DOI] [PubMed] [Google Scholar]
- 13.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 1998;171:201–4 [DOI] [PubMed] [Google Scholar]
- 14.Elsayes KM, Mukundan G, Narra VR, Lewis JS, Jr, Shirkhoda A, Farooki A, et al. Adrenal masses: MR imaging features with pathologic correlation. Radiographics 2004;24:S73–86 [DOI] [PubMed] [Google Scholar]
- 15.Miyake H, Takeoka H, Wakisaka M, Mori H. Accumulation of Tc-99m PMT in adrenal metastasis of hepatoma. Clin Nucl Med 1991;16:940–1 [DOI] [PubMed] [Google Scholar]
- 16.Yamashita N, Fukawa M, Imaizumi N, Matsumoto S, Tabuchi M, Hiroyoshi M, et al. Establishing a diagnosis of adrenal metastasis from hepatocellular carcinoma by 99mTc-PMT hepatobiliary scintigraphy. Surg Today 1992;22:565–7 [DOI] [PubMed] [Google Scholar]
- 17.Shiozaki T, Hayakawa K, Tanikake M, Oida T, Hida S, Yasui H. Accumulation of 99mTc-PMT in renal metastasis of hepatocellular carcinoma. Ann Nucl Med 2003;17:333–6 [DOI] [PubMed] [Google Scholar]