Abstract
Objectives
The aim of this study was to describe our experience of imaging following hysteroscopic sterilisation with the Essure (Conceptus Inc., Mountain View, San Carlos, CA) microinsert, and to underline the importance of a carefully performed follow-up hysterosalpingogram (HSG) in the management of these patients.
Methods
18 women underwent the procedure and all returned for follow-up HSG. A standard HSG technique was used and views were acquired to establish microinsert position and tubal occlusion.
Results
In 16 of the 18 women, adequate microinsert positioning and bilateral tubal occlusion was present. In one woman, a unilateral microinsert occluded the fallopian tube, whereas the other fallopian tube was ligated with a clip. The final patient underwent two studies; both showed well-positioned microinserts but unilateral free spill from the right fallopian tube. There are no reported pregnancies thus far.
Conclusion
Essure sterilisation coils have a unique appearance when radiographed and are an effective means of permanently occluding the fallopian tubes. HSG is a rapid and safe method of confirming satisfactory placement and tubal occlusion. Non-HSG imaging techniques are suboptimal at detecting patent fallopian tubes and expose patients to the risk of an unwanted and potentially complicated pregnancy.
Although there have been numerous advances in the methods available for contraception, female tubal sterilisation represents one of the most popular. It is the method of choice for birth control for approximately 180 million couples worldwide [1]. 700 000 procedures are performed annually in the USA [2] and 49 000 procedures annually in the UK [3]. Traditionally, tubal “interruption” has been performed laparoscopically, at laparotomy or minilaparotomy, vaginally via the posterior fornix or transcervically via the hysteroscope. The procedure may be performed under local anaesthetic with sedation or under general anaesthetic and employs any combination of cautery, electrocoagulation, ligation, clipping, division of the fallopian tubes, intratubal devices or chemicals. All of these methods have an associated morbidity and failure rate, with the subsequent risk of an unwanted pregnancy. The United States Collaborative Review of Sterilization (CREST) reported a cumulative probability of pregnancy 5 years after tubal surgery of 13.1 per 1000 procedures and 18.5 per 1000 procedures after 10 years, with 33% of these pregnancies being ectopic [4]. Some methods offer the chance of reversal at a later date if desired.
To date, the main methods of transcervical sterilisation have involved introduction of chemicals such as quinacrine, electrodiathermy or mechanical obstruction, all with varying rates of success [2]. The most recent and promising advance in sterilisation techniques involves the concept of transcervical tubal cannulation and placement of an intrafallopian implant. Tubal sterilisation using the Essure microinsert (Conceptus Inc., Mountain View, San Carlos, CA) offers a permanent, irreversible alternative which is performed hysteroscopically under mild sedation in an outpatient setting [5-8]. The device was approved by the United States Food and Drugs Administration (US FDA) in November 2002 and by the UK National Institute for Health and Clinical Excellence (NICE) in February 2004. To date, over 200 000 women have undergone sterilisation with Essure microinserts worldwide.
The Essure microinsert consists of a stainless steel inner coil, a radially expanding nickel–titanium alloy (nitinol) outer coil and polyethylene terephthalate (PET) fibres wound in and around the inner coil (Figure 1) [8]. The ends of the inner and outer coils are delineated by radio-opaque markers. There is no hormonal element to the system. The insert is 4 cm long and the outer coil expands to 1.5–2 mm to anchor it in the fallopian tube. The manufacturers recommend that between three and eight coils of the outer coil are left trailing into the endometrial cavity. The microinsert is delivered via the hysteroscope using a single-handed ergonomic handle containing a delivery wire, and delivery and release catheters. After placement, the presence of the PET fibres induces an inflammatory reaction that leads to intraluminal fibrosis over a 3 month period; this achieves the dual effect of fallopian tube anchorage of the microinsert as well as tubal occlusion [8]. Although far more commonly performed hysteroscopically, placement under fluoroscopic guidance has been described [9]. The microinserts have been shown to be MRI-compatible up to a field strength of 1.5 T [10].
Figure 1.

Essure hysteroscopic sterilisation device. From Essure [8], courtesy of Conceptus, Inc (Mountain View, San Carlos, CA).
The device is usually inserted in an outpatient day-care setting, with most patients requiring mild sedation and/or local anaesthesia, ideally during the early proliferative phase of the menstrual cycle. When performed by an experienced operator, the procedure usually takes less than 10 min to complete [11,12]. Patients are advised to use non-steroidal anti-inflammatory drugs (NSAIDs) for analgesia, are discharged within hours of the procedure and most return to normal daily activities within 24 h [13]. They are advised to use additional contraception for 3 months prior to imaging to demonstrate satisfactory tubal occlusion.
Methods and materials
18 patients undergoing Essure sterilisation were counselled on the risks and benefits, as well as the requirement for additional contraception for 3 months and the need for a 3 month hysterosalpingogram (HSG) examination to check for tubal occlusion [14]. All 18 patients returned following microinsert insertion for HSG examination. A modified standard HSG technique was employed [15], with standard fluoroscopic equipment (Axiom Artis MP, Siemens, Germany).
Prior to instillation of contrast (Ultravist 300 (iopromide), Bayer, UK), a control image was obtained to demonstrate the locations of the microinserts. After instillation of contrast (usually maximum 10 ml), a minimum of 4 further images was obtained. Special attention was paid to ensuring complete distension of the uterine cavity with a good seal on the endocervical canal to ensure that intrauterine pressure was high enough to reveal tubal patency if present. Standard views included an early filling anteroposterior image, right and left anterior oblique views and a delayed en face view to provide an accurate assessment of the relation of the proximal end of the inner coil to the uterine cornua, and to verify that the microinsert spanned the uterotubal junction and to optimise detection of any intraperitoneal spill. Care was taken to ensure that the fluoroscopy beam was as perpendicular as possible to the uterine cavity to obtain a true en face view. On occasion this involved moving the fluoroscopy tube or performing traction on the cervix to manipulate uterine position. Extreme care was taken to minimise the risk of introducing of air bubbles into the endometrial cavity, as bubbles may obscure the view of the uterine cornu [16].
HSG studies were evaluated by experienced practitioners, working in consensus with the two main parameters evaluated (Table 1). Following confirmation of satisfactory position and tubal occlusion, patients were instructed to discontinue additional contraception. For patients with satisfactory coil position but persisting tubal patency, additional contraception was advised for a further 3 months pending repeat HSG examination. For patients with unsatisfactory coil position, additional contraceptive use was recommended.
Table 1. Criteria for evaluation of satisfactory placement and tubal occlusion on hysterosalpingogram (HSG).
| Placement | |
| Satisfactory | • Distal end of the inner coil being within the tube with less than half of the length of the inner coil trailing into the uterine cavity |
| • Proximal end of the inner coil is up to 30 mm into the tube from the cornua | |
| Unsatisfactory | • Absence of the microinsert on imaging (implying expulsion) |
| • More than half of the length of the inner coil trailing into the uterine cavity | |
| • Proximal end of the microinsert in the tube more than 30 mm from the cornua | |
| • Presence of the microinsert within the peritoneal cavity | |
| Occlusion | |
| Satisfactory (grade 1) | • Complete non-filling of the tubes |
| Satisfactory (grade 2) | • Filling of the tubes but not beyond the distal-most aspect of the outer coil |
| Tubal patency (grade 3) | • Contrast beyond the outer coil |
| • Spill into the peritoneal cavity | |
Results
18 patients treated with Essure sterilisation were assessed with HSG. The mean age of the patients at the time of sterilisation was 40.2 years (range 32–45 years). The mean time from undergoing the microinsert placement procedure to the first HSG was 14.0 weeks (range 7–21 weeks). This variation in part reflected the need to perform the procedure during a specific part of the menstrual cycle, a factor which could vary the date of the appointment by up to 3 weeks. All 18 patients attended their HSG appointment, and, thus far, there have been no reported pregnancies in those with successful placement.
There were a total of 19 HSG studies for these 18 patients. In 16 of the 18 patients, the post-procedure HSG demonstrated well-positioned microinserts and bilateral tubal occlusion, allowing these women to rely on the devices for future contraception (Figure 2). In 1 of the 16 patients (Figure 3), there was filling of the proximal aspect of one of the tubes but not beyond the outer coil, thus meeting the criteria for satisfactory tubal occlusion.
Figure 2.
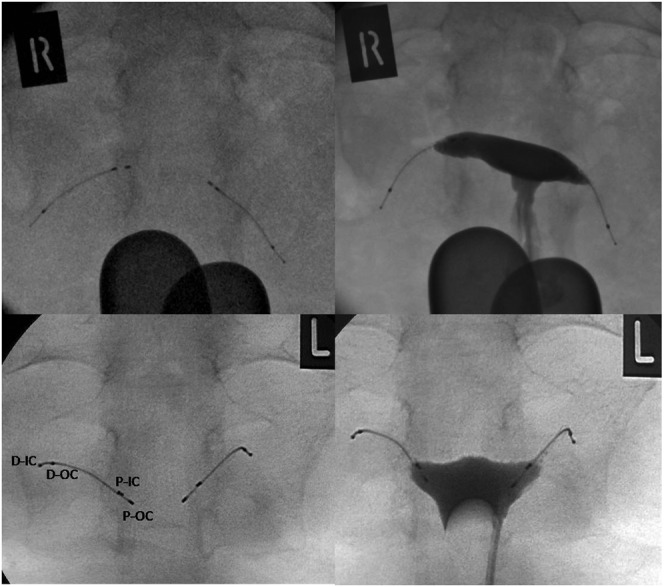
Normal control and post-contrast infusion hysterosalpingogram (HSG) images. D-IC, distal inner coil; D-OC, distal outer coil; P-IC, proximal inner coil; P-OC, proximal outer coil.
Figure 3.
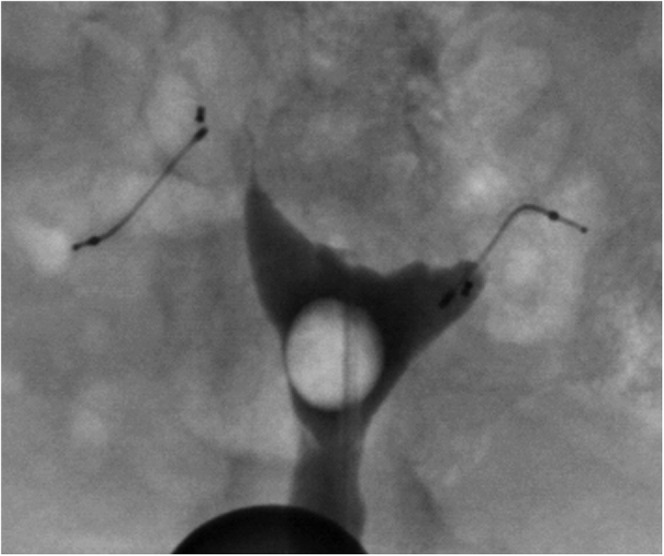
Filling of the proximal aspect of the right fallopian tube, but not beyond the distal-most aspect of the outer coil; Grade 2 tubal occlusion.
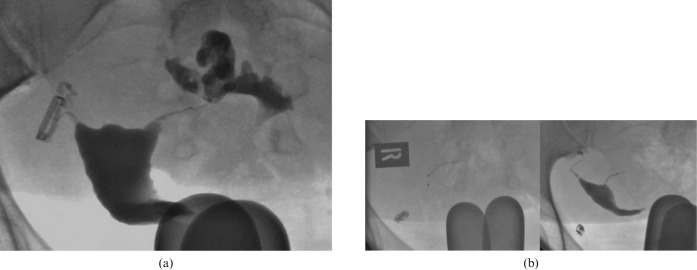
One patient had unilateral Essure coil placement as previous imaging had demonstrated detachment of one previously placed sterilisation clip but satisfactory occlusion of the contralateral tube with a well-placed clip (Figure 4a). On the HSG study following unilateral Essure placement, this single device was demonstrated to be satisfactorily placed with bilateral tubal occlusion (Figure 4b). In this particular case, the control radiograph is misleading; the apparent demonstration of a right-sided microinsert is due to the anatomical orientation of the uterus. Following instillation of contrast, the true axis of the uterus became apparent, and the left-sided microinsert-induced tubal occlusion is demonstrated. A static plain pelvic radiograph would not have adequately assessed coil position in this case.
Figure 4.
(a) The right fallopian tube is occluded by a clip; the left fallopian tube is patent with free spill demonstrated. (b) Repeat hysterosalpingogram (HSG) following placement of a microinsert in the left fallopian tube, with bilaterally occluded fallopian tubes.
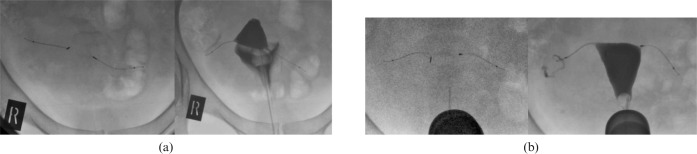
One of the patients attended twice for HSG. The microinsert placement procedure was reported as being uncomplicated. The first HSG, at 11 weeks, demonstrated unilateral filling of the right tube and spill into the peritoneal cavity (Figure 5a). The microinserts were noted to be well positioned bilaterally. The patient was advised to continue alternative contraception and another appointment for HSG was made 7 weeks later. The microinserts were again noted to be well positioned. Spill from the same tube was again noted but only after a short delay, reinforcing the importance of meticulous technique in performing these post-sterilisation investigations (Figure 5b). The patient was referred back to the gynaecologist for further management.
Figure 5.
(a) Well-positioned microinserts bilaterally, but there is spill from the right fallopian tube on delayed imaging (Grade 3). (b) Repeat study 3 months later demonstrates spill from the right fallopian tube at an earlier phase of imaging (Grade 3).
Thus, 17 of 18 patients were successfully treated; a rate of 94%, which is comparable with other reported series [11]. Correct interpretation of the HSG images was dependent on meticulous technique, having correctly localised the site of coil placement and given due attention to identifying intraperitoneal spill.
Discussion
Hysteroscopic sterilisation is a major advance in the field of contraception. Essure microinserts have been commercially available in Europe since 2001 and were approved by NICE in February 2004. They have been shown to be a safe, reliable method of permanent sterilisation with minimal morbidity. A 5 year effectiveness rate of 99.8% has been reported [17]. Patients are able to tolerate their insertion extremely well and high levels of patient satisfaction have been reported; it has also been shown to be less painful than laparoscopic sterilisation [3,18]. Use of microinserts has been shown to be as or more cost-effective than laparoscopic sterilisation in several studies [19-21]. There has been a rapid rise in the use of the devices synchronous to a decline in laparoscopic sterilisation techniques [22]. The characteristics of safety, minimal invasiveness, short procedure time and effectiveness led to a recent case series of device use in women with severe cardiac disease which would preclude successful pregnancy [23].
Placement of the device under fluoroscopic control rather than hysteroscopically has been described [9]. The authors describe one of the advantages of performing the procedure in this way as being able to track the hydrophilic guidewires into the fallopian tubes to successfully deploy the microinserts. The radiographic markers on the devices can be used to evaluate position during the procedure. However, there was no follow-up of the patients to document tubal occlusion at 3 months, and long-term data regarding fertility and possible pregnancies are awaited.
There is an increasing volume of literature describing the use of hysteroscopic sterilisation in conjunction with intrauterine contraceptive devices and minimally invasive gynaecological techniques, such as endometrial ablation. In a small series of women with contraindications to use of, or poor compliance with, oral contraceptives, Agostini et al [24] placed the Essure microinserts with an intrauterine contraceptive device in situ. They found that the procedure was uncomplicated, allowed the women to rely on the intrauterine contraceptive device for additional contraception during the 3 month follow-up period and HSG examination following removal of the intrauterine contraceptive device showed tubal occlusion in all cases.
Endometrial ablation, performed hysteroscopically as an outpatient, has also been used with microinsert placement as an “all-in-one” procedure [25-27]. Hopkins et al [27] used HSG as the assessment tool at 3 month follow-up. They found that the ablation had not interfered with the ability to either perform or interpret the HSG, with mild intrauterine synechiae demonstrated in only 5 out of 21 patients. However, an HSG delayed until 9 months post-procedure showed severe synechiae, with the ability to confirm tubal occlusion compromised. Combined procedures are not without risk; bilateral cornual abscesses requiring laparotomy and salpingectomy have been described in a patient following endometrial ablation with microinserts in situ [28]. Recently, the devices have also been used to treat hydrosalpinges in women about to undergo in vitro fertilisation (IVF) for whom laparoscopic treatment of hydrosalpinges was contraindicated owing to extensive previous abdominopelvic surgery [29,30]. Successful pregnancies have been reported using this technique [30].
Although hysteroscopic sterilisation is associated with reduced morbidity in comparison with other sterilisation techniques, specific complications and adverse events have been described. Immediate post-procedure cramping and pain in the first hour occurs in up to 30% of patients [31]. Other complications include tubal or cornual perforation by the microinsert at the time of placement [32,33], changes in menstrual bleeding patterns, a dislodged or expelled microinsert [34] and persistent post-procedure pain either with a misplaced microinsert or in the setting of appropriately positioned microinserts [35]. Fortunately, most complications can be dealt with hysteroscopically [33,34] or laparoscopically [35].
Although the device is designed to offer permanent sterilisation, removal has been described between 6 weeks and 8 months following the procedure as a treatment for persisting pain [35,36]. In these cases endoluminal fibrosis was “soft” enough to allow removal of the devices. It is not yet clear for how long post-procedure the devices may be successfully retrieved, or what impact removal would have on tubal function. Further studies are required to characterise the temporal relationship between tubal fibrosis and functional occlusion.
Initial trials showed an 88% success rate for bilateral placement of the device [6,7]. Success rates of 95% have now been demonstrated with experienced operators [11,12,37]. The most common reasons for failure to place the microinserts successfully are failure to visualise tubal ostia, tubal obstruction or stenosis. Confirmation of bilateral tubal occlusion on HSG is achieved in 92–96% of patients at 3 months and 99–100% at 6 months.
A variety of imaging techniques, including plain pelvic radiographs, ultrasound (transabdominal and transvaginal), hysterosonography and hysterosalpingography, have been described to assess successful implantation following microinsert placement. Although plain pelvic radiography may demonstrate appropriately positioned microinserts, it takes no account of the mechanism by which the device acts as a contraceptive [38]. Endoluminal fibrosis of the fallopian tubes leads to tubal occlusion; occlusion can only be confirmed or excluded by a dynamic examination such as HSG that specifically examines the patency of the tubes. This principle is illustrated by our case of free unilateral tubal spill, as well as the case reported by Karthigasu et al [39], with an apparently well-positioned microinsert with free peritoneal spill demonstrated on both the 3 and 6 month HSGs. The manufacturers of the device recommend an HSG at 3 months following placement to confirm adequate positioning and tubal occlusion, and, therefore, successful implantation. As use of the device increases, more radiologists will be involved in the assessment of satisfactory placement, and more radiologists will come across images of patients with Essure microinserts being imaged for other reasons; it is therefore important to be aware of the range of radiographic, sonographic and HSG appearances of the microinserts.
The many advocates of non-HSG techniques as the primary follow-up investigation cite patient intolerance of the HSG procedure, exposure to ionising radiation, cost and pain [32]. Most of the studies using non-HSG procedures are based in Europe or Australia; in the USA, HSG is a mandatory aspect of the microinsert placement procedure as part of its FDA approval. NICE guidelines, which were published in consultation with general practitioners and gynaecologists but not radiologists, state “at 3 months, an imaging procedure is performed to confirm correct placement of the microinserts and to check that occlusion has been achieved” [40]. In many European centres, HSG is only performed if there were fewer than three or greater than eight coils trailing into the uterine cavity at the end of the procedure, if only a single microinsert could be placed or if the plain radiograph is not satisfactory [11]. In one of the largest series published from a UK centre, the primary follow-up investigation was a plain radiograph, with an HSG performed according to the above criteria [21].
On transabdominal and transvaginal sonography, the device is seen as a linear echogenic structure extending from the uterine cornua into the proximal fallopian tube (Figure 6) [41,42]. Advocates of ultrasound suggest that a well-positioned device can be assumed to have a contraceptive effect because of cornual inflammation and because, in laparoscopic tubal sterilisation procedures, failure of tubal occlusion does not equate to failure of the sterilisation procedure, and that ultrasound can be used to seek this information without exposing the patient to radiation [43-45]. However, although microinsert position in the region of the tubes can be easily confirmed by pelvic radiography or ultrasound [46,47], these imaging modalities make no assessment of tubal occlusion, leaving the patient with a risk of unwanted pregnancy. Similarly, they do not assess whether the device has been placed in an intratubal position; there are many cases of “normal” post-procedure ultrasound scans in which tubal or cornual perforations have not been identified, leading to unwanted pregnancy in some cases [32,47]. In addition, because the device is usually curved in configuration after placement, visualisation of the entire device on a single plane is difficult with ultrasound, and the distal ends of the microinserts are often difficult to identify owing to obscuration by bowel gas [42,43]. Newer ultrasound-based techniques using contrast agents have been developed in an attempt to evaluate the patency of the tubes in a dynamic fashion. Contrast infusion sonography (CIS) has evolved from hysterosalpingo-contrast-sonography (HyCoSy) [48]. A small volume of ultrasound contrast is infused via an endocervical balloon catheter and real-time tubal flow assessed with transvaginal ultrasound. A recent study, although with a small sample size, showed good correlation between HSG and CIS in demonstrating tubal occlusion, with no reported pregnancies at 15 months follow-up [49]. However, there are several drawbacks to this technique: only one tube at a time can be examined with CIS, whereas a wider field of view permits evaluation of both tubes together with HSG. With CIS, it was found that there was sometimes no detectable real-time flow in the tubes but contrast agent pooled in the cul-de-sac. This implies tubal patency and the patient should be advised to continue with alternative contraception; however, HSG identifies specifically which tube is patent. This is important in cases of unilateral occlusion when the patient may require a second procedure to occlude the patent tube, such as laparoscopic ligation. The presence of echogenic bowel loops in the region of the cul-de-sac also makes identification of free contrast agent difficult; the same difficulty is not encountered with HSG.
Figure 6.
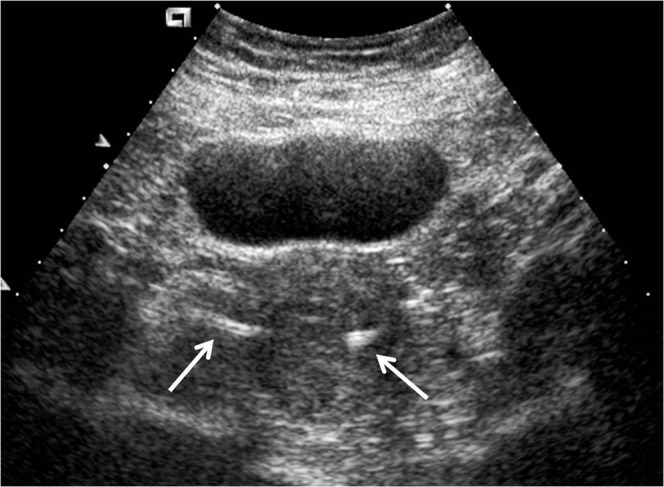
Transabdominal ultrasound image showing echogenic inserts (white arrows) at the uterine cornua.
The phase II and III trials reported a 3 month HSG showing tubal occlusion in 96% and 92% of patients, respectively. This means that there is a substantial proportion of women who will be at risk of unwanted pregnancy at 3 months, and false reassurance would have been provided if device position was assessed using only radiography or two-dimensional sonography.
Despite optimal positioning of the device and HSG demonstrating bilateral tubal occlusion, pregnancies following apparently successful microinsert insertion have been reported [50,51]. Most cases of pregnancy are avoidable [52]. Causes include non-compliance by patients as regards post-procedure contraception, non-attendance for post-procedure HSG, incorrect post-procedure advice provision by the gynaecologist, undetected pre-procedure pregnancies, improper insertion of the device and use of assisted reproductive techniques such as IVF [52,53]. In a recent case series, among the causes identified were misinterpretation of the HSG and failure to detect abnormal device position by ultrasound [47]. In the few cases of pregnancy, surgical exploration of the uterus and fallopian tubes following delivery have shown that perforation of the uterine wall or of the proximal tubal wall had occurred. This mimics proper placement of the microinserts. A review of the HSG images from the case reports of patent tubes following apparently successful implantation and of pregnancy following demonstration of tubal occlusion reveals that tubal or cornual perforation may have been detectable at the time of HSG with careful image review by experienced operators [54,55]. 28% of reported unintended pregnancies were attributed to improperly read or interpreted HSG results [52]. In a recent study, 3 out of 93 women with bilaterally placed microinserts were found to have incorrectly positioned microinserts (expelled or perforated) on 3 month imaging [37]. In all three cases, the placement procedure was noted to be difficult. The authors of that study recommend that, in this select group of patients, pelvic radiography at 4 weeks may be appropriate to identify expelled microinserts such that appropriate surgical action is taken sooner than after the 3 month HSG.
Tubal spasm at the time of HSG may also mimic tubal occlusion; although there has been no validation by published research data, it is postulated that administration of NSAIDs 30–60 min before HSG might avoid tubal spasm, as is the case during the insertion procedure. Slow injection of the HSG contrast medium will also help to avoid tubal spasm.
A low (12.7%) compliance rate for return for HSG was found by Shavell et al [56] in an urban teaching hospital population; this resulted in at least 1 unintended pregnancy. In the study by Wittmer et al [16], 19 of 32 patients attended for the 3 month HSG. A reported post-Essure pregnancy occurred after the patient declined the 3 month HSG on financial grounds [51]. In an analysis of pregnancies reported following use of the Essure system, it was found that 22% could be attributed to failure to attend for the 3 month HSG [52]. All these examples were from studies in populations who rely on insurance-based healthcare. The importance of the HSG as an essential part of the procedure, not a separate voluntary component, needs to be emphasised both to insurers and to prospective patients [52].
Conclusion
Hysteroscopic sterilisation by placement of Essure microinserts is an increasingly popular method of permanent sterilisation. Its uptake in the UK lags behind that of the rest of Europe, Australia and the USA. 77% of women in a UK study were found to favour laparoscopic sterilisation over a hysteroscopic technique [57]. With increasing knowledge of the procedure, through the medical literature and non-controlled sources such as the internet, the procedure is likely to become more popular. Radiologists will play a key role in the management of these patients as the efficacy of tubal occlusion must be confirmed by post-procedure imaging at 3 months to avoid rare but avoidable unplanned pregnancies.
References
- 1.Peterson HB. Sterilization. Obstet Gynecol 2008;111:189–203 [DOI] [PubMed] [Google Scholar]
- 2.Chapman L, Magos A. Female sterilization. Expert Rev Med Devices 2008;5:525–37 [DOI] [PubMed] [Google Scholar]
- 3.Duffy S, Marsh F, Rogerson L, Hudson H, Cooper K, Jack S, et al. Female sterilisation: a cohort controlled comparative study of ESSURE versus laparoscopic sterilisation. BJOG 2005;112:1522–8 [DOI] [PubMed] [Google Scholar]
- 4.Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol 1996;174:1161–70 [DOI] [PubMed] [Google Scholar]
- 5.Kerin JF, Carignan CS, Cher D. The safety and effectiveness of a new hysteroscopic method for permanent birth control: results of the first Essure pbc clinical study. Aust N Z J Obstet Gynaecol 2001;41:364–70 [DOI] [PubMed] [Google Scholar]
- 6.Kerin JF, Cooper JM, Price T, Herendael BJ, Cayuela-Font E, Cher D, et al. Hysteroscopic sterilization using a micro-insert device: results of a multicentre phase II study. Hum Reprod 2003;18:1223–30 [DOI] [PubMed] [Google Scholar]
- 7.Cooper JM, Carignan CS, Cher D, Kerin JF, Selective Tubal Occlusion Procedure 2000 Investigators Group Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol 2003;102:59–67 [DOI] [PubMed] [Google Scholar]
- 8.ESSURE Health Professional Website Mechanism of action [accessed 16 November 2009]. Available from http://www.essuremd.com/Home/bTheEssureProcedureb/bMechanismsofActionb/tabid/57/Default.aspx [Google Scholar]
- 9.McSwain H, Shaw C, Hall LD. Placement of the Essure permanent birth control device with fluoroscopic guidance: a novel method for tubal sterilization. J Vasc Interv Radiol 2005;16:1007–12 [DOI] [PubMed] [Google Scholar]
- 10.Muhler M, Taupitz M. How safe is magnetic resonance imaging in patients with contraceptive implants? Radiologe 2006;46:574–8 [DOI] [PubMed] [Google Scholar]
- 11.Mino M, Arjona JE, Cordon J, Pelegrin B, Povedano B, Chacon E. Success rate and patient satisfaction with the Essure sterilisation in an outpatient setting: a prospective study of 857 women. BJOG 2007;114:763–6 [DOI] [PubMed] [Google Scholar]
- 12.Ubeda A, Labastida R, Dexeus S. Essure: a new device for hysteroscopic tubal sterilization in an outpatient setting. Fertil Steril 2004;82:196–9 [DOI] [PubMed] [Google Scholar]
- 13.Scarabin C, Dhainaut C. The ESTHYME study. Women's satisfaction after hysteroscopic sterilization (Essure micro-insert). A retrospective multicentre survey. Gynecol Obstet Fertil 2007;35:1123–8 [DOI] [PubMed] [Google Scholar]
- 14.ESSURE U.S. Physician Training Manual HSG protocol [accessed 16 November 2009]. Available from http://www.essuremd.com/Portals/0/Skins/Conceptus_Skin/PDFs/TR-0671-101-HSG-Protocol.pdf [Google Scholar]
- 15.Hemingway A. Hysterosalpingography. In: Grainger RG, Allison DJ, Adam A, Dixon AK, editors. Grainger & Allison's diagnostic radiology, a textbook of medical imaging, 4th edition London, UK: Churchill Livingstone, 2001: 2227–37 [Google Scholar]
- 16.Wittmer MH, Famuyide AO, Creedon DJ, Hartman RP. Hysterosalpingography for assessing efficacy of Essure microinsert permanent birth control device. AJR Am J Roentgenol 2006;187:955–8 [DOI] [PubMed] [Google Scholar]
- 17.Connor VF. Essure: a review six years later. J Minim Invasive Gynecol 2009;16:282–90 [DOI] [PubMed] [Google Scholar]
- 18.Syed R, Levy J, Childers ME. Pain associated with hysteroscopic sterilization. JSLS 2007;11:63–5 [PMC free article] [PubMed] [Google Scholar]
- 19.Thiel JA, Carson GD. Cost-effectiveness analysis comparing the essure tubal sterilization procedure and laparoscopic tubal sterilization. J Obstet Gynaecol Can 2008;30:581–5 [DOI] [PubMed] [Google Scholar]
- 20.Hopkins MR, Creedon DJ, Wagie AE, Willaims AR, Famuyide AO. Retrospective cost analysis comparing Essure hysteroscopic sterilization and laparoscopic bilateral tubal coagulation. J Minim Invasive Gynecol 2007;14:97–102 [DOI] [PubMed] [Google Scholar]
- 21.Vellayan M, Baxter A, Connor M, Brown V. The Essure hysteroscopic sterilisation procedure: initial experience in Sheffield, UK. Gynecol Surg 2006;3:303–7 [Google Scholar]
- 22.Shavell VI, Abdallah ME, Shade GH, Jr, Diamond MP, Berman JM. Trends in sterilization since the introduction of Essure hysteroscopic sterilization. J Minim Invasive Gynecol 2009;16:22–7 [DOI] [PubMed] [Google Scholar]
- 23.Famuyide AO, Hopkins MR, El-Nashar SA, Creedon DJ, Vasdev GM, Driscoll DJ, et al. Hysteroscopic sterilization in women with severe cardiac disease: experience at a tertiary centre. Mayo Clin Proc 2008;83:431–8 [DOI] [PubMed] [Google Scholar]
- 24.Agostini A, Crochet P, Petrakian M, Estrade JP, Cravello L, Gamerre M. Hysteroscopic tubal sterilization (essure) in women with an intrauterine device. J Minim Invasive Gynecol 2008;15:277–9 [DOI] [PubMed] [Google Scholar]
- 25.Donnadieu AC, Deffieux X, Gervaise A, Faivre E, Frydman R, Fernandez H. Essure sterilization associated with endometrial ablation. Int J Gynaecol Obstet 2007;97:139–42 [DOI] [PubMed] [Google Scholar]
- 26.Donnadieu AC, Fernandez H. The role of Essure sterilization performed simultaneously with endometrial ablation. Curr Opin Obstet Gynecol 2008;20:359–63 [DOI] [PubMed] [Google Scholar]
- 27.Hopkins MR, Creedon DJ, El-Nashar SA, Brown DL, Good AE, Famuyide AO. Radiofrequency global endometrial ablation followed by hysteroscopic sterilization. J Minim Invasive Gynecol 2007;14:494–501 [DOI] [PubMed] [Google Scholar]
- 28.Jansen NE, Vleugels MP, Kluivers KB, Vierhout ME. Bilateral cornual abscess after endometrial ablation following Essure sterilization. J Minim Invasive Gynecol 2007;14:509–11 [DOI] [PubMed] [Google Scholar]
- 29.Hitkari JA, Singh SS, Shapiro HM, Leyland N. Essure treatment of hydrosalpinges. Fertil Steril 2007;88:1663–6 [DOI] [PubMed] [Google Scholar]
- 30.Mijatovic V, Veersema S, Emanuel MH, Schats R, Hompes PG. Essure hysteroscopic tubal occlusion device for the treatment of hydrosalpinx prior to in vitro fertilization-embryo transfer in patients with a contraindication for laparoscopy. Fertil Steril 2010;93:1338–42 [DOI] [PubMed] [Google Scholar]
- 31.Hastings-Tolsma M, Nodine P, Teal SB, Embry J. Pregnancy outcome after transcervical sterilization. Obstet Gynecol 2007;110:504–6 [DOI] [PubMed] [Google Scholar]
- 32.Langenveld J, Veersema S, Bongers MY, Koks CA. Tubal perforation by Essure: three different clinical presentations. Fertil Steril 2008;90:2011.e5–10 [DOI] [PubMed] [Google Scholar]
- 33.Thoma V, Chua I, Garbin O, Hummel M, Wattiez A. Tubal perforation by ESSURE microinsert. J Minim Invasive Gynecol 2006;13:161–3 [DOI] [PubMed] [Google Scholar]
- 34.Booker CJ, Yarwood RL, Dodson WC. Dislodged Essure microinsert. Fertil Steril 2008;89:964–5 [DOI] [PubMed] [Google Scholar]
- 35.Hur HC, Mansuria SM, Chen BA, Lee TT. Laparoscopic management of hysteroscopic essure sterilization complications: report of 3 cases. J Minim Invasive Gynecol 2008;15:362–5 [DOI] [PubMed] [Google Scholar]
- 36.Lannon BM, Lee SY. Techniques for removal of the Essure hysteroscopic sterilisation tubal occlusion device. Fertil Steril 2007;88:497.e13–14 [DOI] [PubMed] [Google Scholar]
- 37.Gerritse MB, Veersema S, Timmermans A, Brolmann HA. Incorrect position of Essure microinserts 3 months after successful bilateral placement. Fertil Steril 2009;91:930.e1–5 [DOI] [PubMed] [Google Scholar]
- 38.Veersema S, Mol BW, Brolmann HA. Reproducibility of the interpretation of pelvic x-ray 3 months after hysteroscopic sterilization with Essure. Fertil Steril 2010;94:1202–7 [DOI] [PubMed] [Google Scholar]
- 39.Karthigasu KA, Garry R, Hart R. Case report of failed tubal occlusion using Essure pbc (permanent birth control) hysteroscopic sterilisation procedure. Aust N Z J Obstet Gynaecol 2006;46:365–7 [DOI] [PubMed] [Google Scholar]
- 40.National Institute for Health and Clinical Excellence. Hysteroscopic sterilisation by tubal cannulation and placement of intrafallopian implant. NICE guidance no. 44 [accessed 16 November 2009]. Available from http://www.nice.org.uk/Guidance/IPG44. [Google Scholar]
- 41.Teoh M, Meagher S, Kovacs G. Ultrasound detection of the Essure permanent birth control device: a case series. Aust N Z J Obstet Gynaecol 2003;43:378–80 [DOI] [PubMed] [Google Scholar]
- 42.Wittmer MH, Brown DL, Hartman RP, Famuyide AO, Kawashima A, King BF. Sonography, CT, and MRI appearance of the Essure microinsert permanent birth control device. AJR Am J Roentgenol 2006;187:959–64 [DOI] [PubMed] [Google Scholar]
- 43.Thiel JA, Suchet IB, Lortie K. Confirmation of Essure microinsert tubal coil placement with conventional and volume-contrast imaging three-dimensional ultrasound. Fertil Steril 2005;84:504–8 [DOI] [PubMed] [Google Scholar]
- 44.Weston G, Bowditch J. Office ultrasound should be the first-line investigation for confirmation of correct ESSURE placement. Aust N Z J Obstet Gynaecol 2005;45:312–15 [DOI] [PubMed] [Google Scholar]
- 45.Kerin JF, Levy BS. Ultrasound: an effective method for localization of the echogenic Essure sterilization micro-insert: correlation with radiologic evaluations. J Minim Invasive Gynecol 2005;12:50–4 [DOI] [PubMed] [Google Scholar]
- 46.Veersema S, Vleugels MP, Timmermans A, Brolmann HA. Follow-up of successful bilateral placement of Essure microinserts with ultrasound. Fertil Steril 2005;84:1733–6 [DOI] [PubMed] [Google Scholar]
- 47.Veersema S, Vleugels MP, Moolenaar LM, Janssen CA, Brolmann HA. Unintended pregnancies after Essure sterilization in the Netherlands. Fertile Steril 2010;93:35–8 [DOI] [PubMed] [Google Scholar]
- 48.Connor VF. Contrast infusion sonography to assess microinsert placement and tubal occlusion after Essure. Fertile Steril 2006;85:1791–3 [DOI] [PubMed] [Google Scholar]
- 49.Connor V. Contrast infusion sonography in the post-Essure setting. J Minim Invasive Gynecol 2008;15:56–61 [DOI] [PubMed] [Google Scholar]
- 50.Ory EM, Hines RS, Cleland WH, Rehberg JF. Pregnancy after microinsert sterilization with tubal occlusion confirmed by hysterosalpingogram. Obstet Gynecol 2008;111:508–10 [DOI] [PubMed] [Google Scholar]
- 51.Moses AW, Burgis JT, Bacon JL, Risinger J. Pregnancy after Essure placement: report of two cases. Fertile Steril 2008;89:724.e9–11 [DOI] [PubMed] [Google Scholar]
- 52.Levy B, Levie MD, Childers ME. A summary of reported pregnancies after hysteroscopic sterilization. J Minim Invasive Gynecol 2007;14:271–4 [DOI] [PubMed] [Google Scholar]
- 53.Hastings-Tolsma M, Nodine P, Teal SB. Essure: hysteroscopic sterilization. J Midwifery Womens Health 2006;51:510–14 [DOI] [PubMed] [Google Scholar]
- 54.Valle RF. Re: Case report of failed tubal occlusion using Essure pbc (permanent birth control) hysteroscopic sterilisation procedure. Aust N Z J Obstet Gynaecol 2007;47:155–6 [DOI] [PubMed] [Google Scholar]
- 55.Nichols JE., Jr Re: Pregnancy after microinsert sterilization with tubal occlusion confirmed by hysterosalpingogram. Obstet Gynecol 2008;112:185–6 [DOI] [PubMed] [Google Scholar]
- 56.Shavell VI, Abdallah ME, Diamond MP, Kmak DC, Berman JM. Post-Essure hysterosalpingography compliance in a clinic population. J Minim Invasive Gynecol 2008;15:431–4 [DOI] [PubMed] [Google Scholar]
- 57.Baxter N, Hudson H, Rogerson L, Duffy S. Hysteroscopic sterilisation: a study of women's attitudes to a novel procedure. BJOG 2005;112:360–2 [DOI] [PubMed] [Google Scholar]