Abstract
Objective
Consistency in target organ and organ at risk position from planning to treatment is an important basic principle of radiotherapy. This study evaluates the effectiveness of bladder-filling instructions in achieving a consistent and reproducible bladder volume at the time of planning CT and daily during the course of radical radiotherapy for prostate cancer. It also assessed the rate of bladder filling before and at the end of radiotherapy.
Methods
30 men attending for radiation therapy planning for prostate cancer received written and verbal bladder-filling instructions. They had their bladder volume assessed using a bladder ultrasound scanner post-void, immediately prior to planning CT scan and then daily immediately prior to treatment while in the therapy position. The inflow was calculated using the void and full bladder volumes and the time for the bladder to fill.
Results
The mean bladder volume at the time of planning was 282 ml (range 89–608 ml, standard deviation (SD) = 144.5 ml). This fell during treatment, with a mean value for all treatments of 189 ml (range 11–781 ml, SD = 134 ml). During radiotherapy, 76% (828/1090), 53% (579/1090) and 36% (393/1090) of bladder volumes had >50 ml, >100 ml and >150 ml difference, respectively when compared with their volume at the time of planning. Inflow reduced from 4.6 ml min–1, SD = 2.9 min–1 at planning to 2.5 min–1, SD = 1.8 min–1 after radiotherapy.
Conclusion
The Bladderscan device (BVI 6400 Bladderscan, Verathon Medical UK, Sandford, UK) provides an effective means of assessing bladder volume prior to radiotherapy for prostate cancer. The evaluated bladder-filling protocol does not produce consistent, reproducible bladder volumes for radiotherapy.
Reproducibility of target volume position is a fundamental component of external beam radiotherapy at any site, but is of particular importance where a dose-escalated regimen is being employed and where the surrounding organs are both dose and volume sensitive. For men receiving dose-escalated radical prostate radiotherapy, a consistent bladder volume between planning and treatment is vital. Although there are differing opinions on the influence of bladder volume on interfraction prostate position [1-7], the influence of the irradiated bladder volume on acute and late urinary [8] and bowel toxicity [2,9] has been well documented.
Despite the apparent importance of controlling bladder volume for prostate radiotherapy, there is surprisingly limited research into the provision of bladder-filling instructions that produce acceptable dose–volume histograms (DVHs) and provide a reproducible bladder volume from planning through to treatment [1-2,10,11] rather than an unreliable and misleading snapshot at the time of planning.
This study was designed to first validate bladder volume measurements using a non-invasive transabdominal bladder ultrasound device (BVI 6400 Bladderscan, Verathon Medical UK, Sandford, UK) before using it to evaluate the effectiveness of standardised bladder-filling instructions in achieving a consistent and reproducible bladder volume from the time of planning and during each daily fraction of radical radiotherapy for prostate cancer. The data obtained would then be used to inform changes in bladder-filling instructions before future re-evaluation.
There is a basic assumption in trying to obtain a standardised bladder volume that the rate at which a bladder fills after a defined fluid load is relatively constant. This has been previously confirmed in healthy volunteers [12] but not in men receiving radiotherapy for prostate cancer. The study was also designed to assess bladder inflow rate in such a group at the time of radiotherapy planning and on the final fraction of radiotherapy.
Methods and materials
Patients
After ethical approval (REC ref: 08/NIRO3/70) and based on a review of previous literature [1,10] and a small pilot study in our centre, 30 consecutive men attending for radical external beam radiotherapy planning for their localised prostate cancer between August 2008 and January 2009 gave written informed consent to enter the study. They received written and verbal advice on bladder filling prior to their CT planning appointment. The advice was to “void the bladder and then drink 500 ml of water within the next 15 minutes. 30 minutes later proceed with the radiotherapy planning scan. This process should then be repeated daily prior to each treatment”.
All men were being treated with radiotherapy as the primary definitive therapy for their prostate cancer, and received 3 months of neo-adjuvant hormonal therapy prior to radiation planning. All had an anatomically normal functioning bladder and had minimal lower urinary tract symptoms defined as a pre-treatment international prostate symptom score (IPSS) of less than 7.
Bowel preparation with a single microenema was administered prior to voiding at the time of planning and repeated daily prior to each treatment. The use of α-blockade during radiotherapy, weekly IPSS and acute Radiation Therapy Oncology Group (RTOG) toxicity criteria from baseline were recorded.
Radiotherapy planning and treatment
Planning and treatment were performed with the patient in a supine position with a knee rest. CT slices were obtained at 2.5 mm intervals on a Siemens Emotion 6 CT Scanner (Siemens Healthcare, Camberley, UK). Target and critical structures including the bladder and rectum were delineated by the attending radiation oncologist and reviewed by a singe physicist on the Oncentra Masterplan treatment planning system (Nucletron, Veenendal, The Netherlands).
Three-dimensional (3D) conformal radiotherapy was delivered using a 15 MV accelerator in a 2 phase plan treating the prostate and base of seminal vesicles to 64 Gy in 32 fractions with a 10 mm margin, except posteriorly where 8 mm was used. A final 10 Gy in 5 fractions was delivered with no margin.
Bladder ultrasound scan protocol
A bladder ultrasound scan was performed by a therapy radiographer directly after voiding and then immediately before the planning CT scan while on the CT couch (approximately 45 min post-fluid load). The time of each ultrasound scan was documented. The bladder volume was also calculated from the CT planning scan. Patients followed current bladder-filling instructions and then had an ultrasound scan performed immediately prior to each daily fraction of radiotherapy while on the treatment couch. A post-void volume was also recorded on the final fraction. Patients were not informed of their volume results to prevent falsely consistent volumes occurring via a biofeedback mechanism.
The therapy radiographers were trained and supervised for their first 30 scans before being deemed competent. Each ultrasound scan took approximately 1–2 min and had no impact on overall treatment time.
Analysis
Validation of the ultrasound bladder volume measurements was performed by means of comparison with the corresponding CT scan volume using a Pearson correlation, a paired Student’s t-test (assuming normality) and a Bland–Altman plot. Statistical analysis was performed using SPSS (Version 17; Chicago, IL).
Linear regression was used to calculate the average reduction in bladder volume per session using robust standard errors to account for the lack of independence of observations from the same person (Kirkwood and Sterene Medical Statistics; STATA version 9).
Pearson’s correlation coefficients were used to investigate the association between different characteristics (IPSS, age, Gleason, prostate-specific antigen (PSA) and glomerular filtration rate (GFR)) and average bladder volume and standard deviation. Spearman’s correlation coefficient was used to investigate the association between ranked characteristics such as Gleason and T stage and explanatory variables.
The spread of bladder volumes measured over the course of treatment was compared with the pre-treatment volume for each patient.
A linear regression analysis adjusting for the lack of independence to account for the fact that the same 30 patients were scanned on each session was performed to analyse the changes in bladder volume over the course of treatment.
Inflow was calculated by taking the difference between the post-void volume on ultrasound scan and the full bladder ultrasound scan volume and dividing by the time between scans. This was performed during the CT planning appointment and on the final day of treatment. A Student’s t-test was performed to assess the statistical significance of any differences (p<0.05). Any changes between these inflows were correlated with the change in bladder volumes on these specific days.
Results
30 men with a median age of 67 years (range 56–78 years) were assessed. The hand-held ultrasound device was well tolerated, with 98% (1090/1110) of proposed scans performed and no men requiring α-blockade during radiotherapy.
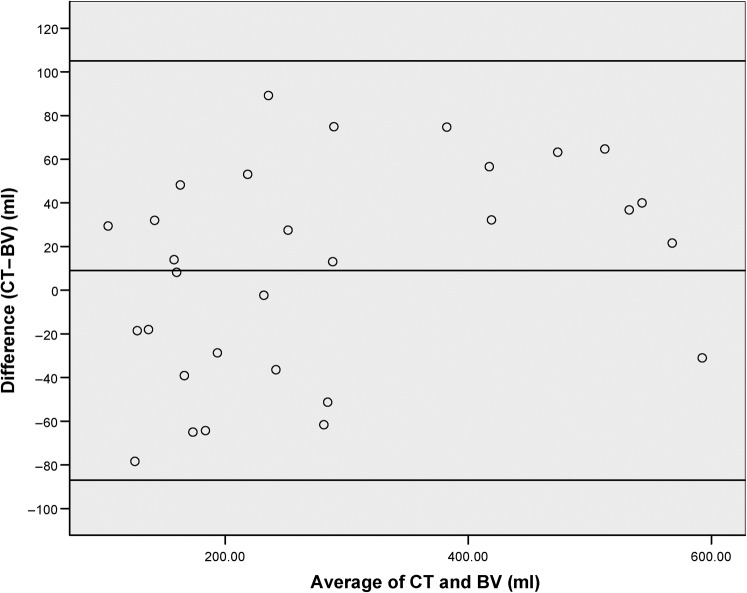
The validity of the volume measured by the ultrasound scanner was confirmed by comparison of the volume it calculated immediately prior to CT planning and the volume calculated on the CT scan data set using Oncentra Masterplan (Pearson correlation coefficient 0.91). Figure 1 demonstrates the relationship between the average of the bladder volumes (CT and ultrasound scan) and the difference between these readings, which is not constant, with larger volumes demonstrating a greater difference (Spearman’s correlation coefficient 0.38, p = 0.04).
Figure 1.
A plot demonstrating the relationship between the average of the bladder volumes (CT and Bladderscan BV) at the time of planning and the difference between these values with the limits of agreement added.
Patient bladder volumes
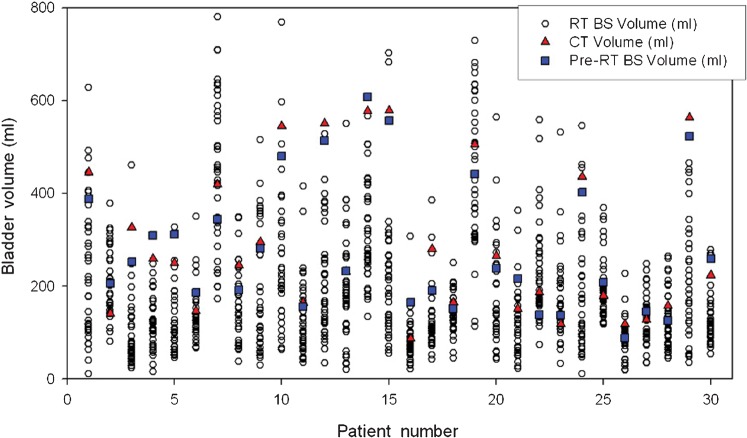
The mean volume on ultrasound at the time of planning was 282 ml (range 89–608 ml, SD = 144.5 ml) and the mean CT volume was 291 ml (range 87–579 ml, SD = 162 ml). A paired Student’s t-test showed that these were not statistically different (p = 0.29). The average volume fell during treatment, with a mean value for all treatments of 189 ml (range 11–781 ml, SD = 134 ml) and on the final day of treatment the mean value was 165 ml (range 64–339 ml, SD = 138 ml). Figure 2 shows the spread of bladder volumes measured over the course of the treatment compared with the pre-treatment volumes for each patient. Based on the coefficient of variation (CoV), the most and least variable patients had median values of 62 ml (range 29–461 ml, SD = 83 ml, CoV = 86) and 473 ml (range 124–728, SD = 159 ml, CoV = 35), respectively.
Figure 2.
Individual patient bladder volumes from both ultrasound (pre-RT BS volume) and CT at the time of planning and daily immediately prior to radiotherapy (RT BS volume). RT, radiotherapy; BS, bladder scanning.
During radiotherapy, 76% (828/1090), 53% (579/1090) and 36% (393/1090) of bladder volumes had a >50 ml, >100 ml and >150 ml difference, respectively, when compared with their volume at the time of planning. All men had a >50% reduction in their ultrasound volume on at least 1 occasion during treatment when compared with their planning volume.
No correlations were observed between patient characteristics (age, Gleason, PSA and T stage) or GFR compared with the average bladder volume, standard deviation (ml) or standard deviation (%). IPSS score at each week was also compared with the average volumes for that week for each patient, but revealed no correlation.
Bladder volume and inflow over time
The mean time from void to bladder ultrasound immediately before the CT planning was 47.3 min (range 25–70 min) and from void to bladder ultrasound immediately before the final treatment was 45.5 min (range 10–139 min), p = 0.8.
There was an average decrease in bladder volume per visit of 1 ml (p = 0.026; 95% CI 0.1–2.1) from linear regression using robust standard errors to adjust for correlated observations in patients. While the majority of cases had no apparent trend in bladder volume reduction over the course of their radiotherapy, a few had a clear reduction, with the most obvious having a 7 ml decrease in bladder volume per day.
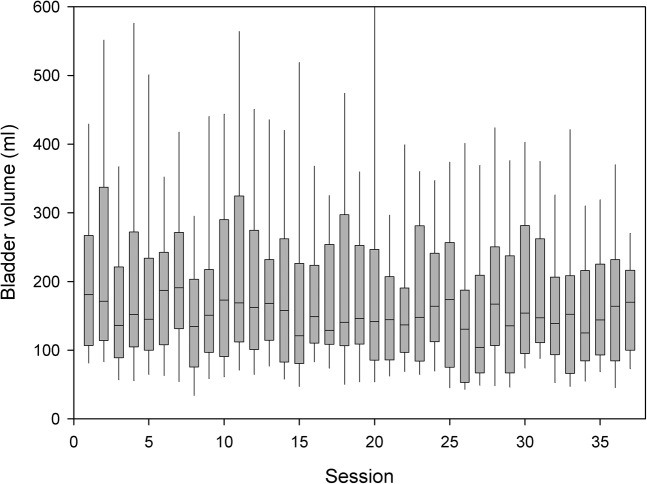
Average pre-treatment inflow was found to be 4.6 ± 2.9 min–1 and was 2.5 ± 1.8 min–1 after radiotherapy, which was a statistically significant reduction (p = 0.0003). It can be seen in Figure 3 that bladder volumes decrease over the course of radiotherapy. When the difference in inflow rate and the difference in bladder volume pre- and post-radiotherapy were assessed (Figure 4), a Pearson correlation of 0.79 was noted. The reduction in bladder inflow rate was not related to the calculated GFR (Cockcroft–Gault formula).
Figure 3.
A box plot demonstrating the median, interquartile range and whisker’s indicating the 90th and 10th percentile of daily bladder volume in all patients through the course of radiotherapy.
Figure 4.
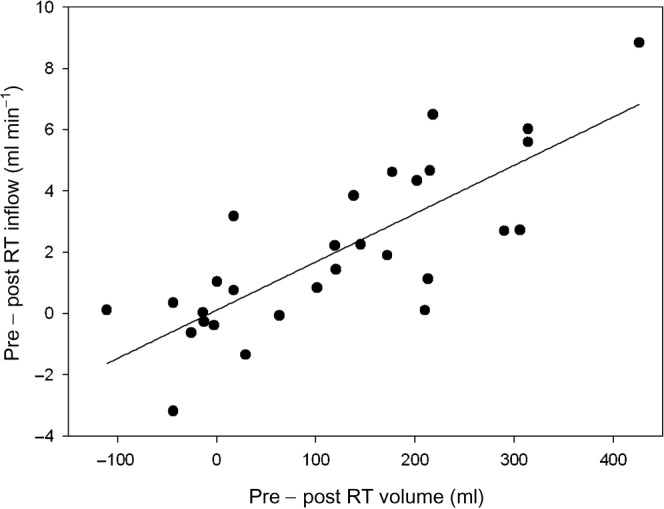
Direct relationship between the difference in inflow before and after radiotherapy compared with bladder volume before and after radiotherapy for prostate cancer patients.
Discussion
Daily ultrasound assessment using a hand-held bladder ultrasound scanner is an effective, well-tolerated and non-invasive method of evaluating the bladder volume prior to radiotherapy treatment in the studied group.
It provides an accurate assessment of bladder volume when compared with volumes calculated on a CT planning system, but may slightly underestimate the volume where the values are greater than 300 ml.
In this cohort of men, the evaluated bladder-filling instructions fail to provide consistent and reproducible bladder filling from CT planning through a course of radiotherapy, with a progressive reduction in mean bladder volume over the duration of treatment. This finding concurs with other published work [1,8]. None of the evaluated factors predicted for this inconsistency.
While this study does not examine the effect of changing bladder volume on prostate position for radiotherapy, it clearly demonstrates the fallibility of DVHs in providing an accurate assessment of the actual radiation dose delivered to the bladder in this study population. In 21 of 30 (70%) men, the average bladder volumes were lower than the planning scan, and in 8 of 30 (27%) the average bladder volume over the course of treatment was more than 50% lower than the volume at the time of planning. The DVH data provided by the CT planning scan would therefore have underestimated the dose delivered to the bladder in 70% of treatments.
There are three ways to apply this information. First, assuming that patients had no post-void residual volume, asking men to empty their bladder immediately prior to radiotherapy would remove any potential effect of variable bladder filling on prostate motion [7]. However, the dose delivered to the bladder and small bowel particularly in the dose escalation era would be expected to lead to increased acute and late toxicity in these organs [2]. Unfortunately, post-void residual volumes are often seen and provide a further source of variability.
Second, continuing to plan radiotherapy with a large fluid load that, although difficult to reproduce through treatment, provides acceptable but unreliable DVHs. Assuming prostate position is not influenced by the change in bladder volume, then this could be a valid strategy. If, however, the prostate position is influenced by bladder filling, then as the bladder volume reduces over the course of treatment and if rectal volume is unchanged, the prostate would move anteriorly. Given the more generous anterior margins typically applied in expanding the clinical target volume (CTV) to planning target volume (PTV), the possibility of a geometric miss would be much less likely [3].
Finally, applying the general principle of consistency in patient set-up, a bladder-filling protocol should be developed that is reproducible and has limited variability with DVHs representative of the course of treatment. Several methods of reducing the variability of bladder volume have been examined in this setting. Stam et al [1] reduced the variability of the bladder volume through the use of a biofeedback mechanism with daily ultrasound assessment. The average bladder volume during treatment was 94.6% of the CT planning volume with biofeedback compared with 83.2% without biofeedback (p = 0.38). O’Doherty et al [10] demonstrated how the provision of written bladder-filling instructions to patients helped to keep the average bladder filling during treatment closer to the volume at CT planning than patients who had not received the written instructions.
This study has described in detail how the bladder volume can change on a daily basis. This has been invaluable in gaining an insight into daily variations. We are also now in the position where we can examine the frequency of bladder scanning that would be required to assess the overall bladder-filling protocol. Taking a weekly reading shows that the average 193.9 ml ± 136.6 ml is not significantly different from the daily average of 189.1 ml ± 134.8 ml (p = 0.83). Three readings at the start, middle and end of treatment would yield a significant difference (p = 0.02) from the daily analysis and therefore may not be adequate for full analysis of the bladder-filling protocol. This information can be transferred for use by in-line soft-tissue imaging such as cone beam CT, with weekly scans required to evaluate organ position and filling.
The hypothesis that the rate at which the bladder fills is constant after a defined fluid load has not been supported by this study. Although the intravolunteer variation (SD = 2.9 ml min–1 pre-radiotherapy and 1.8 ml min–1 post-radiotherapy) is similar to those quoted by Lotz et al [12] (SD = 3.0 ml min–1), the younger healthy volunteers studied by Lotz had minimal variation in their inflow rate compared with the significant reduction in inflow rate from planning to the end of treatment in the men in our study.
Bladder filling is not a simple input–output calculation, as it is affected by the patient’s state of hydration, time of day and concomitant medication, and the complexities of renal blood flow must be considered. Non-compliance with the protocol resulted from men either reducing their fluid load as bladder irritation increases over the course of treatment or reducing the period of time from void to load to treatment as they became more concerned about maintaining the fluid load for the recommended time.
It is important that this reduction in inflow is taken into account when assessing bladder-filling protocols. Most studies have seen a reduction in bladder volume over the course of radiotherapy [1,8]. We acknowledge the limited size of our population and recommend that this finding should be confirmed in a larger population, with inflow calculations performed more frequently during treatment. This study would also be improved by using cone beam CT, providing daily DVH data as well as assessing organ motion, which is not available with the ultrasound bladder scanner.
While there should be continued focus on compliance with bladder-filling instructions, the reduction in inflow over treatment is a useful finding, suggesting that patients should be encouraged to progressively increase their fluid load as their treatment progresses.
Conclusion
The bladder-filling protocol evaluated in this study fails to provide reproducible and consistent bladder volumes from the time of planning through to daily treatment for prostate cancer, with the volume at CT planning generally overestimating the volume achieved during treatment. The reduction in bladder volume over the course of radiotherapy may not only be due to poor compliance to bladder-filling instructions, but also due to the inability to fill the bladder within a specified time, as demonstrated by the reduction in inflow. Further investigative research is planned to compare the effectiveness of different bladder-filling protocols in achieving a reproducible bladder volume for prostate radiotherapy.
Acknowledgment
A donation provided by the Friends of the Cancer Centre purchased and maintained the Bladderscan BVI 6400 device used in the study.
References
- 1.Stam MR, Van Lin ENJ, Van DerVight LP, Kaanders JHAM, Visse AG. Bladder filling variation during radiation treatment of prostate cancer: can the use of a bladder ultrasound scanner and biofeedback optimize bladder filling? Int J Rad Oncol Biol Phys 2006;65:371–7 [DOI] [PubMed] [Google Scholar]
- 2.Pinkawa M, Asadpour B, Gagel B, Piroth MD, Holy R, Eble MJ. Prostate position variability and dose-volume histograms in radiotherapy for prostate cancer with full and empty bladder. Int J Radiat Oncol Biol Phys 2006;64:856–61 [DOI] [PubMed] [Google Scholar]
- 3.Langen KM, Jones DTL. Organ motion and it’s management. Int J Radiat Oncol Biol Phys 2001;50:265–78 [DOI] [PubMed] [Google Scholar]
- 4.Antolak JA, Rosen II, Childress CH, Zagars GK, Pollack A. Prostate target volume variation during a course of radiotherapy. Int J Radiat Oncol Biol Phy 1998;42:661–72 [DOI] [PubMed] [Google Scholar]
- 5.Zellars RC, Roberson PL, Stawderman M, Zhang D, Sandler HM, Ten Haken RK, et al. Prostate position late in the course of external beam radiotherapy: patterns and predictors. Int J Rad Oncol Biol Phys 2000;47:655–60 [DOI] [PubMed] [Google Scholar]
- 6.Roeske JC, Forman JD, Mesina CF, He T, Pelizzari CA, Fontenla E, et al. Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder and rectum during a course of external beam radiotherapy. Int J Rad Oncol Biol Phys 1995;33:1321–9 [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Crean D, Mageras GS, Lyass O, Happersett L, Ling C, et al. Quantification and predictors of prostate position variability in 50 patients evaluated with multiple CT scans during conformal radiotherapy. Radiother Oncol 1999;50:225–34 [DOI] [PubMed] [Google Scholar]
- 8.Lebesque JV, Bruce AM, Kroes APG, Touw A, Shouman T, van Herk M. Variation in volumes, dose-volume histograms, and estimated normal tissue complications probabilities of rectum and bladder during conformal radiotherapy of T3 prostate cancer. Int J Rad Oncol Biol Phys 1995;33:1109–19 [DOI] [PubMed] [Google Scholar]
- 9.Storey MR, Pollack A, Zagars G, Smith L, Antolak J, Rosen I. Complications from radiotherapy dose escalation in prostate cancer: preliminary results of a randomized trial. Int J Radiat Oncol Biol Phys 2000;48:635–42 [DOI] [PubMed] [Google Scholar]
- 10.O’Doherty UM, McNair HA, Norman AR, Miles E, Hooper S, Davies M, et al. Variability of bladder filling in patients receiving radical radiotherapy to the prostate. Radiother Oncol 2006;79:335–40 [DOI] [PubMed] [Google Scholar]
- 11.Moiseenko V, Liu M, Kirstensen S, Gelowitz G, Berthelet E. Effect of bladder filling on doses to prostate and organs at risk: a treatment planning study. J Appl Clin Med Phys 2007;8:55–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotz HT, van Herk M, Betgen A, Pos F, Lebesque JV, Remeijer P. Reproducibility of the bladder shape and bladder shape changes during filling. Med Phys 2005;32:2590–7 [DOI] [PubMed] [Google Scholar]