Abstract
Objectives
Imaging of the pleura by multidetector CT (MDCT) can be challenging. There is no clear evidence or guidelines on contrast infusion parameters for imaging pleura. We compared two contrast protocols for assessing pleural pathology on MDCT.
Methods
This was a prospective study in which consecutive patients with MDCT for suspected pleural disease on chest radiograph were randomised into two groups. The first group received 150 ml of intravenous contrast at a rate of 2.5 ml s–1 and the second group received 100 ml at 2 ml s–1. Images were acquired after a 60 s delay. Hounsfield units of the pleura, thoracic aorta, main pulmonary artery, portal vein and superior mesenteric artery were measured and analysed by two independent readers.
Results
40 patients (20 in each group) who had pleural enhancement on MDCT were included for final analysis. The mean pleural enhancement value was 83 HU (Group A) vs 59 HU (Group B) (p = 0.0004). The mean aortic enhancement was 241 HU (A) vs 141 HU (B) (p<0.0001); main pulmonary artery enhancement was 208 HU (A) vs 139 HU (B) (p<0.0002); portal venous enhancement was 169 HU (A) vs 115 HU (B) (p<0.0001); and the superior mesenteric artery enhancement was 215 HU (A) vs 128 HU (B) (p<0.0001).
Conclusion
Enhancement of the pleura and major vessels was significantly higher in the group receiving more contrast at a greater infusion rate. This technique of a single scan through the entire pleural surface with a delayed acquisition is promising. When pleural disease is suspected, contrast infusion protocols should be modified to achieve the best results and clinicians should be encouraged to specifically request a “pleural CT”.
Diseases of the pleura can be broadly classified into benign and malignant. The incidence of malignant pleural mesothelioma is increasing worldwide. Projections suggest that the number of men dying from mesothelioma in western Europe each year will almost double over the next 20 years, from 5000 in 1998 to approximately 9000 in around 2018 [1].
Contrast-enhanced multidetector CT (MDCT) is an established modality for investigating suspected pleural disease by allowing thorough scrutiny of the various pleural surfaces within the thorax [2]. Pleural thickening, enhancement, effusions and other associated findings on MDCT help in further characterisation of disease into a benign or malignant process.
There is a relative lack of published studies and guidelines on MDCT imaging of the pleura, specifically looking at different contrast infusion protocols. In this study we compare two contrast infusion protocols, used in our centre, for assessing suspected pleural disease.
Method and materials
Study design
This prospective study was conducted in our centre between August and October 2007. The aim of the study was to compare two contrast infusion protocols used in MDCT imaging of suspected pleural disease. The study design was discussed and accepted by the institutional review board.
Consecutive patients who had MDCT imaging for suspected pleural disease on their chest radiograph were randomised into two groups (A and B) and received different infusion protocols. Patients who did not receive contrast because of either impaired renal function or previous allergy were excluded from the study. Two independent observers, who were blinded to the patient group classification, collected the data. Observation bias was minimised by taking the mean values of both observers for final analysis. MDCT scans with no pleural enhancement and/or pleural thickening of <2 mm were excluded from the final analysis.
Scan technique
Standard scan protocols were used to ensure uniformity and comparability among the two groups. Iopamidol 61.2% wt/vol. was injected using a power injector (Injektron CT2, Medtron Saarbrüken, Germany) through an intravenous cannula (either 22 G or 20 G) placed in the antecubital fossa. All scans were performed, with a 60 s delay from the start of injection, on a 16-slice MDCT (Somatom Sensation 16, Siemens AG, Erlangen, Germany) with 1.5 mm collimation and a reconstruction interval of 2 mm. The scan range extended from the lung apices to the inferior border of the liver. Group A received 150 ml of contrast at 2.5 ml s–1; Group B received 100 ml of contrast at 2 ml s–1.
Image interpretation
Images were reviewed on a Siemens Leonardo workstation and enhancement values (Hounsfield units) of the pleura, main pulmonary artery (MPA), thoracic aorta (TA), portal vein (PV) and superior mesenteric artery (SMA) were measured using a circular region-of-interest cursor on mediastinal window settings (window level 40–50 HU; width 400–500 HU). The mean of the HU values calculated by two authors was used for analysis.
For uniformity, pleural enhancement values were measured posteriorly in the paraspinal region at the level of the inferior pulmonary vein. If there was no enhancement at this site, HU values were measured at the level of most intense pleural enhancement. MPA enhancement was measured immediately before its division into right and left branches. The thoracic aortic measurement was carried out at the same level as the MPA. PV enhancement was measured at the porta hepatis prior to its bifurcation and SMA enhancement was measured at its origin.
Analysis
SPSS software (version 14, SPSS Inc, Chicago, IL) was used for statistical analysis. The Mann–Whitney test was used to compare the data between groups; p-values of <0.05 were considered statistically significant.
Results
A total of 94 patients underwent MDCT for suspected pleural disease during this period. 54 were excluded owing to a lack of pleural enhancement and/or pleural thickening of <2 mm on MDCT.
Patient demographics
20 of the total of 40 patients analysed were assigned to each group; Group A had a 16:4 (male:female) distribution in comparison with 17:3 in Group B. The mean age of the patients in Group A was 65 years old (range 24–86) and in Group B it was 64 years old (31–82). No statistically significant difference was found in the age or sex distribution.
Enhancement
The mean enhancement of the pleura was significantly higher in the group receiving 150 ml of contrast at 2.5 ml s–1 (83 HU) than in the group receiving 100 ml at 2 ml s–1 (59 HU), p<0.001. The mean enhancement of the MPA was 208 HU (Group A) compared with 139 HU in Group B, p<0.001. The mean enhancement of the TA was 241 HU in Group A and 141 HU in Group B, p<0.001. The mean PV enhancement was 169 HU in Group A and 115 HU in Group B, p<0.001. The mean SMA enhancement was 215 HU in Group A and 128 HU in Group B, p<0.001.
Discussion
MDCT allows detailed evaluation of the pleura and differentiation of benign from malignant pleural disease [2]. Adequate enhancement of the pleura enables differentiation of the thickened pleura from adjacent effusion or aerated or collapsed lung. There is a lack of consensus regarding the optimal infusion protocol for imaging suspected pleural disease primarily because of the paucity of published evidence and guidelines. The results of this study demonstrate that imaging with 150 ml of contrast infused at 2.5 ml s–1 leads to significantly higher enhancement of the pleura and major vessels than 100 ml of contrast infused at 2 ml s–1.
Multiple studies have examined the relationship of volume of contrast, its infusion rate, scan delay and the patient’s weight with enhancement [3-7]. Most have indicated a positive correlation between contrast enhancement and higher contrast volumes and faster injection rates. Based on these findings, a protocol in which more contrast (150 ml) is infused at a higher rate (2.5 ml s–1) is practised at our institution. Prior to this, MDCT imaging of the pleura was performed with 100 ml of contrast infused at 2 ml s–1. All imaging was performed with a 60 s delay from the start of contrast injection as pleural enhancement is delayed compared with lung parenchyma [2].
It is vital to have adequate enhancement of the pathological pleura so that it can be seen in patients with empyema and malignant effusions [3,8-10]. Waite et al [9] demonstrated that 96% of patients with empyema and up to 10% of patients with malignant effusions showed parietal pleural enhancement; 95% of the latter are metastatic in origin, with common primaries being lung and breast, while mesothelioma accounts for 5% [11]. Localised fibrous tumours involving the pleura also exhibit intense homogeneous contrast enhancement on CT [11-13]. Our study demonstrates significantly higher pleural enhancement (83 HU) in patients receiving more contrast at a higher rate than in patients receiving a smaller traditional volume (59 HU) (Figure 1).
Figure 1.
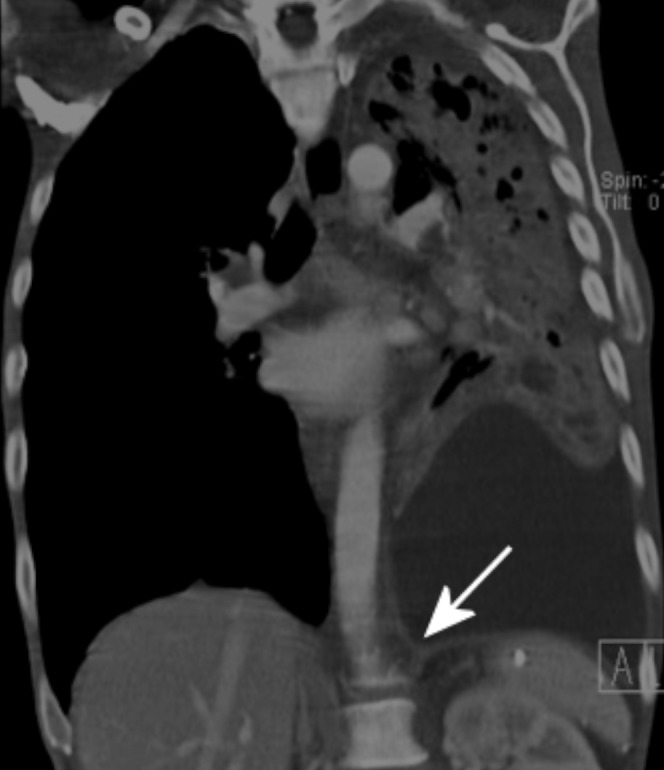
Coronal reconstruction of a contrast-enhanced “pleural” CT (150 ml of contrast at 2.5 ml s–1) showing pleural thickening and enhancement (arrow) in a left-sided empyema secondary to a necrotising pneumonia.
Pulmonary embolism (PE) is a relatively common condition and patients with malignant pleural pathology are at a higher risk of developing thromboembolic events [14]. Up to 1.5% of patients undergoing routine chest CT are found to have an unsuspected PE [15,16]. This incidence increases to 4% in inpatients and 5% in patients with neoplastic disease [16,17]. Wittram [18] suggested that the mean attenuation values for acute and chronic emboli were 78 HU and 87 HU, respectively. Wittram derived the highest possible attenuation value of a chronic embolus as 180 HU, and suggested that the minimum attenuation of opacified blood to be able to identify this embolus should be 211 HU. This value was nearly achieved in the group receiving higher contrast (208 HU). Although a CT pulmonary angiogram is the gold standard for imaging suspected PE, our modified technique perhaps further aids the detection of incidental emboli in patients with suspected pleural disease (Figure 2).
Figure 2.
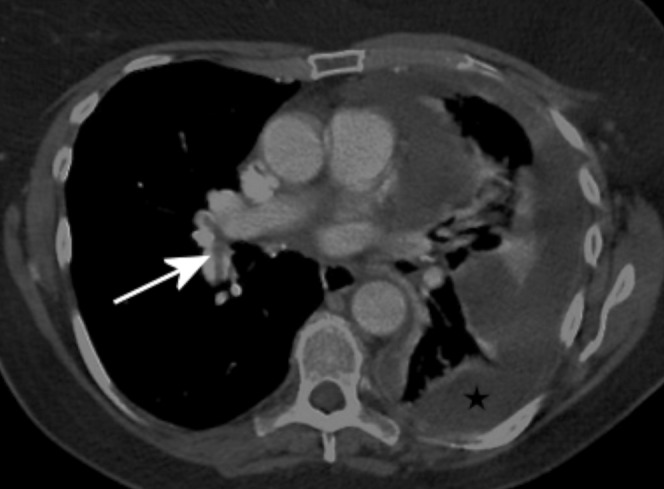
Contrast-enhanced CT with 150 ml of contrast infused at 2.5 ml s–1. This shows a left-sided malignant pleural collection (star). There is sufficient enhancement of the pulmonary arteries to diagnose pulmonary emboli in the right lobar arteries (arrow).
Most liver lesions are hypovascular and are well demonstrated during the portal venous phase of liver enhancement [3,5]. In our study, portal vein enhancement was significantly better in Group A than in Group B. Although not a substitute for two- or three-phase assessment of the liver, our technique allows confident interpretation of unexpected liver lesions (Figure 3). This has also been corroborated by Chambers et al [6], who concluded that greater hepatic enhancement results from a faster rate of infusion of a greater volume of contrast. In a similar pattern, enhancement values for both the aorta and SMA were significantly higher (241 vs 141) in Group A.
Figure 3.
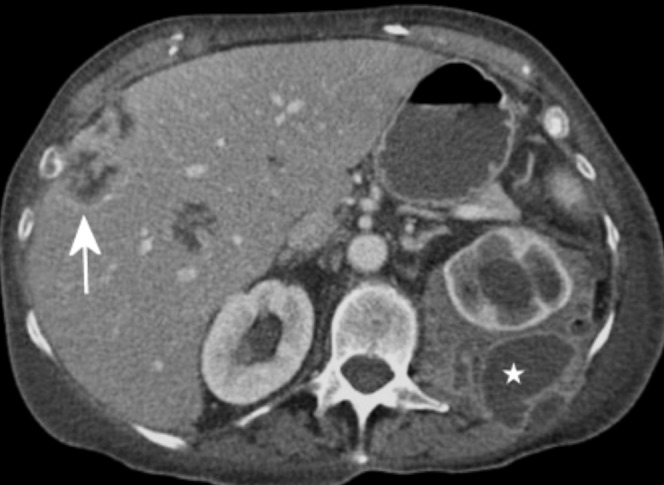
Contrast-enhanced CT (150 ml at 2.5 ml s–1) showing portal venous enhancement of the liver with low-density lesions within the parenchyma that were later confirmed to be liver metastases (arrow). Also note the left retroperitoneal necrotising collection (star).
In many institutions, a two-phase scanning technique used for lung cancer staging is also used for imaging suspected pleural disease. With this technique, the chest and a part of the upper abdomen are scanned in the arterial phase followed by a portovenous phase scan of the upper abdomen. This not only increases the radiation dose, because of scan overlap, but also limits the use of multiplanar reconstruction for overall assessment of the pleura. Our modified technique overcomes this and allows good reformatting of the images for a more thorough assessment, thereby helping to determine the target lesion for image-guided biopsy, which is proven to be highly sensitive and specific [2,19,20].
Limitations of study
This study did not assess factors such as body weight, cardiac output and total iodine dose, which can influence contrast enhancement [5]. All imaging was performed after a delay of 60 s after the start of contrast injection. Therefore, the relationship of the time of the scan and pleural enhancement has not been evaluated. We believe that if a structure enhances better then it is more conspicuous and improves diagnostic confidence, in turn increasing the sensitivity and specificity. This, however, was not tested in the study.
Clinical relevance
We have adopted this technique in our centre for all patients with suspected pleural disease on plain radiographs. Pelvic imaging is included in women with suspected ovarian pathology and in patients with previous known abdominal malignancy. We plan to evaluate further protocols using a lower volume of contrast and different infusion rates.
Conclusion
MDCT has an important role in managing pleural disease. Enhancement of the pleura and major vessels is significantly higher in patients receiving more contrast at a greater infusion rate (150 ml at 2.5 ml s–1). This technique of a single scan through the entire pleural surfaces with a delay in acquisition is promising. Therefore, when pleural disease is suspected, contrast infusion protocols could be modified to achieve maximal enhancement of relevant structures.
References
- 1.Peto J, Decarli A, la Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer 1999;79:666–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benamore RE, O'Doherty MJ, Entwisle JJ. Use of imaging in the management of malignant pleural mesothelioma. Clin Radiol 2005;60:1237–47 [DOI] [PubMed] [Google Scholar]
- 3.Baron RL. Understanding and optimizing use of contrast material for CT of the liver. AJR Am J Roentgenol 1994;163:323–31 [DOI] [PubMed] [Google Scholar]
- 4.Erturk SM, Ichikawa T, Sou H, Tsukamoto T, Motosugi U, Araki T. Effect of duration of contrast material injection on peak enhancement times and values of the aorta, main portal vein, and liver at dynamic MDCT with the dose of contrast medium tailored to patient weight. Clin Radiol 2008;63:263–71 [DOI] [PubMed] [Google Scholar]
- 5.Garcia PA, Bonaldi VM, Bret PM, Liang L, Reinhold C, Atri M. Effect of rate of contrast medium injection on hepatic enhancement at CT. Radiology 1996;199:185–9 [DOI] [PubMed] [Google Scholar]
- 6.Chambers TP, Baron RL, Lush RM. Hepatic CT enhancement. Part II. Alterations in contrast material volume and rate of injection within the same patients. Radiology 1994;193:518–22 [DOI] [PubMed] [Google Scholar]
- 7.Awai K, Hiraishi K, Hori S. Effect of contrast material injection duration and rate on aortic peak time and peak enhancement at dynamic CT involving injection protocol with dose tailored to patient weight. Radiology 2004;230:142–50 [DOI] [PubMed] [Google Scholar]
- 8.Aquino SL, Webb WR, Gushiken BJ. Pleural exudates and transudates: diagnosis with contrast-enhanced CT. Radiology 1994;192:803–8 [DOI] [PubMed] [Google Scholar]
- 9.Waite RJ, Carbonneau RJ, Balikian JP, Umali CB, Pezzella AT, Nash G. Parietal pleural changes in empyema: appearances at CT. Radiology 1990;175:145–50 [DOI] [PubMed] [Google Scholar]
- 10.Traill ZC, Davies RJ, Gleeson FV. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin Radiol 2001;56:193–6 [DOI] [PubMed] [Google Scholar]
- 11.Kuhlman JE, Singha NK. Complex disease of the pleural space: radiographic and CT evaluation. Radiographics 1997;17:63–79 [DOI] [PubMed] [Google Scholar]
- 12.Rosado-de-Christenson ML, Abbott GF, McAdams HP, Franks TJ, Galvin JR. From the archives of the AFIP: localized fibrous tumor of the pleura. Radiographics 2003;23:759–83 [DOI] [PubMed] [Google Scholar]
- 13.Ferretti GR, Chiles C, Choplin RH, Coulomb M. Localized benign fibrous tumors of the pleura. AJR Am J Roentgenol 1997;169:683–6 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen D, Sang-Joon L, Edward L, Claire V. Rate of thromboembolic events in mesothelioma. Ann Thorac Surg 2008;85:1032–8 [DOI] [PubMed] [Google Scholar]
- 15.Cronin CG, Lohan DG, Keane M, Roche C, Murphy JM. Prevalence and significance of asymptomatic venous thromboembolic disease found on oncologic staging CT. AJR Am J Roentgenol 2007;189:162–70 [DOI] [PubMed] [Google Scholar]
- 16.Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol 2005;184:264–7 [DOI] [PubMed] [Google Scholar]
- 17.Gosselin MV, Rubin GD, Leung AN, Huang J, Riczk NW. Unsuspected pulmonary embolism: prospective detection on routine helical CT scans. Radiology 1998;208:209–15 [DOI] [PubMed] [Google Scholar]
- 18.Wittram C. How I do it: CT pulmonary angiography. AJR Am J Roentgenol 2007;188:1255–61 [DOI] [PubMed] [Google Scholar]
- 19.Adams RF, Gray W, Davies RJ, Gleeson FV. Percutaneous image-guided cutting needle biopsy of the pleura in the diagnosis of malignant mesothelioma. Chest 2001;120:1798–802 [DOI] [PubMed] [Google Scholar]
- 20.Adams RF, Gleeson FV. Percutaneous image-guided cutting needle biopsy of the pleura in the presence of a suspected malignant pleural effusion. Radiology 2001;219:510. [DOI] [PubMed] [Google Scholar]


