Abstract
Objective
The preferential use of intensity-modulated radiotherapy (IMRT) over conventional radiotherapy (CRT) in the treatment of head and neck cancer has raised concerns regarding dose to non-target tissue. The purpose of this study was to compare dose-volume characteristics with the brachial plexus between treatment plans generated by IMRT and CRT using several common treatment scenarios.
Method
The brachial plexus was delineated on radiation treatment planning CT scans from 10 patients undergoing IMRT for locally advanced head and neck cancer using a Radiation Therapy Oncology Group-endorsed atlas. No brachial plexus constraint was used. For each patient, a conventional three-g0ield shrinking-g0ield plan was generated and the dose-volume histogram (DVH) for the brachial plexus was compared with that of the IMRT plan.
Results
The mean irradiated volumes of the brachial plexus using the IMRT vs the CRT plan, respectively, were as follows: V50 (18±5 ml) vs (11±6 ml), p = 0.01; V60 (6±4 ml) vs (3±3 ml), p = 0.02; V66 (3±1 ml) vs (1±1 ml), p = 0.04, V70 (0±1 ml) vs (0±1 ml), p = 0.68. The maximum point dose to the brachial plexus was 68.9 Gy (range 62.3–78.7 Gy) and 66.1 Gy (range 60.2–75.6 Gy) for the IMRT and CRT plans, respectively (p = 0.01).
Conclusion
Dose to the brachial plexus is significantly increased among patients undergoing IMRT compared with CRT for head and neck cancer. Preliminary studies on brachial plexus-sparing IMRT are in progress.
Although intensity-modulated radiotherapy (IMRT) is widely considered the current standard in the radiotherapeutic management of head and neck cancer, investigators are increasingly recognising that this technology is associated with significant beam path doses to non-target structures that previously received little dose using previous, less conformal techniques [1]. Indeed, since the clinical implementation of IMRT at our institution, we have observed a striking number of patients returning for follow-up with symptoms thought to be related to radiation-induced brachial plexopathy. The purpose of this study was to compare dose-volume characteristics to the brachial plexus between treatment plans generated by IMRT and conventional radiotherapy (CRT) using several common head and neck cancer treatment scenarios.
Methods and materials
Patients and simulation
10 patients with locally advanced (stage IV) biopsy-proven head and neck cancer undergoing definitive radiation therapy formed the study population. Table 1 lists patient and disease characteristics. At simulation, the head, neck and shoulders were immobilised in a hyperextended position using a perforated, thermoplastic head mask with the neck supported on a Timo cushion (S-type, MED-TEC, South Plainsfield, NJ) mounted on a carbon fibre board (S-type, MED-TEC, Orange City, IA) that allowed patient positioning to be indexed. The isocentre was placed at the centre of the primary tumour. Axial slices with 3 mm spacing were obtained on a CT simulator (Picker PQ2000, Philips Medical Systems, Andover, MA) and transferred into the Pinnacle treatment planning system (TPS). Two treatment plans were generated for each patient: an IMRT plan with the low neck encompassed in the radiation field, and a CRT plan using initial opposed lateral beams matched to an anterior low neck field.
Table 1. Tumour and target characteristics.
| Pt | Stage | Site | GTV |
| 1 | T4aN2b | R tonsil | R tonsil, R nasopharynx, R level II/III, L level II/III lymph nodes |
| 2 | T1N2c | L base of tongue | L base of tongue, L level II, R level II lymph nodes |
| 3 | T3N2b | R tonsil | R tonsil, R level II/III, L level II lymph nodes |
| 4 | T1N2c | L base of tongue | L base of tongue, R level II lymph nodes |
| 5 | T4aN2b | R tonsil | R tonsil, R arytenoid-epiglottic fold, R level II/III lymph nodes |
| 6 | T1N2b | R base of tongue | R base of tongue, L level II Nodes |
| 7 | T4N2c | R tonsil | R tonsil, R level II, L level II nodes |
| 8 | T4N2 | Nasopharynx | B nasopharynx, clivus, B sphenoids, B ethmoids, R retropharyngeal nodes, R level II/III, L level II nodes |
| 9 | T4N1 | Nasopharynx | B nasopharynx, clivus, B cavernous sinus, L retropharyngeal nodes, R level V nodes |
| 10 | T4N2 | Nasopharynx | B nasopharynx, clivus, R retropharyngeal nodes, R level II/III lymph nodes |
Pt, patient; GTV, gross tumour volume; R, right; L, left; B, bilateral.
IMRT target volume delineation
The gross tumour volume (GTV) was defined as the extent of tumour demonstrated by imaging studies and physical examination including endoscopy. Grossly positive lymph nodes were defined as any lymph nodes greater than 1 cm or those with a necrotic centre. MRI registered with the CT image was used to assist in defining the parapharyngeal and superior extent of tumour for patients with nasopharyngeal carcinoma (Figures 1–4). Positron emission tomography (PET) was not used. Three clinical target volumes (CTVs) were defined: CTV1, which included the GTV with a 5–10 mm margin to account for microscopic spread (in cases with disease in close proximity to the brain stem and optic apparatus, the expansion could be as small as 1 mm); CTV2, which included nodal areas at high risk for recurrence; and CTV3 for low-risk nodal regions. An additional margin of 3–5 mm was added to the CTVs to compensate for the variabilities of treatment set-up and internal organ motion to create a separate planning target volumes (PTVs) corresponding to PTV1, PTV2, and PTV3, respectively.
Figure 1.
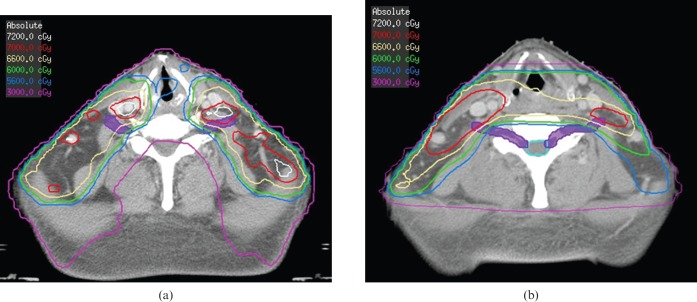
Case illustration: 50-year-old Asian male with T4N2 nasopharyngeal carcinoma who was treated to a total dose of 70 Gy in 35 fractions to gross disease. Axial images at the level of the thyroid notch demonstrating the increased dose to the brachial plexus (contoured in purple) for the intensity-modulated radiotherapy plan (a) compared with the conventional radiotherapy plan (b). Isodose lines are 72 Gy (grey), 70 Gy (red), 66 Gy (khaki), 60 Gy (green), 56 Gy (light blue) and 30 Gy (purple).
Figure 4.
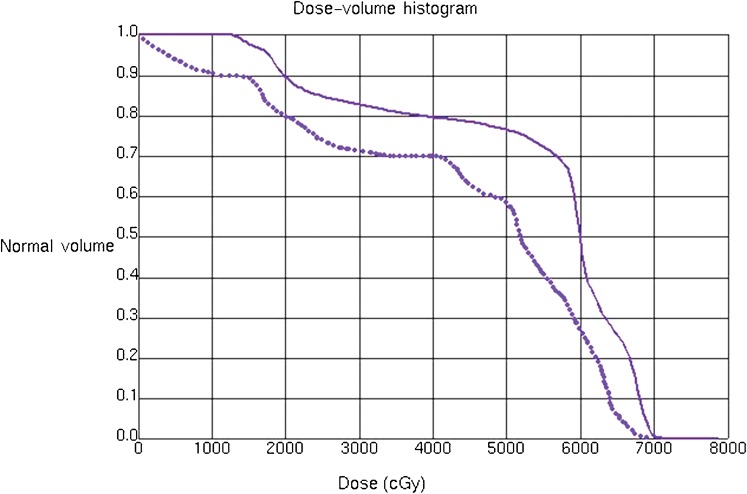
Dose-volume histograms of the brachial plexus from the intensity-modulated radiotherapy plan (solid line) and the conventional radiotherapy plan (dotted line) for the patient from Figure 1.
Figure 2.
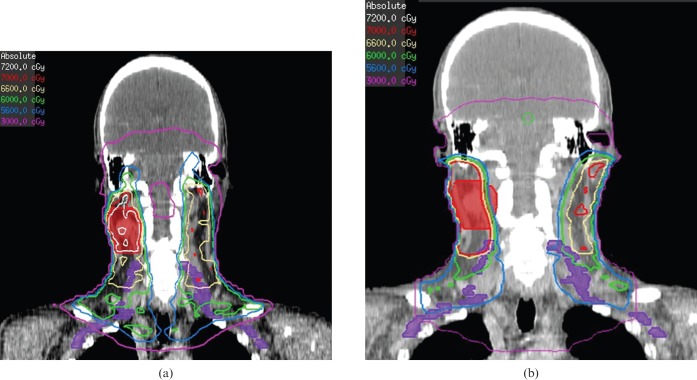
Coronal views demonstrating increased dose to the brachial plexus (contoured in purple) from the intensity-modulated radiotherapy plan (a) compared with the conventional radiotherapy plan (b) for the patient from Figure 1. Isodose lines are 72 Gy (grey), 70 Gy (red), 66 Gy (khaki), 60 Gy (green), 56 Gy (light blue), and 30 Gy (purple).
Figure 3.
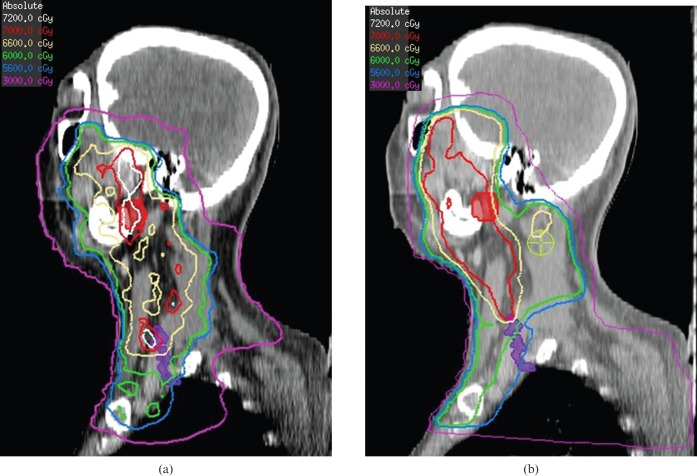
Sagittal views demonstrating increased dose to the brachial plexus (contoured in purple) from the intensity-modulated radiotherapy plan (a) compared with the radiotherapy plan (b) for the patient from Figure 1. Isodose lines are 72 Gy (grey), 70 Gy (red), 66 Gy (khaki), 60 Gy (green), 56 Gy (light blue) and 30 Gy (purple).
IMRT treatment planning and dose specification
IMRT was planned and delivered using a simultaneous integrated boost technique. For patients treated by IMRT, the treatment goal was to deliver a prescribed dose of 70 Gy to at least 95% of the PTV70 and 60–63 Gy to at least 95% of the PTV2 over 33–35 treatments with once-daily fractionation, 5 days per week. Other absolute planning parameters were no more than 20% of PTV1 receives >110% of the prescribed dose; no more than 1% of any PTV1 and any PTV2 receive <93% of the prescribed dose; no more than 1% or 1 cc of the tissue outside the PTVs receives >110% of the dose prescribed to the PTV1. The IMRT plan used nine coplanar beams equally distributed with heterogeneity correction. Beam weightings were based on the optimisation output. Plans were optimised using an inverse planning module that used a conjugate gradient optimisation algorithm, which permitted real-time modification of the optimisation parameters, encouraging user interactivity to minimise the overall optimisation time. The goals were to generate a plan with the prescription isodose lines conformed to the defined PTVs while minimising the dose delivered to the specified avoidance structures. The dose prescription was based on a dose distribution corrected for heterogeneities. The plans were evaluated both quantitatively with dose-volume histogram (DVH) analysis and qualitatively by visually inspecting isodose curves on axial slices. Organs at risk (OARs) and dose constraints used in IMRT planning are outlined in Table 2. Notably, no brachial plexus constraint was used.
Table 2. Dose constraints for intensity-modulated radiotherapy planning.
| Structure | Constraint | Priority |
| Spinal cord | Max. <48 Gy | High |
| Brainstem | Max. <54 Gy | High |
| Temporal lobe | Max. <60 Gy | High |
| Optic chiasm | Max. <50 Gy | High |
| Optic nerve | Max. <54 Gy | High |
| Retina | Max. <45 Gy | High |
| Parotid gland (spared) | Mean <26 Gy or V30 <50% | Intermediate |
| Cochlea/vestibule | Max. <50 Gy | Intermediate |
| Larynx | Mean 40 Gy | Intermediate |
| Oral cavity | Mean 35 Gy | Intermediate |
| Mandible | Max. >70 Gy | Intermediate |
Max., maximum.
CRT treatment planning
For patients treated by CRT, a shrinking field technique was employed with initial fields consisting of opposed lateral fields to treat the primary tumour bed and upper neck lymph nodes. In general, the anterior border included the posterior third of the nasal cavities and the anterior tonsillar pillars; the posterior border was placed behind the spinous process, the superior border was placed at the mid-sphenoid sinus or bottom of the pituitary fossa to encompass the nasopharynx and base of skull; the inferior border was placed just superior to the arytenoids or above the shoulders depending on whether the hypopharynx was part of the target volume. The lower neck nodes were treated with a matched low anterior neck field. An isocentric technique was used to eliminate divergence into treatment fields. The spinal cord was limited to 45–50 Gy. Electrons were used as appropriate to boost areas overlying or posterior to the spinal cord after field reductions. Wedges were used, as appropriate, to maintain dose homogeneity within 10% of the prescribed dose. Total prescribed dose to gross tumour was 70 Gy in 2 Gy fractions. Dose to microscopic disease ranged from 54 to 60 Gy.
Statistical analysis
The brachial plexus was subsequently contoured as a single structure on both radiation treatment plans for each patient using guidelines previously described by our group [2]. IMRT plans were evaluated for acceptability using quality assurance guidelines of dose distribution as set forth in Radiation Therapy Oncology Group (RTOG) protocols 0225 (nasopharynx) and 0522 (oropharynx). Dose-volume statistics to the contoured brachial plexus were compared using a paired Student's t-test. The differences were reported as statistically significant at the p<0.05 level (two-tailed).
Results
IMRT plan evaluation
No major protocol deviations were observed when the IMRT plans were judged using quality assurance guidelines of dose distribution as proposed by RTOG protocols 0225 and 0522. Three separate incidences (two with RTOG 0522 and one with RTOG 0522) of minor deviations involving PTV70 coverage were identified. For two patients with oropharynx cancer, minor protocol deviations resulted because 1.8% and 1.4%, respectively, of the PTV70 received a dose exceeding 77 Gy (110% of the prescription dose). For an additional patient with nasopharyngeal carcinoma, a minor protocol deviation resulted from failure of the 93% isodose surface to entirely encompass the PTV70. Other than for these noted deviations, the generated IMRT plans were deemed acceptable with respect to PTV coverage, DVH analysis and homogeneity.
Brachial plexus evaluation
The mean brachial plexus volume was 32±5 ml (range 25.1–34.7 ml). The absolute volume of brachial plexus receiving greater than 50 Gy, 60 Gy and 66 Gy was significantly greater for the IMRT plan than the CRT plan (p<0.05, for all). The mean irradiated volumes of the brachial plexus using the IMRT vs the CRT plan, respectively, were as follows: V50 (18±5 ml) vs (11±6 ml), p = 0.01; V60 (6±4 ml) vs (3±3 ml), p = 0.02; V66 (3±1 ml) vs (1±1 ml), p = 0.04; V70 (0±1 ml) vs (0±1 ml), p = 0.68. These values represented the following percentages to the whole brachial plexus: V50 (56% vs 34%); V60 (19% vs 9%); V66 (9% vs 3%); V70 (0% vs 0%).
The maximum point dose to the delineated brachial plexus was 68.9 Gy (range 62.3–78.7 Gy) for the IMRT plan compared with 66.1 Gy (range 60.2–75.6 Gy) for the CRT plan (p = 0.01). The corresponding maximum dose to 1% of the brachial plexus volume was 67.2 Gy (range 61.5–74.0 Gy) and 64.3 Gy (range 57.0–71.5 Gy), respectively (p = 0.02). The mean dose to the brachial plexus was 60.5 Gy (range 52.5–67.2 Gy) and 58.5 Gy (range 50.9–66.7 Gy) for the IMRT and CRT plans, respectively (p = 0.49).
Figures 1–4 serve as an example of how the brachial plexus may receive a higher dose using IMRT compared with conventional radiation fields.
Discussion
The results of the present study illustrate how the generation of more conformal dose distributions and steep gradients between target and non-target tissues using IMRT may come at the expense of a higher dose to non-delineated extra-target organs. In this particular case, our study is the first to demonstrate an increased dose to the brachial plexus with the use of IMRT over CRT in the treatment of head and neck cancer.
Although the benefits of IMRT with respect to salivary gland sparing and the creation of eloquent dose distributions around critical structures, such as the central nervous system and optic structures, have been widely heralded, the data reporting on the potential costs of this technology with respect to the phenomenon of “dose dumping” are relatively limited [3–5]. However, data are starting to emerge that suggests rates of mucositis and dysphagia may be higher with IMRT than with traditional CRT, particularly if the oral cavity and swallowing structures are not delineated as avoidance structures during IMRT planning [6]. Given concerns of high doses to the brachial plexus, we previously published a basic atlas to delineate this organ as an avoidance structure for the purpose of IMRT planning [2].
The literature reporting on dose tolerance for the brachial plexus is scarce, and, consequently, the sensitivity of this structure to irradiation is poorly understood. Nevertheless, radiation-induced brachial plexopathy has been described as a potentially debilitating and irreversible condition characterised by upper extremity paraesthesias, weakness and motor dysfunction [7–9]. Although most reports exist in anecdotal form, clinicians generally agree that total dose and fraction size are important. Emami et al [10] suggested TD5/5 (dose resulting in 5% probability of complications at 5 years) values of 62, 61 and 60 Gy; and TD50/5 (dose resulting in 50% probability of complications at 5 years) of 77, 76 and 75 Gy for one-third, two-thirds and whole organ, respectively. More recent protocols from the RTOG recommend a limit of 60–66 Gy. Clearly, additional studies analysing these tolerances are needed.
Notably, ongoing debate exists regarding the optimal IMRT technique for head and neck cancer [11]. Some investigators have proposed a split-g0ield technique matched to a superior IMRT field as a means of minimising dose to critical structures within the low neck (e.g. larynx, oesophagus) [12, 13]. We believe that the use of this technique probably would not have changed our study findings since the brachial plexus emerges anatomically from C5–6, which would lie superiorly to the match line. At our institution, we currently favour an extended field IMRT technique, particularly among patients with low neck lymphadenopathy, given concerns regarding dosimetric uncertainties at the junction line [14]. Regardless of the technique used, current RTOG protocols now require limiting dose to the brachial plexus to between 60 and 66 Gy for patients undergoing IMRT for head and neck cancer.
Conclusions
The results of the present study illustrate how the generation of more conformal dose distributions and steep gradients between target and non-target tissues using IMRT may come at the expense of a higher dose to non-delineated extra-target organs. In particular, this study clearly demonstrates that dose to the brachial plexus is significantly increased among patients undergoing IMRT compared with CRT for head and neck cancer. These findings should serve as a warning to users and suggest that the incidence of radiation-induced brachial plexopathy may increase with the use of this technology. Although the exact clinical repercussions remain uncertain, these findings have prompted us to routinely contour the brachial plexus and use it as a constraint among all patients treated with IMRT. Preliminary studies analyzing the feasibility of brachial plexus sparing IMRT are encouraging and will be reported separately.
Footnotes
This work was presented in part at the 2008 Annual Meeting of the Radiological Society of North America (RSNA), Chicago, IL.
References
- 1.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NC, Garcia J, Kies MS, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2008;72:747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall WH, Guiou M, Lee NY, Dublin A, Narayan S, Vijayakumar S, et al. Development and validation of a standardized method for contouring the brachial plexus: Preliminary dosimetric analysis among patients treated with IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2008;72:1362–7 [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A. Reducing xerostomia by IMRT: What may, and may not, be achieved. J Clin Oncol 2007;25:4863–4 [DOI] [PubMed] [Google Scholar]
- 4.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: Promises and pitfalls. J Clin Oncol 2006;24:2618–23 [DOI] [PubMed] [Google Scholar]
- 5.Lee N, Puri DR, Blanci AI, Chao KS. Intensity-modulated radiation therapy in head and neck cancers: an update. Head Neck 2007;29:387–400 [DOI] [PubMed] [Google Scholar]
- 6.Garden AS, Morrison WH, Wong PF, Tung SS, Rosenthal DI, Dong L, et al. Disease-control rates following intensity-modulated radiation therapy for small primary oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2007;67:438–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajrovic A, Rades D, Fehlauer F, Tribius S, Hoeller U, Rudat U, et al. Is there a life-long risk of brachial plexopathy after radiotherapy of supraclavicular lymph nodes in breast cancer patients? Radiother Oncol 2004;71:297–301 [DOI] [PubMed] [Google Scholar]
- 8.Johansson S, Svensson H, Denekamp J. Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. Int J Radiat Oncol Biol Phys 2002;52:1207–19 [DOI] [PubMed] [Google Scholar]
- 9.Powell S, Cooke J, Parsons C. Radiation-induced brachial plexus injury: Follow-up of two different fractionation schedules. Radiother Oncol 1990;18:213–20 [DOI] [PubMed] [Google Scholar]
- 10.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int. J Radiation Oncology Biol. Phys. 1991;21:109–22 [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Mechalakos J, Puri DR, Hunt M. Choosing an intensity-modulated radiation therapy technique in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2007:1299–309 [DOI] [PubMed] [Google Scholar]
- 12.Amdur RJ, Li JG, Liu C, Mendenhall WM. Unnecessary laryngeal irradiation in the IMRT era. Head Neck 2004;26:257–63 [DOI] [PubMed] [Google Scholar]
- 13.Dabaja B, Salehpour MR, Rosen I, Tung S, Morrison WH, Ang KK, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-g0ield and whole-g0ield techniques. Int J Radiat Oncol Biol Phys 2005;63:1000–5 [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Xia P, Fischbein NJ, Akazawa C, Quivey JM, et al. Intensity-modulated radiation therapy for head-and-neck cancer: The UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys 2003;57:49–60 [DOI] [PubMed] [Google Scholar]