Abstract
Two functionally distinct sets of meristematic cells exist within root tips of pea (Pisum sativum): the root apical meristem, which gives rise to the body of the root; and the root cap meristem, which gives rise to cells that differentiate progressively through the cap and separate ultimately from its periphery as border cells. When a specific number of border cells has accumulated on the root cap periphery, mitosis within the root cap meristem, but not the apical meristem, is suppressed. When border cells are removed by immersion of the root tip in water, a transient induction of mitosis in the root cap meristem can be detected starting within 5 min. A corresponding switch in gene expression throughout the root cap occurs in parallel with the increase in mitosis, and new border cells begin to separate from the root cap periphery within 1 h. The induction of renewed border cell production is inhibited by incubating root tips in extracellular material released from border cells. The results are consistent with the hypothesis that operation of the root cap meristem and consequent turnover of the root cap is self-regulated by a signal from border cells.
The root cap has been a favored model system for cell biological studies because of its defined structure and easy accessibility to experimental manipulation (Loening, 1961; Brown, 1963; López-Sáez et al., 1975; Heyes, 1977; Steeves and Sussex, 1989). Meristematic cells within the cap give rise by mitosis to new cells that differentiate progressively through a series of developmental stages (Esau, 1977; Luxová and Ciamporová, 1992). Each stage is associated with well-described changes in cellular morphology and ultrastructure that reflect transient functional specialization for traits such as starch synthesis and degradation, gravity sensing, and polysaccharide secretion (Juniper, 1972; Barlow, 1975, 1984; Moore and McClelen, 1983). Eventually, the cells at the periphery of the cap separate into the external environment. This dynamic process has been assumed to be a constitutive process that, like root growth, is continuous as long as the root is healthy and has access to water and nutrients (Clowes, 1976; Barlow, 1978). The separation of cells from the periphery of the cap was thus considered to be an inevitable by-product of the continuous turnover of the cap (Clowes, 1972, 1994; Barlow, 1973). Consistent with such a model was the assumption that such so-called “sloughed root cap cells” are waste products that are programmed to die and in fact begin to degenerate even before separation from the root (Haberlandt, 1914; Rougier, 1981; Rost et al., 1988). Although the time required for a cell to progress from a newly synthesized product of the meristem through the cap proper and into the external milieu has been controversial, radiolabeling studies have demonstrated conclusively that such turnover does occur (Barlow, 1973; Clowes, 1980). Little is known about the molecular mechanisms underlying root cap development, including the structural genes that give specific cell types their unique properties, the regulatory genes and receptors that control the process, or the signals that trigger it (Jacobs, 1994).
Studies in our laboratory have confirmed an observation first documented in 1919: the sloughed root cap cells, which separate in large numbers from the caps of species such as cereals and legumes, are not a degenerate waste product (Knudson, 1919). Instead, they represent the ultimate step in root cap development. Upon separation from the cap, these unusual cells develop into a uniquely differentiated and little-understood part of the root system whose function is unknown (for review, see Hawes and Brigham, 1992; Hawes et al., 1998). The ability of these cells to influence gene expression and behavior of soil-borne pathogens and symbionts is the basis for the hypothesis that they protect plant health by affecting the ecology of the rhizosphere surrounding vulnerable young root tips (for review, see Hawes and Brigham, 1992; Hawes et al., 1998). We have termed the cells “root border cells” to emphasize that, by definition, they are not a part of the root cap, and to highlight their specialized position at the root-soil interface. Border cells are more metabolically active than their progenitor cells in the root cap, and they express a distinct set of mRNAs and proteins (Brigham et al., 1995). Many of the newly synthesized proteins are exported rapidly into the external environment, as might be expected for cells that function to modulate the properties of that environment.
Studies describing the separation of border cells during development have yielded surprising results that are not consistent with a model in which root cap turnover is continuous during the life of the root (Barlow, 1973; Clowes, 1980, 1994). In the absence of free water, border cells do not separate but remain appressed to the root periphery, so it is possible to determine the number of cells that accumulate over time by the simple procedure of washing them into water and counting them (Hawes, 1990). During germination, when roots of pea (Pisum sativum) first emerge, border cells can be collected by the time the root is 5 mm in length (Hawes and Lin, 1990). Cell number then increases linearly with increasing root length, as would be expected if meristematic activity were continuous. However, when the root reaches 24 mm in length, the cell number stops increasing. A species-specific set point is reached, and unless the existing cells are removed, no new border cells are shed, even though linear root growth continues. Therefore, the same set of several thousand cells remains in a sheath surrounding the tip as the root elongates. Two explanations can account for such results. First, meristematic activity leading to root cap turnover could be continuous, according to a long-standing model of root cap development (Clowes, 1994), but border cell separation is not. In that case, the number of cells within the cap would increase continuously, but unknown mechanisms would prevent their separation from the cap. Alternatively, the meristematic activity of cells that give rise to the root cap may not be continuous, but rather may be turned off as border cell development proceeds.
The purpose of this study was to distinguish between these two possibilities by directly testing the hypothesis that mitosis in the root cap meristem ceases when border cell separation stops, and that mitosis is induced when border cell separation is induced.
MATERIALS AND METHODS
Plant Material
Seeds of pea (Pisum sativum L. cv Little Marvel; Royal Seed, Kansas City, MO) were surface-sterilized by immersion in 95% (v/v) ethanol for 10 min, then 5.25% sodium hypochlorite (full-strength commercial bleach) for 30 min. During five rinses in sterile distilled water, seeds contaminated with bacteria or fungi (those that floated) were discarded. The remaining seeds were allowed to imbibe in sterile water for 6 h, after which they were placed on 1.2% water agar overlaid with sterile germination paper (Anchor Paper, Hudson, WI) in plastic Petri dishes and incubated in the dark at 24°C. Radicles emerged by 24 h and roots reached a length of 24 mm by 48 h. All experiments described in this paper were performed on 24-mm roots.
Root-Tip Sectioning and Staining for Mitotic Figures
Border cells were removed from the root cap by immersion in water and gently agitated with a Pasteur pipette (Brigham et al., 1995). Border cells were not removed from control roots. At 5, 15, and 30 min and 1, 2, 3, 4, 6, 8, 10, 12, 16, 18, 20, and 24 h, root tips were excised 1 cm from the apex into tissue fixative (HistoChoice MB, Amresco, Solon, OH), and dehydrated in an ethanol series and then a butanol series. Sections were embedded in Paraplast (Sigma), sectioned in 10-μm sections on a rotary microtome (model 820, American Optical, Southbridge, MA), dried on slides, and stained with 2% aqueous Safranin O and 0.5% Fast Green in 95% ethanol. Sections through the transverse meristem (Popham, 1955) were identified microscopically based on morphology. For each time point in each replicate experiment, at least five roots were analyzed. At least two and as many as six replicate experiments were performed for each time point. Three to five sections per root tip were identified as containing the transverse meristematic region. A mitotic event was scored if the nucleus was clearly in metaphase, anaphase, or telophase. Ambiguous figures were not scored. Total figures identified per time point were divided by the number of sections analyzed for that time point to yield the number of mitotic events per section.
Preparation of Probes for in Situ Hybridization and Gel-Blot Analysis
The plasmids carrying cDNAs corresponding to the genes for starch-synthase enzyme II (GBSSII; Dry et al., 1992), starch-branching enzyme II (SBEII; Dry et al., 1992), ubiquitin-conjugating enzyme (PsUBC4; Woo et al., 1994), H1 histone (PsH1-41; Woo et al., 1995), and pectin methylesterase (rcpme1; Zhu et al., 1997) were linearized for SP6, T3, or T7 polymerase-directed RNA synthesis using the Maxiscript kit (Ambion, Austin, TX), and sense and antisense strands were synthesized for each. RNA was labeled by incorporating digoxigenin-conjugated UTP (Boehringer Mannheim). cDNAs for pea starch-synthase enzyme and starch-branching enzyme were gifts from Cathie Martin (John Innes Centre, Norwich, UK).
In Situ Hybridization
Border cells were removed from root tips by washing in water and then subjected to in situ northern-blot analysis using whole-mount preparations (Hemmati-Brivanlou et al., 1990). Root tips were excised 1 cm from the apex 15 min after border cell removal and placed in 3% glutaraldehyde in 0.1 m phosphate buffer at pH 7.0 for 4 h with nutation. (A 0 time point was attempted, but because of the aqueous nature of the fixative, genes were induced within the time of fixation. Pectin methylesterase was shown to be induced within 5 min.) All procedures were done in 1.7-mL microfuge tubes with 10 tips per tube. If tips were not used immediately, they were placed in a series of ethanol concentrations (from 25%, 50%, 75%, to 100%) and stored at −20°C. Just before hybridization, the tips were rehydrated by placing in 75% ethanol and 25% water for 5 min, then 50% ethanol and 50% water for 5 min, then 25% ethanol and 75% phosphate buffer for 5 min. The tips were then rinsed twice for 5 min each in 100% phosphate buffer. Tips were rinsed twice for 5 min each in 0.1 m triethanolamine (Sigma T-1502), and 2.5 μL of acetic anhydride was added to the last rinse. An additional 2.5 μL of acetic anhydride was added and the tips were incubated for another 5 min. Tips were washed twice in phosphate buffer and then placed in prehybridization buffer (50% formamide, 5× SSC, 1 mg/mL RNA, 1× Denhardt's solution, 0.1% Tween 20, 5 mm EDTA, pH 8.0) for at least 6 h at 55°C. Both sense and antisense probes (100–500 ng probe/mL) were used for each gene analyzed. Tips were hybridized to both sense and antisense probes overnight at 55°C. Tips were washed in decreasing concentrations of prehybridization buffer diluted with increasing concentrations of 2× SSC. The final rinse was in 0.2× SSC at 55°C. After two rinses in maleate buffer (100 mm sodium maleate, pH 7.5, 150 mm NaCl), tips were incubated in northern block (5% blocking reagent from Boehringer Mannheim in maleate buffer) at 55°C for 60 min. Northern block was replaced with fresh northern block with 1:2000 dilution of anti-digoxigenin-alkaline phosphatase (Boehringer Mannheim) at 4°C overnight. Tips were rinsed in two changes of maleate buffer for 30 min each, incubated in buffer no. 3 (100 mm Tris-Cl, pH 9.5, 100 mm NaCl, 50 mm MgCl2) and 5 mm levamisole (Sigma) for 5 min, and then placed in color solution (buffer no. 3, 5 mm levamisole, 4.5 μL/mL nitroblue tetrazolium; Boehringer Mannheim) and 3.5 μL/mL X-phosphate solution (Boehringer Mannheim). Tips were placed in the dark and color development was monitored from 1 to 24 h. Tips were then split laterally, mounted on glass slides in water, and photographed.
RNA Isolation and Gel-Blot Analysis
Total RNA was extracted from 1-mm sections of root caps at various times after removal of border cells using an SDS-phenol method with lithium chloride precipitation. For RNA gel-blot analysis, 20 μg of total RNA was separated on a 1% agarose-formaldehyde gel and transferred onto N+ nylon membrane (Amersham). Blots were hybridized with digoxigenin-labeled RNA probes as described above according to the published protocol (Boehringer Mannheim). Visualization was by the colorimetric method using nitroblue tetrazolium and X-phosphate (Boehringer Mannheim). Quantitation of mRNA level was carried out using the public domain NIH-Image program (developed at the National Institutes of Health, Springfield, VA, part no. PB95-500195GEI).
In Situ Induction of Renewed Border Cell Production
Border cells from 24-mm roots were either given no treatment or were dipped in water for 1 s, and both groups were then incubated on filter paper for 24 h. Border cells were then collected as described above and cell number was determined by direct counts. Values represent the means from at least three experiments, with at least five replicate roots in each experiment. se values were less than 15% of the mean.
Inhibition of Border Cell Production by Extracellular Products from Border Cells
Root tips were placed in 3 mL of distilled water for 2 min and gently pipetted to remove border cells as described above. The eluate and border cells from 100 tips was centrifuged (600g) to sediment the border cells. The supernatant was concentrated by vacuum evaporation at room temperature to an equivalent of four tips per microliter. This material was designated as root exudate. The pelleted border cells were washed, resuspended in 1 mL of distilled water, incubated at room temperature for 24 h, and again separated from the extracellular material by centrifugation. The supernatant was concentrated by vacuum evaporation to an equivalent of four tips per microliter. This material was designated the border cell exudate. To test the effect of root exudate or border cell exudate, each root tip was immersed in a total volume of 10 μL of root exudate or border cell exudate in a microcentrifuge tube and incubated overnight. The total number of border cells produced overnight was obtained by direct microscopic counts of the cells present on root tips or in the surrounding medium. Values represent the means from at least three independent experiments, with at least five replicate seedlings per test. se values were less than 15% of the mean.
RESULTS
Cell Division Is Not Active in Root Caps with a Full Set of Border Cells, but Is Induced When Border Cells Are Removed
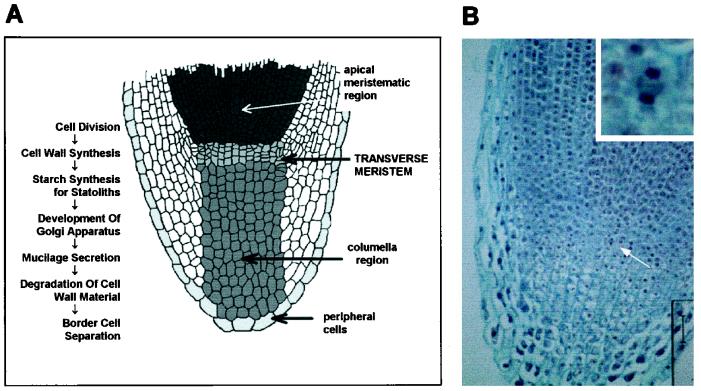
Under the experimental conditions used in this study, the number of border cells on roots of pea seedlings increases until the root is approximately 24 mm long, at which point a mean of approximately 4500 cells are present. No further increase in cell number occurs, even though linear root growth continues. When the existing border cells are washed from the root cap, new border cells begin to separate from the cap periphery almost immediately, and within 24 h a new generation of approximately 4500 cells is again present and new border cell production ceases. This phenomenon was exploited experimentally to test the hypothesis that when border cell separation stops, mitosis leading to root cap development is no longer active. Sections containing the transverse meristem of the root cap (Fig. 1A) were used to count mitotic events, because cell lineages can be traced from this region directly to cells within the columella region of the root cap (Popham, 1955). Therefore, this region provides the basis for an assay in which mitosis leading to root cap development can be distinguished reliably from mitotic events generating other cell types (Luxová and Murín, 1973). In a medial transverse section, the upper boundary of the transverse meristem is morphologically distinct from the portion of the root apex that gives rise to root development (Fig. 1B). Cells across the transverse meristem that were visibly undergoing cell division were counted as positive (Fig. 1B, inset).
Figure 1.
Root cap structure and development. A, Dynamics of root cap development (from Barlow, 1975). As cell division occurs in the meristem of the root cap, cell tiers are displaced toward the periphery of the cap. In the columella region, cell tiers exhibit distinct morphologies reflecting their specialized physiological functions. As each cell tier is displaced, previous functions cease and new functions are initiated within the progressively differentiating cells. The time required for the entire cap to be replaced by a new set of cells ranges from 24 h to 7 d, depending on growth conditions (Hawes and Lin, 1990). (Diagram adapted from de Janczewski, 1874.) B, Medial transverse section of pea root tip. Dividing cells are visible in the area of the transverse meristem (arrow and inset). Bar = 50 μm.
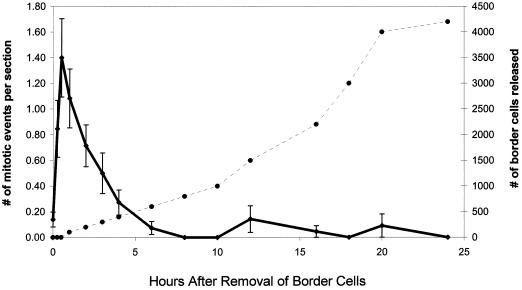
The results were consistent with the hypothesis that root cap meristematic activity stops when border cell separation stops, and that it begins again when separation is reinitiated. In roots with a full complement of border cells (0 h), mitotic activity in the transverse meristem was rarely observed (Fig. 2). This was in contrast to cells within the apical meristem of the same root tips, which, as others have reported, exhibited significant levels of mitotic activity at all times (Jensen and Kavaljian, 1958). In sections of roots whose border cells had been removed, renewed mitosis in the transverse meristem was detected almost immediately. Within 5 min a significant increase in the number of mitotic events was detected, and the number increased linearly for 30 min (Fig. 2). After 1 h the number of mitotic events began to decline, and by 6 h after border cell removal, mitosis had returned to preinduction background levels and remained low for the duration of the experiment. This pattern of a rapid, transient, meristem-specific induction of mitosis in response to border cell removal occurred regardless of the time of day the experiment was initiated.
Figure 2.
Correlation of mitosis in the root cap with border cell separation. Border cell numbers were determined by direct counts, and the number of mitotic events was determined by direct microscopic observation of dividing cells within the transverse meristem. Error bars are 95% confidence intervals (se). Solid line, Number of mitotic events; dashed line, number of border cells released.
Specific Genes, Expressed in Three Discrete Regions of the Root Cap, Show Differential Responses to Border Cell Removal
Previous models have suggested that root cap development is a coordinately regulated process whose terminal steps are linked to its initiation in mitosis (Barlow, 1975). The ability to induce root cap development experimentally by manipulating border cells provides an opportunity to test predictions of such models. If correct, the observed changes in gene expression would not be limited to those involved directly in cell division, but would include genes throughout the cap. Transcripts for genes associated with physiological functions known to occur within specific regions of the cap would be predicted to be localized within those regions. Based on the known distribution pattern of specific physiological processes within the root cap, we obtained probes for genes likely to play a role in such functions. The spatial and temporal distribution of their messages was analyzed using whole-mount in situ hybridization and RNA gel-blot analysis, respectively. Processes predicted to be localized within the root cap in three different regions were chosen. A gene encoding H1 histone (PsH1-41) (Woo et al., 1995) was selected as a marker for dividing cells within the meristem; genes encoding starch-synthase enzyme (GBSSII) and starch-branching enzyme (SBEII; Dry et al., 1992) were chosen as markers for gravity-sensing cells within the columella; and rcpme1 (Stephenson and Hawes, 1994), a gene encoding a cell wall-degrading enzyme, was selected as a marker for cell separation at the root cap periphery. Experiments focused on the changes occurring in the expression of these genes within 1 h after border cell removal, since this time frame includes the period of maximum mitotic activity. If mitosis is not regulated coordinately with other processes within the cap, then activation of gene expression would be expected to be confined to the region of meristematic activity.
Spatial Distribution of Genes Expressed in the Root Cap
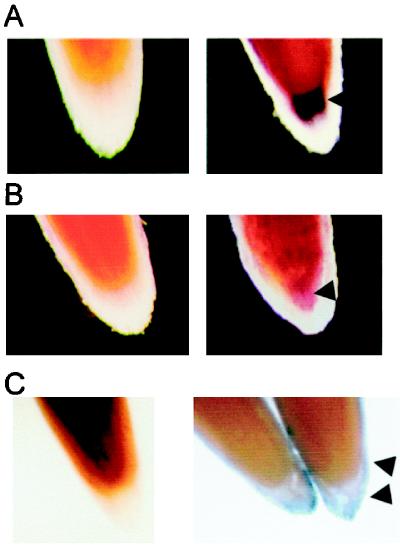
Expression of the selected marker genes was localized within the regions predicted based on previous cell biological assays (Barlow, 1975), as shown in Figure 3. PsH1-41 was localized in the meristematic region of the cap (Fig. 3A), expression of a gene homologous with GBSSII was localized within the central columella (Fig. 3B), and rcpme1 was expressed in cells at the periphery of the cap (Fig. 3C).
Figure 3.
Localization of expression of specific genes within the root cap. Whole-mount in situ hybridization was used to visualize expression of marker genes for cells within the meristematic region (A), the columella (B), and the root cap periphery (C). Roots were bisected longitudinally to display the interior surface of the root. Probes from genes encoding H1 histone (A), starch synthase (B), and pectin methylesterase (C) were used. Treatments included sense (left) and antisense (right) mRNA probes. The boundaries of positive reactions are highlighted with triangles.
Temporal Changes in Gene Expression
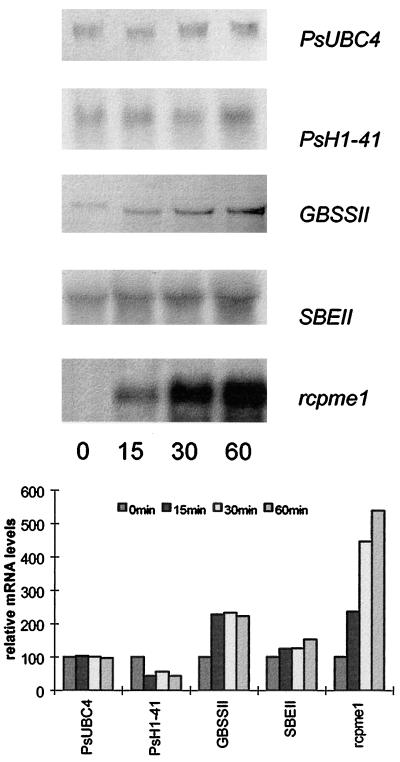
Total RNA from uninduced root caps and root caps from which the border cells had been removed 15, 30, and 60 min previously was subjected to RNA-blot analysis using probes from PsH1-41, GBSSII, SBEII, and rcpme1 (Fig. 4). PsUBC4, a gene encoding a ubiquitin-conjugating enzyme that is constitutively expressed in all tissues tested, was used as a control (Woo et al., 1994). The genes represented three categories identified in differential display: those that showed no change over time, those that changed quantitatively, and those that were induced to a very high abundance. PsH1-41 steady-state levels of transcript decreased in 15 min and remained low during the 1-h period (Fig. 4). In contrast, expression of mRNAs with homology to GBSSII and SBEII increased within 15 min and remained high. rcpme1 mRNA was undetectable in uninduced root tips, but its abundance increased progressively during the course of 1 h.
Figure 4.
Changes in specific gene expression in the root cap after removal of border cells. Northern-blot analysis was used to compare levels of expression of genes with homology to H1 histone, starch-synthesizing and starch-branching enzymes, and pectin methylesterase within the root cap (top). Samples were tested before (time 0) and after (15, 30, or 60 min) induction of root-cap development by removing border cells (bottom). Ubiquitin-conjugating enzyme (PsUBC4) was used as a control for loading equal amounts of RNA.
An Extracellular Water-Soluble Product Inhibits Root Cap Mitosis and Border Cell Production
When a full set of border cells accumulates on root caps of pea, mitosis and border cell production virtually ceases. When the existing border cells are removed by immersing the tip in water and gently washing, mitosis recommences and a new set of border cells is produced. One explanation for these observations is that the physical manipulation of the root during border cell removal activates pathways leading to renewed border cell development. Thigmotropic responses by the root tip can signal developmental changes (Braam et al., 1996). The physical manipulation involved in border cell removal was approximated by tapping the tip gently with a forceps 10 times, without removing border cells. This treatment did not result in a change in border cell number. The mean number of border cells present before touching was 5081 ± 596; 24 h after touching, mean border cell number was 5178 ± 197. The similarity in these values suggests that touch alone does not induce renewed border cell production.
An alternative hypothesis to account for the results is that the presence of border cells or associated extracellular material (root exudate) inhibits border cell development. If so, then removing these cells not only by washing but by any means would be predicted to result in renewed border cell production. When border cells and their associated root exudates were removed completely by wiping the tip manually with a tissue, without immersion in water, renewed mitosis and border cell production was evident by the presence on the roots of a new set of 3864 ± 250 border cells after 16 h. A factor in the root exudate that influences border cell development could explain this result. Such a factor may accumulate within the external mucilage that accompanies border cell separation, until it reaches a level that is inhibitory to further development. Removal of this factor by washing or wiping off border cells then acts as a trigger to induce renewed mitosis, leading to renewed border cell separation. If so, then any treatment that dilutes its concentration would be expected to interfere with its effects.
The unique water-holding properties of the extracellular matrix were exploited to develop an assay to test this hypothesis. Root cap mucilage quickly absorbs up to 1000 times its weight in water (Guinel and McCully, 1987). In the absence of free water, however, the material remains dry, causing its encased border cells to remain appressed to the root tip (Hawes and Brigham, 1992). When water is added, border cells disperse as the mucilage hydrates and swells. However, up to 60 s of immersion in water is required before this process leads to border cell dispersal into suspension. Thus, root tips can be immersed briefly in liquid to allow dilution of the extracellular mucilage without dislodging any existing border cells. When so treated, the normal dynamics of border cell development changed dramatically. Instead of remaining suppressed as long as the species-specific set number of border cells was present at the root periphery, border cell development resumed. When roots with a full set of border cells were dipped for 1 s in water, they synthesized a new set in addition to the existing cells; the mean number of new border cells present 24 h after dipping was 4262 (Table I). This result suggests that a transitory dilution of the extracellular material by a 1-s immersion in water is sufficient to overcome the normal inhibition of border cell development that occurs when a full set of border cells is present on root tips.
Table I.
Stimulation of border cell production by in situ dilution of root-cap mucilage
| Time | Control | Dipped |
|---|---|---|
| no. | ||
| 0 h | 4192 | 4192 |
| 24 h | 4236 | 8454 |
| Percent stimulation | 0 | 100 |
These results are consistent with the hypothesis that a factor inhibitory to border cell development accumulates to a threshold level on the surface of root tips as border cells accumulate on the root tip. If correct, then adding such root exudates back to root tips would be predicted to prevent the activation of mitosis and border cell production that normally occurs when existing border cells are removed. This prediction was tested by removing border cells from 24-mm root tips and then incubating the induced tips overnight without agitation in water or in root exudate. Control tips incubated in water alone made 3513 new border cells within 24 h (Table II). This number was about 84% of that of roots maintained on filter paper. This slight reduction presumably was attributable to deleterious effects of incubating tips in water without aeration. The number produced by root tips incubated in root exudate was reduced by nearly 60% compared with the water control. The results suggest that an inhibitory factor is a component of the water-soluble extracellular material that is washed from roots when border cells are removed. Such a factor could be derived by secretion from the root or by secretion from border cells. To test the possibility that border cells are a source of the inhibitory factor, border cells were washed to remove all extracellular material and then incubated for 24 h in water before the supernatant (border cell exudate) was collected by centrifugation. On induced roots incubated overnight in border cell exudate, renewed border cell production was inhibited by 80%. Border cells apparently secrete a product that we call factor B, which somehow acts to inhibit root cap turnover, leading to border cell separation from the cap periphery.
Table II.
Inhibition of border cell production by root cell exudates
| Time | Water | Root Exudate | Border Cell Exudate |
|---|---|---|---|
| no. | |||
| 0 h | 0 | 0 | 0 |
| 24 h | 3507 | 1493 | 750 |
| Percent inhibition | 0 | 57 | 79 |
DISCUSSION
The results of this study are consistent with the following model. The process of root cap development is “end-product” regulated, and the end product of root cap development is border cells. Meristematic cells within the root cap but not those within the root apex may be competent to receive a signal(s) from border cells that regulates mitosis. How one group of meristematic cells can be impervious to a signal that activates mitosis in cells only a few layers away is not known. However, regulation of border cell production independent of root growth undoubtedly offers substantial benefits to the plant. Continuous synthesis of thousands of living cells to be shed externally would be a costly process, and might be prohibitively energy draining under natural conditions. If border cells function in a specialized capacity to regulate rhizosphere ecology at the root tip, as proposed, continuous production is unnecessary (Hawes and Brigham, 1992; Hawes et al., 1998). In the absence of free water there is little threat from soil-borne microbial populations because in the absence of free water, microorganisms are inactive (Curl and Truelove, 1986).
Although root cap turnover always has been assumed to proceed constantly, the dynamics of root cap turnover in diverse soil environments are unknown and the results of our study suggest that the plant regulates this process. Nevertheless, continuous turnover of the root cap has been demonstrated by growing maize roots under conditions in which border cells continuously disperse into suspension (Clowes, 1976). This observation is the basis for a long-standing controversy regarding how rapidly root cap turnover proceeds (Hawes and Lin, 1990). When roots are maintained in hydroponic conditions with agitation, mitosis apparently is continuous and root cap turnover can be completed within 1 d (Clowes, 1971, 1976). In contrast, the rate of root cap turnover slows to 7 d when roots are grown without exposure to free water, such as in damp moss (Barlow, 1978). In the absence of free water, border cell dispersal away from the root does not happen readily. Thus, when roots are maintained on damp filter paper, as in this study, removing the cells manually requires repeated direct wiping with a tissue. Similarly, border cells remain in a sheath around the root periphery when plants are grown in sand, vermiculite, or clay, and border cells disperse from the root only when free water is introduced (Hawes et al., 1998; M.C. Hawes, unpublished results).
The chemical nature of factor B, the extracellular signal that appears to suppress root cap turnover, is not known. Border cells synthesize and export a diverse array of molecules, ranging from small proteins, amino acids, and sugars, to phenolic and flavonoid antibiotics (Hawes and Brigham, 1992; Brigham et al., 1995; Zhu et al., 1997; Hawes et al., 1998), but we are unaware of any known chemicals with a comparable capacity to suppress mitosis in any organism. Molecules such as colchicine and caffeine can cause arrest of the cell cycle, as factor B apparently does, but their effects are relatively slow and nonspecific, and both can cause cellular toxicity at the concentrations required to inhibit mitosis. Levels of factor B sufficient to inhibit root cap mitosis by virtually 100% have no apparent deleterious effects on cellular function, and altering its concentration constitutes a signal that meristematic cells respond to almost instantaneously. The rapid transmission of this signal across the entire cap may be explained by a model that proposes that hydrated root cap mucilage acts as a high-speed cellular “bypass” conduit throughout the root tip (Miller and Moore, 1990). This contiguous apoplastic pathway facilitates rapid movement of molecules from the periphery of the root into its interior (Enstone and Peterson, 1992; van der Bayliss et al., 1996). This may explain the results of older studies that revealed that crowding roots in hydroponic culture slows mitosis and root cap turnover; continuous release of border cells within small vessels presumably would allow an eventual increase of factor B to inhibitory levels (Clowes, 1980). Irrespective of how factor B functions, the cells that respond to its removal by dividing within 5 to 15 min must have in place all of the necessary machinery to complete a cell cycle (Van't Hof, 1985; Jacobs, 1994). Such a rapid activation of cell division would not be likely to occur otherwise.
The discovery that mitosis is induced in parallel with a global switch in gene expression throughout the cap validates a long-standing model of root cap development (Barlow, 1975, 1984). This model proposed that root cap differentiation is a dynamic, coordinately regulated process that is initiated in the meristem and progresses continuously to completion by cell separation at the periphery. A surprising conclusion from this study is that cells within the cap remain for some time in each specialized fate, depending on the state of border cells on the surface. Once the accumulation of border cells inhibits cap turnover, the entire cap must remain in a steady-state condition in which, for example, starch-synthesizing cells remain as starch-synthesizing cells rather than progressing into secretory cells. Genes needed for cell function would be expressed, but genes needed for cell development would not be active because development would be temporarily static. Upon renewal of cap turnover, the genes needed for development are induced as well. This model explains the large changes in root cap gene expression observed using differential display assays (data not shown) or specific marker genes such as pectin methylesterase and starch synthase. Induction of cap turnover synchronously by manipulating border cells provides a convenient method to identify new genes needed for specific cellular processes (Woo et al., 1994, 1995; Woo and Hawes, 1997). Also consistent with that prediction is that genes expressed within meristematic cells of the cap have sequences that suggest a possible role in mitosis or cell wall biosynthesis (Woo and Hawes, 1997).
Among the most compelling questions in plant biology are those relating to signaling and response to environmental stimuli to produce appropriate adaptive responses. The results presented here demonstrate that one unique adaptive mechanism allows plants to not merely respond to signals from the external environment, but to change it dramatically by producing thousands of living cells programmed to separate from the root cap. The root tip is known to be sensitive to external stimuli such as light, pH, moisture, and electrical and chemical gradients (for review, see Curl and Truelove, 1986). Indeed, the root tip's response to environmental cues by directed growth plays a role in all aspects of plant development by virtue of its crucial role in establishing a stable underground architecture with access to nutrients and water (Aiken and Smucker, 1996). Less appreciated is the plant's potential to modify such environments by the regulated delivery of thousands of border cells. The results of this study provide a molecular framework from which to begin dissecting the interplay between signals from the rhizosphere and gene expression in the root cap, and to determine its effect on metabolic function within the whole plant.
ACKNOWLEDGMENTS
We thank Dr. Cathie Martin for providing the starch-synthase clone GBSSII and the starch-branching clone SBEII; Dr. Elizabeth A. Pierson for statistical analysis of the mitosis data; and Drs. Leland S. Pierson III and Elizabeth Vierling for helpful criticism of the paper.
Footnotes
This work was supported by grants from the U.S. Department of Education, the U.S. Department of Agriculture, Pioneer Seed, and the Storkan-Hanes Foundation.
LITERATURE CITED
- Aiken RM, Smucker AJM. Root system regulation of whole plant growth. Annu Rev Phytopathol. 1996;34:325–346. doi: 10.1146/annurev.phyto.34.1.325. [DOI] [PubMed] [Google Scholar]
- Barlow PW (1973) Mitotic cycles in root meristems. In M Balls, FS Billett, eds, The Cell Cycle in Development and Differentiation. British Society for Developmental Biology, Cambridge, UK, pp 133–165
- Barlow PW. The root cap. In: Torrey JG, Clarkson DT, editors. The Development and Function of Roots. London: Academic Press; 1975. pp. 21–54. [Google Scholar]
- Barlow PW. Cell displacement through the columella of the root cap of Zea mays L. Ann Bot. 1978;42:783–790. [Google Scholar]
- Barlow PW. Positional controls in root development. In: Barlow PW, Carr DJ, editors. Positional Controls in Plant Development. Cambridge, UK: Cambridge University Press; 1984. pp. 281–318. [Google Scholar]
- Braam J, Sistrunk ML, Polisensky DH, Xu W, Purugganan MM, Antosiewicz DM, Campbell P, Johnson KA. Life in a changing world: TCH gene regulation of expression and responses to environmental signals. Physiol Plant. 1996;98:909–916. [PubMed] [Google Scholar]
- Brigham LA, Woo H-H, Nicoll SM, Hawes MC. Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiol. 1995;109:457–463. doi: 10.1104/pp.109.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. Cellular differentiation in the root. Symp Soc Exp Biol. 1963;17:1–17. [PubMed] [Google Scholar]
- Clowes FAL. The proportion of cells that divide in root meristems of Zea mays L. Ann Bot. 1971;35:249–261. [Google Scholar]
- Clowes FAL (1972) The control of cell proliferation within root meristems. In MW Miller, CC Kuehnert, eds, The Dynamics of Meristem Cell Populations: Advances in Experimental Medicine and Biology, Vol 18. Plenum Press, New York, pp 133–147
- Clowes FAL. Cell production by root caps. New Phytol. 1976;77:399–407. [Google Scholar]
- Clowes FAL. Mitosis in the root cap of Zea mays. New Phytol. 1980;85:79–87. [Google Scholar]
- Clowes FAL. Origin of the epidermis in root meristems. New Phytol. 1994;127:335–347. doi: 10.1111/j.1469-8137.1994.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Curl EA, Truelove B. The Rhizosphere. Berlin: Springer-Verlag; 1986. [Google Scholar]
- de Janczewski E. Recherches sur l'accroissement terminal des racines dans les Phanerogames. Ann Sci Bot Nat Ser V. 1874;20:162–201. [Google Scholar]
- Dry I, Smith A, Edwards A, Bhattacharyya M, Dunn P, Martin C. Characterization of cDNAs encoding two isoforms of granule-bound starch synthase which show differential expression in developing storage organs of pea and potato. Plant J. 1992;2:193–202. [PubMed] [Google Scholar]
- Enstone DE, Peterson CA. The apoplastic permeability of root apices. Can J Bot. 1992;70:1502–1512. [Google Scholar]
- Esau K. Anatomy of Seed Plants. London: John Wiley & Sons; 1977. [Google Scholar]
- Guinel FC, McCully ME. Some water-related physical properties of maize root cap mucilage. Plant Cell Environ. 1987;9:565–566. [Google Scholar]
- Haberlandt G, Drummond M. Physiological Plant Anatomy. London: MacMillan; 1914. [Google Scholar]
- Hawes MC. Living plant cells released from the root cap: a regulator of microbial populations in the rhizosphere? Plant Soil. 1990;129:19–27. [Google Scholar]
- Hawes MC, Brigham LA. Impact of root border cells on microbial populations in the rhizosphere. Adv Plant Pathol. 1992;8:119–148. [Google Scholar]
- Hawes MC, Brigham LA, Wen F-S, Woo H-H, Zhu Y-M. Function of root border cells in plant health: pioneers in the rhizosphere. Annu Rev Phytopathol. 1998;36:311–327. doi: 10.1146/annurev.phyto.36.1.311. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Lin H-J. Correlation of pectolytic enzyme activity with the programmed release of cells from root caps of pea. Plant Physiol. 1990;94:1855–1859. doi: 10.1104/pp.94.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Frank D, Bolce ME, Brown BD, Sive HL, Harland RM. Localization of specific messenger RNAs in Xenopus embryos by whole-mount in-situ hybridization. Development. 1990;110:325–330. doi: 10.1242/dev.110.2.325. [DOI] [PubMed] [Google Scholar]
- Heyes JK. Metabolic aspects of cell growth and development in the root. In: Sutcliffe JF, Pate JS, editors. The Physiology of the Garden Pea. London: Academic Press; 1977. pp. 153–181. [Google Scholar]
- Jacobs TW. Cell cycle control. Annu Rev Plant Physiol Plant Mol Biol. 1994;46:317–339. [Google Scholar]
- Jensen WA, Kavaljian LG. An analysis of cell morphology and the periodicity of division in the root tip of Allium cepa. Am J Bot. 1958;45:365–372. [Google Scholar]
- Juniper B (1972) Mechanisms of perception and patterns of organization in root caps. In MW Miller, CC Kuehnert, eds, The Dynamics of Meristem Cell Populations: Advances in Experimental Medicine and Biology, Vol 18. Plenum Press, New York, pp 119–131
- Knudson L. Viability of detached root cap cells. Am J Bot. 1919;6:309–310. [Google Scholar]
- Loening UE. Changes in microsomal components accompanying cell differentiation of pea-seedling roots. Biochem J. 1961;81:254–260. doi: 10.1042/bj0810254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Sáez JF, González-Fernández A, De La Torre C, Diez JL, Fernández-Gomez ME, Navarette MH, García-Herdugo G, Giménez-Martin G. A model for cell cycle and growth kinetics in roots. J Theor Biol. 1975;53:463–473. doi: 10.1016/s0022-5193(75)80016-6. [DOI] [PubMed] [Google Scholar]
- Luxová M, Ciamporová M. Root structure. In: Kolek J, Kozinka V, editors. Physiology of the Plant Root System. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 45–49. [Google Scholar]
- Luxová M, Murín A. The extent and differences in mitotic activity of the root tip of Vicia faba L. Biol Plant. 1973;15:37–43. [Google Scholar]
- Miller I, Moore R. Defective secretion of mucilage is the cellular basis for agravitropism in primary roots of Zea mays cv Ageotropic. Ann Bot. 1990;66:169–178. doi: 10.1093/oxfordjournals.aob.a088012. [DOI] [PubMed] [Google Scholar]
- Moore R, McClelen CE. Ultrastructural aspects of cellular differentiation in the root cap of Zea mays. Can J Bot. 1983;61:1566–1572. [Google Scholar]
- Popham RA. Zonation of primary and lateral root apices of Pisum sativum. Am J Bot. 1955;42:267–273. [Google Scholar]
- Rost TL, Jones TL, Falk RH. Distribution and relationship of cell division and maturation events in Pisum sativum (Fabaceae) seedling roots. Am J Bot. 1988;75:1571–1583. [Google Scholar]
- Rougier M. Secretory activity of the root cap. In: Tanner W, Loewes FA, editors. Encyclopedia of Plant Physiology, New Series, Plant Carbohydrates II, Vol 13B. Berlin: Springer-Verlag; 1981. pp. 542–574. [Google Scholar]
- Steeves TA, Sussex IM. Patterns in Plant Development. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- Stephenson M, Hawes MC. Correlation of pectin methylesterase activity in root caps of pea with root border cell separation. Plant Physiol. 1994;106:739–745. doi: 10.1104/pp.106.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bayliss C, Weele C, Canny MJ. Determinations of dye diffusivities in the cell wall apoplast of roots by a rapid method. New Phytol. 1996;134:1–4. [Google Scholar]
- Van't Hof J. Control points within the cell cycle. In: Bryant JA, Francis D, editors. The Cell Division Cycle in Plants. Cambridge, UK: Cambridge University Press; 1985. pp. 1–13. [Google Scholar]
- Woo H-H, Brigham LA, Hawes MC. Molecular cloning and expression of mRNAs encoding H1 histone and an H1 histone-like sequence in root tips of pea. Plant Mol Biol. 1995;28:1143–1147. doi: 10.1007/BF00032675. [DOI] [PubMed] [Google Scholar]
- Woo H-H, Brigham LA, Pape M, Hawes MC. Primary structure of the mRNA encoding a 16.5 kDa ubiquitin conjugating enzyme of Pisum sativum. Gene. 1994;148:369–370. doi: 10.1016/0378-1119(94)90715-3. [DOI] [PubMed] [Google Scholar]
- Woo H-H, Hawes MC. Cloning of genes whose expression is correlated with mitosis and localized in dividing cells in root caps of Pisum sativum L. Plant Mol Biol. 1997;35:1045–1051. doi: 10.1023/a:1005930625920. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pierson LS, III, Hawes MC. Induction of microbial genes for pathogenicity and symbiosis by chemicals from root border cells. Plant Physiol. 1997;115:1691–1698. doi: 10.1104/pp.115.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]