Abstract
Objectives
The purpose of this study was to correlate sonographic and mammographic findings with prognostic factors in patients with node-negative invasive breast cancer.
Methods
Sonographic and mammographic findings in 710 consecutive patients (age range 21–81 years; mean age 49 years) with 715 node-negative invasive breast cancers were retrospectively evaluated. Pathology reports relating to tumour size, histological grade, lymphovascular invasion (LVI), extensive intraductal component (EIC), oestrogen receptor (ER) status and HER-2/neu status were reviewed and correlated with the imaging findings. Statistical analysis was performed using logistic regression analysis and intraclass correlation coefficient (ICC).
Results
On mammography, non-spiculated masses with calcifications were associated with all poor prognostic factors: high histological grade, positive LVI, EIC, HER-2/neu status and negative ER. Other lesions were associated with none of these poor prognostic factors. Hyperdense masses on mammography, the presence of mixed echogenicity, posterior enhancement, calcifications in-or-out of masses and diffusely increased vascularity on sonography were associated with high histological grade and negative ER. Associated calcifications on both mammograms and sonograms were correlated with EIC and HER-2/neu overexpression. The ICC value for the disease extent was 0.60 on mammography and 0.70 on sonography.
Conclusion
Several sonographic and mammographic features can have a prognostic value in the subsequent treatment of patients with node-negative invasive breast cancer. Radiologists should pay more attention to masses that are associated with calcifications because on both mammography and sonography associated calcifications were predictors of positive EIC and HER-2/neu overexpression.
The three strongest prognostic factors in invasive breast cancer are widely accepted to be lymph node stage, histological grade and the size of histologically invasive cancer [1–4]. Axillary lymph node stage is an important prognostic factor in invasive breast cancer: the prognosis progressively worsens with an increasing number of involved nodes. Although controversial, micrometastatic disease continues to have clinical significance. Most series have shown that nodal micrometastasis appears to have a more or less adverse effect on disease-g0ree and overall survival [5]. The three strongest prognostic factors in invasive breast cancer provide more valuable information when taken into account altogether than when any single individual factor is used alone. The Nottingham Prognostic Index (NPI) uses these three factors and has been externally validated by several studies [2, 6–8]. In addition, histological grade, tumour size and oestrogen receptor (ER) status are usually used as significant factors in guiding adjuvant systemic chemotherapy in node-negative patients [9].
Lymphovascular invasion (LVI) shows a clear relationship with nodal status [10–13] and local recurrence [12, 13]. LVI is also related to distant metastasis and overall survival in node-negative breast cancer [14, 15]. Patients with breast cancers that exhibit a high proportion of intraductal components have a higher risk of local recurrence after conservative surgery [16, 17]. Hence, accurate evaluation of intraductal spread is considered to be a key issue in determining tumour margins before planning breast-conserving surgery [18]. HER-2/neu overexpression in node-negative cancers is related to disease relapse and to disease-related death, regardless of tumour size, histological grade and ER status [19].
In terms of treatment, most patients with node-positive invasive breast cancers measuring greater than 2 mm are offered adjuvant chemotherapy, with additional hormone therapy or trastuzumab (Herceptin) based upon necessity according to their hormone receptor and HER-2/neu status. On the other hand, patients with node-negative invasive cancer might not be offered adjuvant therapy, adjuvant hormone therapy or chemotherapy depending on the size, LVI, histological grade, their hormone receptor responsiveness and HER-2/neu status, and their age [20]. Therefore, in patients with node-negative breast cancers, knowing the hormone receptor and HER-2/neu status, histological grade and extent of LVI is very important in guiding the treatment plan and determining the prognosis.
Several studies have looked at the correlation between imaging findings and prognostic factors [18, 21–27]. To our knowledge, however, no report has correlated imaging findings in node-negative invasive breast cancers that were analysed according to the Breast Imaging Report and Data System (BI-RADS) lexicon with prognostic factors. The purpose of our study was to correlate sonographic and mammographic findings with prognostic factors in patients with node-negative invasive breast cancer and to determine whether or not the imaging findings could have prognostic value. We also determined the relative accuracy of mammography and sonography in evaluating the extent of disease in patients with node-negative invasive breast cancer.
Methods and materials
Patient selection
Institutional review board approval was obtained and informed consent was waived. All the data were collected and analysed retrospectively. From January 2005 to December 2006, 2535 breast cancer surgeries were performed at our institution. Of these 2535 cancers, 954 (38%) were node-negative invasive breast cancer. We excluded patients who had ductal carcinoma in situ with microinvasion (n = 90), who underwent excisional biopsy or mammotome excision at another hospital (n = 52), who underwent neoadjuvant chemotherapy before surgery (n = 7) or who had no available pre-operative images (n = 16) because the original features of the malignant lesions were altered after excisional biopsy, mammotome excision or neoadjuvant chemotherapy. We also excluded patients who underwent only mammography (n = 43) and only sonography (n = 31). Five women had bilateral node-negative breast cancers, which we managed as two independent cases. Therefore, we finally included 715 cancers in 710 consecutive patients (age range 21–81 years; mean 49 years) who underwent both mammography and sonography. A total of 457 cancers (64%) were treated by breast-conserving surgery, the other 258 cancers (36%) by modified radical or simple mastectomy.
Mammography
Standard bilateral mammograms, with additional views as necessary, were obtained using a Senographe DMR (GE Medical Systems, Milwaukee, WI). We divided mammographic findings into masses and non-mass lesions. Non-mass lesions included areas of asymmetrical density, focal asymmetry, architectural distortion and calcification without associated mass. We classified all of the mammographic findings, both masses and non-mass lesions, into six categories as follows: spiculated masses without calcifications, spiculated masses with calcifications, non-spiculated masses without calcifications, non-spiculated masses with calcifications, calcifications alone and other lesions. We defined a spiculated mass as a central mass with four or more marginal spicules. All masses without four or more spicules were classified as non-spiculated masses regardless of the shape of the mass. Areas of symmetrical density, focal asymmetry and architectural distortion were classified as “other lesions”. When a mass was present, the mammographic findings were also evaluated according to the BI-RADS [28] mammography lexicon, which relies on the following tumour descriptors: shape (oval-to-round, lobular or irregular), margin (circumscribed or obscured, microlobulated, indistinct or spiculated), density (low density, fat-containing radiolucent, isodense or hyperdense), associated calcifications (none, calcifications within a mass or segmental calcifications) and size. Among the associated calcifications, segmental calcifications were defined as calcifications that were distributed in-or-out of a mass. Mammograms were retrospectively reviewed by one breast radiologist with 5 years' experience. At the radiological review, the radiologist was unaware of the pathological prognostic features of the tumour. The largest diameter of the lesion was also measured.
Sonography
One of six radiologists performed bilateral whole-breast sonography. Sonography was performed with 5–12 MHz transducers on an HDI-5000 or IU-22 (Philips Medical Systems, Bothell, WA) ultrasound unit. In our institution, we routinely perform bilateral whole-breast sonography, rather than targeted sonography, in order to evaluate the mammographic or clinical findings. Sonograms were also retrospectively reviewed again by the same breast radiologist who analysed the mammograms and recorded the sonographic features. When the reviewer's evaluation disagreed with the evaluation made at the time of imaging, we chose the reviewer's later evaluation.
We also classified all sonographic findings into mass and non-mass lesions. Non-mass lesions were defined as calcifications without associated mass. Therefore, the types of lesions seen on sonograms were also classified into five categories: oval mass without calcifications, oval mass with calcifications, irregular mass without calcifications, irregular mass with calcifications and calcifications alone (non-mass lesions). When a mass was present, the sonographic findings were evaluated according to the BI-RADS [29] ultrasound lexicon using the following tumour descriptors: shape (oval-to-round or irregular), orientation (parallel to the skin surface or not), margin (circumscribed, microlobulated, indistinct, angular or spiculated), echo pattern (isoechoic-to-hyperechoic, hypoechoic, complex cystic or mixed hyperechoic–hypoechoic), posterior acoustic features (none, enhancement or shadowing), surrounding tissue change (absent or present), the presence of associated calcifications (none or microcalcifications in-or-out of a mass), vascularity (none, focal or penetrating flow, or diffusely increased flow) and size. Among the descriptors of echo patterns, a mixed hyperechoic–hypoechoic mass was defined as a lesion in which some portions were hyperechoic to fat and some hypo- or isoechoic to fat and without any cystic components.
Pathology
Histopathological findings in breast-conserving surgery or mastectomy specimens have served as the gold standard for tumour evaluation. We reviewed the tumour pathology reports, paying attention to the following histological parameters: histological grade, LVI, extensive intraductal component (EIC), ER status, HER-2/neu status and size. The cancers were histopathologically categorised as follows: invasive ductal carcinoma including tubular carcinoma invasive lobular carcinoma, and a miscellaneous group including papillary, mucinous, medullary, metaplastic, cribriform and neuroendocrine cancers.
Tumour grade was determined using the NPI method described by Elston and Ellis [3]. Grade 3 tumours were considered as high grade and grade 1 or 2 as low grade. Immunohistochemical (IHC) staining with antibodies to HER-2/neu was used to assess HER-2/neu status, with membrane staining being scored semi-quantitatively (1+ to 3+) depending on the intensity. HER-2/neu immunostaining was considered positive only when strong (3+) membranous staining was observed in at least 10% of the tumour cells. LVI was assessed in the peritumoural tissue on haematoxylin and eosin sections. It was defined as the presence of carcinoma cells within a definite endothelium-lined space. EIC was defined as the presence of intraductal components extending both beyond the lesion and within the lesion. Cases were considered positive for ER when strong nuclear staining was observed in at least 10% of the tumour cells tested [30]. Tumour size was measured by a combined macroscopic and microscopic measurement of the greatest diameter of the invasive carcinoma. The sizes of all lesions were classified according to one of the following categories: T1, cancer with a size less than or equal to 1 cm; T2, cancer with a size of 1.1–2.0 cm; T3, cancer with a size of 2.1–5.0 cm; and T4, cancer with a size of more than 5 cm.
Statistical analysis
The mammographic and sonographic findings as well as the pathological prognostic factors were recorded. Univariate and multivariate statistical analyses were performed using a statistical software system (SPSS for Windows, 2002, version 11.0; Microsoft Institute, Chicago, IL) to determine whether there was a correlation between the sonographic or mammographic findings and the pathological prognostic factors. Univariate analysis was used to analyse the association between lesion type, as determined by mammograms and sonograms, and prognostic factors. The association between the imaging findings, using the characters of the BI-RADS lexicon, and prognostic factors was analysed using a multivariate logistic regression model.
Logistic regression analysis, with backward elimination based on likelihood ratio tests (backward LR) as necessary, was used to evaluate the odds ratios (ORs) of the various mammographic findings for a specific prognostic factor. ORs and their 95% confidence intervals (CIs) were estimated using logistic regression. To estimate the OR, we used the prognostic parameters as the outcome (dependent) variables and the mammographic or sonographic findings as the explanatory (independent) variables. We chose one of the mammographic or sonographic findings as the reference for the other findings in order to determine their ORs in predicting a specific prognostic factor. Therefore, the OR represented the magnitude of the association between a specific prognostic factor and a specific mammographic or sonographic finding as compared with the other imaging findings. The most benign characteristic among each of the characteristics possible in each prognostic factor was chosen as a reference category (Table 1).
Table 1. Association between types of lesions on mammograms and prognostic factors.
| Type of lesion on mammogram and prognostic factor | Numbers (%) | OR | 95% CI | p-value |
| Histological grade | <0.001* | |||
| Spiculated masses without calcifications | 16/105 (15) | Reference | NA | NA |
| Spiculated masses with calcifications | 8/23 (35) | 2.97 | 1.08, 8.14 | 0.035* |
| Non-spiculated masses without calcifications | 109/294 (37) | 3.28 | 1.83, 5.87 | <0.001* |
| Non-spiculated masses with calcifications | 55/140 (39) | 3.60 | 1.92, 6.77 | <0.001* |
| Calcifications alone | 11/49 (22) | 1.61 | 0.68, 3.79 | 0.279 |
| Other lesions | 18/96 (19) | 1.28 | 0.61, 2.69 | 0.508 |
| Lymphovascular invasion | 0.029* | |||
| Spiculated masses without calcifications | 12/105 (11) | Reference | NA | NA |
| Spiculated masses with calcifications | 6/23 (26) | 2.94 | 1.09, 8.28 | 0.037* |
| Non-spiculated masses without calcifications | 24/298 (8) | 1.27 | 0.64, 2.52 | 0.491 |
| Non-spiculated masses with calcifications | 42/142 (30) | 3.11 | 1.75, 3.32 | 0.025* |
| Calcifications alone | 4/49 (8) | 0.69 | 0.21, 2.56 | 0.538 |
| Other lesions | 4/98 (4) | 0.33 | 0.10, 1.06 | 0.063 |
| Extensive intraductal component | <0.001* | |||
| Spiculated masses without calcifications | 14/105 (13) | Reference | NA | NA |
| Spiculated masses with calcifications | 9/23 (39) | 4.18 | 1.52, 11.46 | 0.005* |
| Non-spiculated masses without calcifications | 57/298 (19) | 1.54 | 0.82, 2.89 | 0.183 |
| Non-spiculated masses with calcifications | 66/142 (46) | 5.65 | 2.94, 10.84 | <0.001* |
| Calcifications alone | 36/49 (73) | 18.00 | 7.71, 42.02 | <0.001* |
| Other lesions | 22/98 (22) | 1.88 | 0.90, 3.93 | 0.092 |
| Negative oestrogen receptor status | <0.001* | |||
| Spiculated masses without calcifications | 16/105 (15) | Reference | NA | NA |
| Spiculated masses with calcifications | 3/23 (13) | 0.83 | 0.22, 3.14 | 0.789 |
| Non-spiculated masses without calcifications | 128/298 (43) | 4.19 | 2.35, 7.48 | <0.001* |
| Non-spiculated masses with calcifications | 62/142 (44) | 4.31 | 2.30, 8.07 | <0.001* |
| Calcifications alone | 21/49 (43) | 4.17 | 1.92, 9.07 | <0.001* |
| Other lesions | 19/98 (19) | 1.34 | 0.64, 2.78 | 0.435 |
| Positive HER-2/neu status | <0.001* | |||
| Spiculated masses without calcifications | 13/105 (12) | Reference | NA | NA |
| Spiculated masses with calcifications | 4/23 (17) | 1.49 | 0.44, 5.07 | 0.523 |
| Non-spiculated masses without calcifications | 57/298 (19) | 1.67 | 0.88, 3.20 | 0.120 |
| Non-spiculated masses with calcifications | 55/142 (39) | 4.47 | 2.29, 8.76 | <0.001* |
| Calcifications alone | 24/49 (49) | 6.79 | 3.03, 15.22 | <0.001* |
| Other lesions | 12/98 (12) | 0.99 | 0.43, 2.28 | 0.976 |
NA, not applicable; OR, odds ratio.
*Statistically significant parameters.
Note – Logistic regression analysis was used to determine the ORs of the mammographic types for a specific prognostic factor. For the explanatory variables, we chose “spiculated masses without calcifications” as the reference for the other mammographic types in order to determine ORs to predict a specific prognostic factor.
The χ2 test was used to evaluate correlation between the imaging findings and tumour size on histopathology. The intraclass correlation coefficient (ICC) was used to measure the agreement between the disease extent measured by mammography or sonography and that measured by pathology. In other words, ICC evaluates the level of agreement between individuals making measurements and between the disease extent measurements taken from mammography, sonography and the surgical pathology specimen. The ICC represents concordance, where 1 is perfect agreement and 0 is no agreement at all. Findings with a p-value of less than 0.05 were considered statistically significant.
Results
On pathology, 650 (91%) lesions were found to be invasive ductal carcinomas, including 7 (1%) tubular carcinomas, 20 (3%) invasive lobular carcinoma and 45 (6%) miscellaneous cancers. Of the 715 cases in total, 69% (n = 490) were histology grade 1 or 2, 30% (n = 217) were grade 3 and the remaining 1% (n = 8) were of unknown histological grade. A positive EIC was found in 29% of the 715 cases, LVI was identified in 13% (n = 92), 65% (n = 466) were positive for ER and 23% (n = 165) were positive for HER-2/neu.
In the statistical analysis, the prognostic factors were used as dichotomised variables as follows: histology grade 3 tumours were considered as positive and grade 1 or 2 tumours were considered as negative; HER-2/neu immunostaining was considered positive when strong (3+) membranous staining was observed, whereas cases with staining evaluated as 0 to 2+ were regarded as negative; tumours with positive LVI were considered as positive and those with negative LVI were considered as negative; tumours with positive EIC were defined as positive and those without as negative; and tumours with a negative ER status were defined as positive whereas those with a positive ER status were considered as negative.
Mammographic findings
In the univariate analysis, the associations between the types of lesions seen on mammograms and the prognostic factors are shown in Table 1. The association between lesion type, as determined by mammograms and sonograms, and prognostic factors was significant in all models. The predominant types of lesions on mammography were non-spiculated masses without calcifications and non-spiculated masses with calcifications, which accounted for 298 (42%) and 142 (20%), respectively, of the 715 lesions. Other mammographic types were as follows: 105 (15%) spiculated masses without calcifications, 98 (14%) other lesions, 49 (6%) calcifications alone and 23 (3%) spiculated masses with calcifications. Non-spiculated masses with calcifications were associated with all of the poor prognostic factors: high histological grade, positive LVI and EIC, negative ER status and positive HER-2/neu. Spiculated masses with calcifications fared better and were associated with high histological grade and with positive LVI and EIC. Calcifications alone were associated with positive EIC, negative ER status and positive HER-2/neu. Non-spiculated masses without calcifications were associated with high histological grade and negative ER. Lastly, the other lesions were not associated with any poor prognostic factors.
For the multivariate analyses, the association between the mammographic findings and the prognostic factors is shown in Table 2. None of the masses showed low density or fat-containing radiolucency. Hyperdense masses were associated with high histological grade and negative ER status (Figure 1a,b). Associated calcifications, including calcifications within a mass and segmental calcifications, were associated with positive EIC and positive HER-2/neu. Irregularly shaped masses were associated with positive EIC, whereas a circumscribed margin was associated with a negative ER status. Indistinct margins were associated with high histological grade, negative ER status, positive EIC and positive HER-2/neu, whereas circumscribed or microlobulated margins were associated with high histological grade and negative ER status. When we evaluated the association between mammographic findings and tumour size, isodense masses were associated with T1 cancers whereas hyperdense masses were associated with cancers graded T2 and above (p<0.001).
Table 2. Association between mammographic findings according to the BI-RADS lexicon and prognostic factors.
| Mammographic findings and prognostic factor | Number (%) | OR | 95% CI | p-value |
| Histological grade (n = 562) | ||||
| Margin of mass | <0.001* | |||
| Spiculated | 24/128 (19) | Reference | NA | NA |
| Circumscribed or obscured | 22/55 (40) | 3.71 | 1.80, 7.63 | <0.001* |
| Microlobulated | 14/33 (42) | 3.61 | 1.57, 8.31 | 0.003* |
| Indistinct | 128/346 (37) | 2.70 | 1.64, 4.46 | <0.001* |
| Density of mass | <0.001* | |||
| Isodense | 57/220 (26) | Reference | NA | NA |
| Hyperdense | 131/342 (38) | 2.02 | 1.37, 2.97 | <0.001* |
| Lymphovascular invasion (n = 568) | ||||
| Shape of mass | 0.015* | |||
| Lobular | 7/116 (6) | Reference | NA | NA |
| Oval-to-round | 22/142 (15) | 2.86 | 1.17, 6.95 | 0.021* |
| Irregular | 55/310 (18) | 3.36 | 1.48, 7.61 | 0.004* |
| Extensive intraductal component (n = 568) | ||||
| Shape of mass | 0.001* | |||
| Oval-to-round | 25/142 (18) | Reference | NA | NA |
| Lobular | 22/116 (19) | 1.19 | 0.62, 2.29 | 0.593 |
| Irregular | 99/310 (32) | 2.68 | 1.53, 4.72 | 0.001* |
| Margin of mass | 0.011* | |||
| Spiculated | 23/128 (18) | Reference | NA | NA |
| Circumscribed or obscured | 13/55 (24) | 2.39 | 0.99, 5.76 | 0.051 |
| Microlobulated | 4/55 (11) | 0.93 | 0.28, 3.04 | 0.902 |
| Indistinct | 106/350 (30) | 2.21 | 1.31, 2.87 | 0.003* |
| Density of mass | 0.001* | |||
| Hyperdense | 74/348 (21) | Reference | NA | NA |
| Isodense | 72/220 (33) | 1.93 | 1.29, 2.87 | 0.001* |
| Associated calcification | <0.001* | |||
| None | 71/405 (18) | Reference | NA | NA |
| Calcifications in a mass | 57/136 (42) | 3.01 | 1.92, 4.73 | <0.001* |
| Segmental calcifications | 18/27 (67) | 7.36 | 3.08, 17.63 | <0.001* |
| Negative oestrogen receptor status (n = 568) | ||||
| Margin of mass | <0.001* | |||
| Spiculated | 19/128 (15) | Reference | NA | NA |
| Circumscribed or obscured | 28/55 (51) | 7.44 | 3.55, 15.62 | <0.001* |
| Microlobulated | 15/35 (43) | 4.71 | 2.04, 10.88 | <0.001* |
| Indistinct | 14/350 (42) | 4.39 | 2.57, 7.50 | <0.001* |
| Density of mass | 0.002* | |||
| Isodense | 68/220 (31) | Reference | NA | NA |
| Hyperdense | 141/348 (41) | 1.83 | 1.25, 2.67 | 0.002* |
| Positive HER-2/neu status (n = 568) | ||||
| Margin of mass | 0.072 | |||
| Spiculated | 17/128 (13) | Reference | NA | NA |
| Circumscribed or obscured | 9/55 (16) | 1.37 | 0.56, 3.32 | 0.492 |
| Microlobulated | 7/35 (20) | 1.62 | 0.60, 4.33 | 0.340 |
| Indistinct | 96/350 (27) | 2.09 | 1.18, 3.72 | 0.011* |
| Associated calcification | <0.001* | |||
| None | 69/405 (17) | Reference | NA | NA |
| Calcifications in a mass | 50/136 (37) | 2.56 | 1.64, 3.99 | <0.001* |
| Segmental calcifications | 10/27 (37) | 2.52 | 1.09, 5.78 | 0.030* |
NA, not applicable; OR, odds ratio; BI-RADS, Breast Imaging Report and Data System.
*Statistically significant parameters.
Note – For statistical analysis, prognostic factors were used as dichotomised variables as follows: tumours with histology grade 3 were considered as positive and cases with grades 1 and 2 as negative; HER-2/neu immunostaining was considered positive when strong (3+) membranous staining was observed, whereas cases with 0 to 2+ were regarded as negative; tumours with positive LVI were considered as positive and those with negative LVI as negative; tumours with positive EIC were defined as positive and those without as negative; and tumours with negative ER status were defined as positive whereas those with positive ER status were considered as negative.
Figure 1.
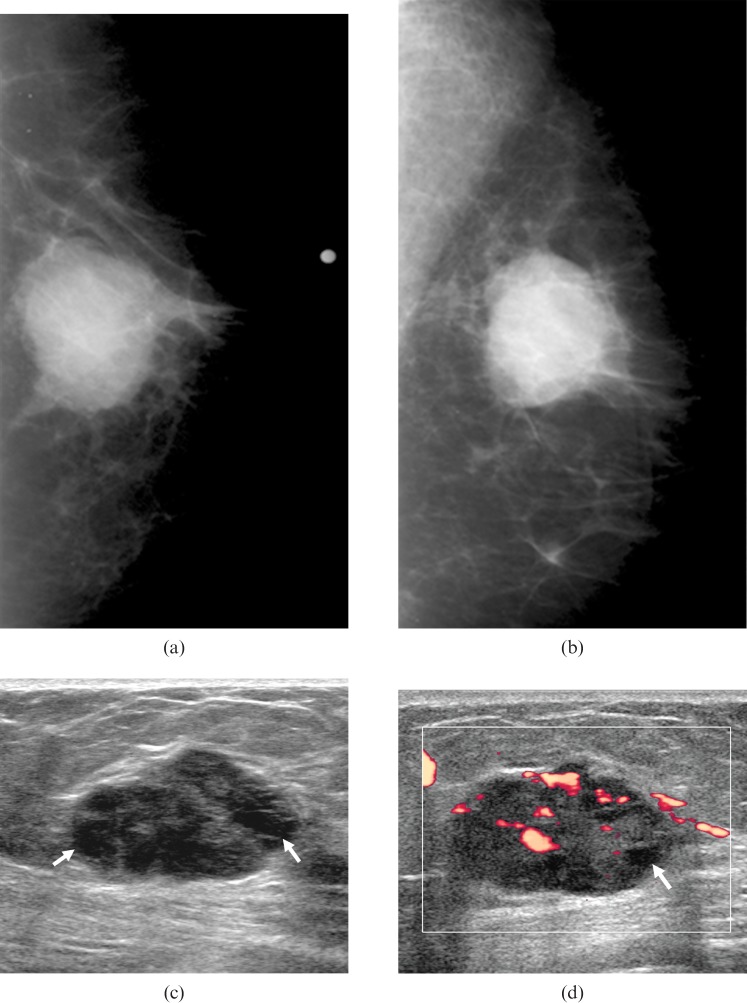
A 70-year-old woman with a palpable lump in the right breast. (a,b) Mammograms show an oval-shaped hyperdense mass with mostly circumscribed and partially obscured margins in the right breast. There are no calcifications associated with the mass. (c,d) Sonograms show an oval-shaped, complex cystic mass (arrows indicate the cystic portions in the solid mass) with mostly circumscribed margin and posterior enhancement in the right breast. This mass showed no associated calcifications and diffusely increased vascularity on power Doppler study. Surgery confirmed an invasive ductal carcinoma with high histological grade and negative oestrogen receptor status, lymphovascular invasion, extensive intraductal component and HER-2/neu status.
Sonographic findings
The predominant findings on sonograms were oval masses without calcifications and irregular masses without calcifications, which accounted for 280 (39%) and 260 (36%), respectively, of 715 lesions. In addition, there were 119 (17%) tumours with irregular masses with calcifications, 48 (7%) tumours with oval masses with calcifications and 8 (1%) tumours with calcifications alone. The associations identified by univariate analysis between the types of lesions found on sonograms and the prognostic factors are shown in Table 3. Oval or irregular masses with calcifications were associated with positive EIC and positive HER-2/neu. Calcifications alone were associated with positive EIC. There was no significant association between sonographic type and histological grade, LVI or ER.
Table 3. Association between types of lesions on sonograms and prognostic factors.
| Type of lesion on sonogram and prognostic factor | Number (%) | OR | 95% CI | p-value |
| Extensive intraductal component | <0.001* | |||
| Oval masses without calcifications | 49/280 (18) | Reference | NA | |
| Oval masses with calcifications | 23/48 (48) | 4.34 | 2.28, 8.26 | <0.001* |
| Irregular masses without calcifications | 59/260 (23) | 1.38 | 0.91, 2.11 | 0.133 |
| Irregular masses with calcifications | 66/119 (55) | 5.87 | 3.65, 9.44 | <0.001* |
| Calcifications alone | 7/8 (88) | 33.00 | 3.97, 274.34 | 0.001* |
| Positive HER-2/neu status | <0.001* | |||
| Oval masses without calcifications | 45/280 (16) | Reference | NA | |
| Oval masses with calcifications | 22/48 (46) | 4.42 | 2.30, 8.48 | <0.001* |
| Irregular masses without calcifications | 50/260 (19) | 1.24 | 0.80, 1.94 | 0.336 |
| Irregular masses with calcifications | 46/119 (39) | 3.29 | 2.02, 5.36 | <0.001* |
| Calcifications alone | 2/8 (25) | 1.74 | 0.34, 8.90 | 0.506 |
NA, not applicable; OR, odds ratio.
*These were considered to be statistically significant parameters.
Note – Logistic regression analysis was used to evaluate the ORs of the sonographic types for a specific prognostic factor. For the explanatory variables, we have chosen “oval masses without calcifications” as the reference for the other sonographic types in order to determine ORs that predict a specific prognostic factor.
In the multivariate analysis, the associations between sonographic findings according to the BI-RADS lexicon and prognostic factors are shown in Table 4. Mixed hyperechoic–hypoechoic masses were associated with high histological grade and negative ER status, whereas hypoechoic masses were associated only with high histological grade. Posterior enhancement was also associated with high histological grade and negative ER status (Figure 1c,d). Similarly, diffusely increased vascularity was associated with high histological grade and negative ER status (Figure 1c,d). Calcifications in or out of a mass were associated with most of the poor prognostic factors: high histological grade, positive EIC, negative ER status and positive HER-2/neu status (Figure 2). None of the shape, margin or orientation characteristics was associated with the prognostic factors. As regards LVI, there was no association between sonographic findings and prognostic factors. When we evaluated the association between sonographic findings and tumour size, no posterior feature was associated with T1 or T2 cancer, whereas posterior enhancement was associated with T3 or T4 cancers (p<0.001). T1 cancers were associated with no increased vascularity, whereas cancers sized T2 or more were associated with focal or penetrating blood flow on Doppler study (p<0.001).
Table 4. Association between sonographic findings according to the BI-RADS lexicon and prognostic factors.
| Sonographic findings and prognostic factor | Number (%) | Odds ratio | 95% CI | p-value |
| Histological grade (n = 699) | ||||
| Echogenicity | 0.004† | |||
| Iso-to-hyperechoic | 14/91 (15) | Reference | NA | |
| Hypoechoic | 116/347 (33) | 3.16 | 1.63, 6.11 | 0.001† |
| Complex cystic | 14/35 (40) | 1.51 | 0.59, 3.89 | 0.391 |
| Mixed hyper-hypoechogenicity | 72/226 (32) | 2.58 | 1.31, 5.06 | 0.006† |
| Posterior feature | <0.001† | |||
| Posterior shadowing | 21/106 (20) | Reference | NA | |
| Posterior enhancement | 111/215 (52) | 3.64 | 1.99, 6.63 | <0.001† |
| None | 84/378 (22) | 1.04 | 0.60, 1.83 | 0.879 |
| Associated calcification | 0.035† | |||
| None | 156/530 (29) | Reference | NA | |
| Calcifications in-or-out of a mass | 60/169 (36) | 1.54 | 1.03, 2.30 | 0.035† |
| Vascularity | 0.010† | |||
| None | 81/308 (26) | Reference | NA | |
| Focal or penetrating | 113/357 (32) | 1.12 | 0.78, 1.61 | 0.552 |
| Diffusely increased | 21/34 (62) | 3.37 | 1.53, 7.45 | 0.003† |
| Extensive intraductal component (n = 707) | ||||
| Posterior feature | <0.001† | |||
| Posterior shadowing | 27/107 (25) | Reference | NA | |
| Posterior enhancement | 33/220 (15) | 0.44 | 0.22, 0.86 | 0.016† |
| None | 137/380 (36) | 1.38 | 0.80, 2.38 | 0.242 |
| Associated calcification | <0.001† | |||
| None | 108/537 (20) | Reference | NA | |
| Calcifications in-or-out of a mass | 90/170 (53) | 3.72 | 2.50, 5.53 | <0.001† |
| Negative oestrogen receptor status (n = 707) | ||||
| Echogenicity | 0.006† | |||
| Iso-to-hyperechoic | 23/92 (25) | Reference | NA | |
| Hypoechoic | 111/350 (32) | 1.37 | 0.77, 2.44 | 0.286 |
| Complex cystic | 14/35 (40) | 0.70 | 0.29, 1.73 | 0.443 |
| Mixed hyper-hypoechogenicity | 99/230 (43) | 2.13 | 1.19, 3.84 | 0.012† |
| Posterior feature | <0.001† | |||
| Posterior shadowing | 24/107 (22) | Reference | NA | |
| Posterior enhancement | 123/220 (56) | 3.85 | 2.14, 6.93 | <0.001† |
| None | 100/380 (26) | 1.01 | 0.59, 1.72 | 0.985 |
| Associated calcification | 0.002† | |||
| None | 173/537 (32) | Reference | NA | |
| Calcifications in-or-out of a mass | 74/170 (44) | 1.86 | 1.26, 2.74 | 0.002† |
| Vascularity | <0.001† | |||
| None | 89/313 (28) | Reference | NA | |
| Focal or penetrating | 131/360 (36) | 1.30 | 0.91, 1.84 | 0.147 |
| Diffusely increased | 26/34 (76) | 6.07 | 2.54, 14.53 | <0.001† |
| Positive HER-2/neu status (n = 707) | ||||
| Associated calcification | <0.001† | |||
| None | 95/537 (18) | Reference | NA | |
| Calcifications in-or-out of a mass | 68/170 (40) | 2.97 | 2.02, 4.35 | <0.001† |
NA, Not applicable
†Statistically significant parameters
Figure 2.
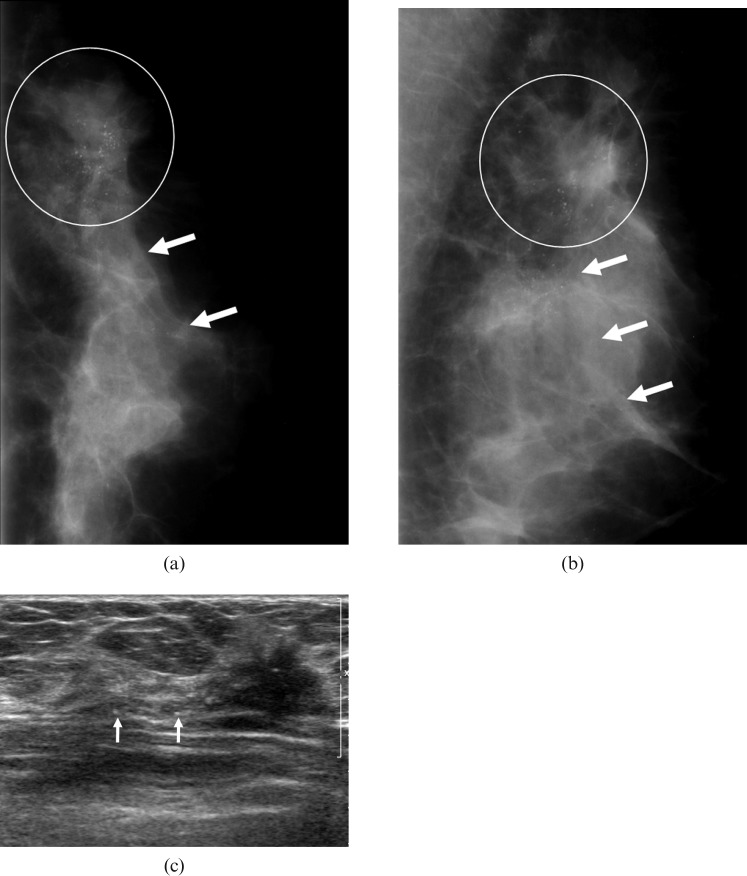
A 45-year-old woman in whom screening at a local clinic had detected a mammographic abnormality in the left breast. (a,b) Mammograms show an irregularly shaped isodense mass with indistinct margin (circle), which is associated with segmentally distributed calcifications (arrows) in the upper outer quadrant of the left breast. (c) The sonogram shows an irregularly shaped hypoechoic mass with an indistinct margin in the left breast, which is associated with calcifications in or out of a mass. Surgery confirmed an invasive ductal carcinoma with high histological grade and positive lymphovascular invasion and extensive introductal component. This mass was also associated with negative oestrogen receptor status and positive HER-2/neu status.
Evaluation of the extent of disease
When we evaluated the extent of disease, we divided the results into three categories as follows: all tumours, tumours with LVI or EIC and tumours without LVI and EIC. There were 270 tumours (38%) with LVI or EIC and 445 tumours (62%) without LVI and EIC. The ICC value for disease extent was 0.60 on mammography and 0.70 on sonography. For the 270 tumours with LVI or EIC, the ICC value was 0.55 on mammography and 0.59 on sonography, whereas for the 445 lesions without LVI and EIC, the ICC value was 0.64 on mammography and 0.81 on sonography. Of the 715 tumours, 258 (36%) were treated by mastectomy. We also divided these 258 tumours into three categories as before. There were 135 tumours (52%) with LVI or EIC, leaving 123 cases (48%) without either. For all 258 tumours, the ICC value was 0.53 on mammography and 0.61 on sonography. For 135 cases with LVI or EIC, the ICC value was 0.48 on mammography and 0.53 on sonography, whereas for 123 cases without LVI and EIC, the ICC value was 0.58 on mammography and 0.78 on sonography. Cases without LVI and EIC showed stronger correlation with pathology than those with LVI or EIC. The difference between ICC values for cases with LVI or EIC and without LVI and EIC was significant on sonography (p<0.001) but not significant on mammography (p = 0.269). In all three categories, sonography provided stronger correlation with pathology than mammography when evaluating the extent of disease.
Discussion
Several previous studies [22, 25] showed that a spiculated mass without calcifications was the most common mammographic feature indicating malignancy. Evans et al [27] demonstrated that mammographic spiculation was an independent, good prognostic factor for screening-detected invasive breast cancer (Figure 3). In our study, we found that non-spiculated masses without calcifications were the most common mammographic finding for node-negative invasive breast cancer (42%), whereas spiculated masses with (n = 23) or without calcifications (n = 105) made up only 18% of lesions (128 of 715). We believe that this difference resulted from the fact that one study [22] included all invasive and non-invasive cancers and the other study [25] included only small invasive breast cancers of less than 15 mm in size, regardless of the lymph node status. By contrast, our study included consecutive patients with node-negative invasive breast cancer of any size.
Figure 3.
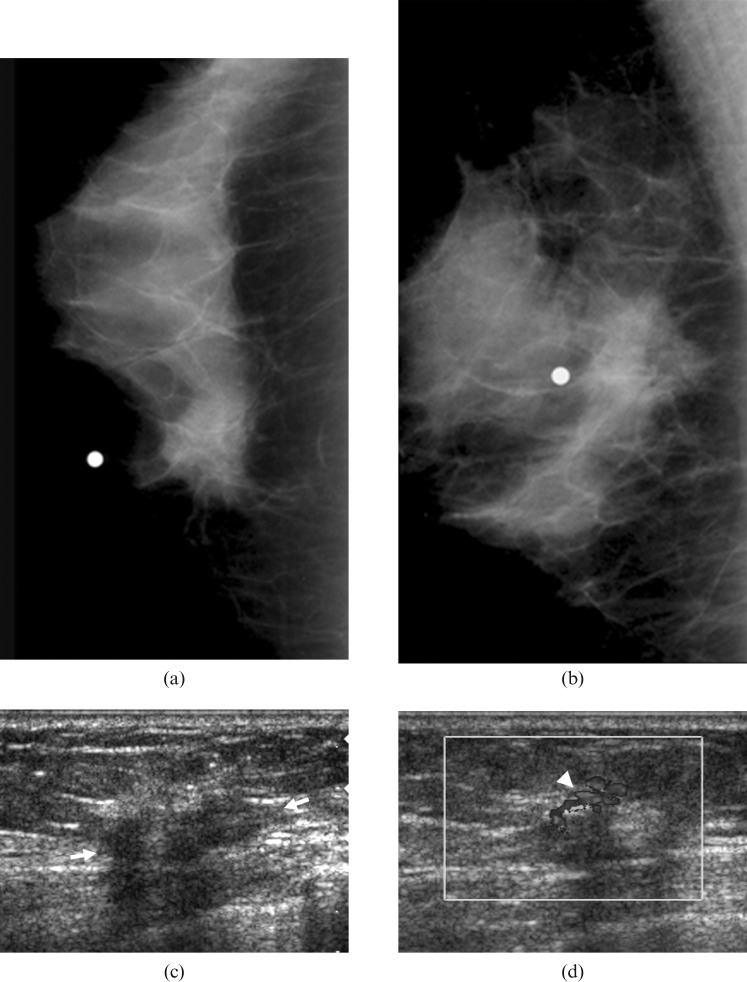
Mammogram of a 64-year-old woman with a palpable lump in the left breast. (a,b) Mammograms show an irregularly shaped hyperdense mass with a spiculated margin in the left breast, which was not associated with calcifications. (c,d) Sonograms show an irregularly shaped hypoechoic mass (arrows) with an indistinct margin and posterior shadowing. The Doppler study shows a penetrating blood flow (arrowhead) in the periphery of this mass. Surgery confirmed an invasive ductal carcinoma with low histological grade and negative lymphovascular invasion and extensive introductal component. This mass was also associated with positive oestrogen receptor status and negative HER-2/neu status.
In our study, we assessed the roles of the mammographic and sonographic features of breast cancer as predictors of prognosis among women with node-negative invasive breast cancers. To our knowledge, there have been several studies on the association between mammographic features and the prognostic or pathological characteristics of breast cancer [22–25, 27], but only two studies have been published on the association between sonographic features and the histological grade of invasive breast cancer [21, 26]. As far as we know, no report has been published on the association between the mammographic and sonographic features analysed according to the BI-RADS lexicon and the pathological characteristics of node-negative invasive breast cancer.
We found that certain mammographic and sonographic features can serve as predictors of both prognosis and pathological characteristics for the subsequent treatment of patients with node-negative invasive breast cancer. The results of our study should be interpreted in the context of whether or not a radiologist can offer pre-operative prognostic predictions when he or she encounters mammographically or sonographically detected malignancies.
When we evaluated the association between the types of lesions and the pathological characteristics, we found that both non-spiculated masses with or without calcifications and spiculated masses with calcifications were associated with a high histological grade.
With regard to the analysis of the imaging findings according to the BI-RADS lexicon, hyperdense masses with non-spiculated margins (e.g. circumscribed, obscured, microlobulated or indistinct margins) on mammography and findings of hypoechoic or mixed hyper-hypoechogenicity, posterior enhancement, calcifications in-or-out of a mass, and diffusely increased vascularity on sonography were also associated with a high histological grade. Several previous studies showed that findings of other lesions with calcifications, comedo calcifications or ill-defined masses were associated with a high histological grade [22, 27]. Lamb et al [21] and Rotstein and Neerhut [26] demonstrated that high-grade invasive ductal carcinomas might paradoxically display features similar to those of benign breast masses, such as posterior enhancement and a well-defined margin. Our results were consistent with the results of these studies. Oken et al [31] demonstrated that invasive ductal carcinoma with a fibrotic focus, which was associated with a significantly worse prognosis than invasive ductal carcinoma without a fibrotic focus, showed focal increased echogenicity within the hypoechoic lesion, i.e. mixed hyper-hypoechogenicity. In our study, mixed hyper-hypoechoic masses were associated with a high histological grade.
In their study of 2760 patients with node-negative invasive breast cancer, Lee et al [11] concluded that LVI was an independent prognostic factor in node-negative breast cancer and should be considered when making decisions regarding adjuvant treatment in this group of patients. Gajdos et al [23] carried out a study of 543 non-palpable breast cancers, and demonstrated that lymphatic invasion was more common in cancers presenting as a mass with calcifications. In our study, mammographic types that were significantly associated with LVI were spiculated or non-spiculated masses with calcifications.
If conservative surgery is performed, intraductal spread can lead to a high risk of local recurrence [17]. Therefore, accurate evaluation of the extent of the intraductal spread is essential in making decisions regarding conservative therapy. According to previous studies [18, 32, 33], the sensitivity of MRI and sonography for the diagnosis of intraductal spread was high, although sonography had a tendency to underestimate intraductal spread when compared with MRI [18]. In our study, the presence of either calcifications alone or masses associated with calcifications on either mammography and sonography were significantly associated with positive EIC. In addition, findings of irregular shape, an indistinct margin, calcifications within a mass or segmental calcifications on mammography, and the presence of posterior shadowing and calcifications in or out of a mass on sonography were associated with a positive EIC. Specifically, associated calcifications on both mammography and sonography were the most important finding in detecting an EIC.
Gajdos et al [23] showed that no specific mammographic finding was significantly related to ER or progesterone receptor (PR) status. However, we found that mammographic findings of lesions with non-spiculated margins (e.g. circumscribed, obscured, microlobulated or indistinct margins) or hyperdense masses and sonographic findings of mixed hyper/hypoechogenicity, posterior enhancement, calcifications in or out of a mass and diffusely increased vascularity were associated with negative ER status. These findings overlapped with those predicting a high histological grade. ER and PR status served both as markers of endocrine responsiveness and as harbingers of increased recurrence risk. ER-positive breast tumours have been shown to provide patients with both longer disease-g0ree survival and overall survival. Oestrogen antagonism treatments, such as selective ER modulators (SERMs) including tamoxifen or antioestrogens, may be effective in the treatment of ER-positive breast cancers. If the tumours are ER and PR negative, hormonal therapy such as SERMs may have less efficacy.
Gajdos et al [23] also indicated that calcifications were associated with greater HER-2/neu immunoreactivity, and our study agreed with this result. In other words, the presence of calcifications in a mass or segmental calcifications on mammography, and of calcifications in-or-out of a mass on sonography, was significantly associated with positive HER-2/neu status. This result might suggest that when HER-2/neu staining is 1+ to 2+, associated calcifications on both sonography and mammography may predict HER-2/neu overexpression. HER-2/neu overexpression may be an independent prognostic variable for the risk for relapse and patient survival. The presence of HER-2/neu overexpression is a predictive factor that may indicate success with the use of trastuzumab (Herceptin) [19].
In our study, sonography provided better correlation with pathology measured in terms of disease extent than mammography, especially in tumours without LVI and EIC. In their prospective study of 40 patients with known breast cancer, Berg and Gilbreath [34] demonstrated that whole-breast sonography would be a useful complement to mammography in the pre-operative evaluation of patients with breast cancer, providing a more accurate assessment of disease extent, which is particularly important when breast conservation is contemplated. In tumours with LVI or EIC, however, both mammography and sonography findings showed worse correlation with pathology than in tumours without LVI and EIC. Our result might suggest that associated calcifications, if found in association with masses on both mammography and sonography, may be predictive of positive EIC. Therefore, tumours suspected of having LVI or EIC should be given careful attention, and further evaluation with other modalities such as MRI might be helpful in evaluating the accurate extent of disease.
Our study had several limitations. First, the mammograms and sonograms were reviewed by a single radiologist retrospectively. Reproducibility was not addressed, and moreover the reviewer was not blinded to the mammographic results when analysing the sonograms. However, the reviewer was at least blinded to the pathology results. Second, we did not evaluate the difference between the screening-detected and symptomatic patient groups. We were interested solely in node-negative invasive breast cancer, regardless of the symptoms. Third, there was a limitation to the accurate evaluation of disease extent in tumours that underwent breast-conserving surgery — 457 (64%) of the total of 715 tumours. In these tumours, the real state of disease extent might not be addressed because all of the disease has not been detected. Furthermore, the disease extents for most tumours were not determined by breast MRI. For the 258 (36%) tumours that were treated by mastectomy and thus were considered to have undergone a thorough excision, however, sonography findings showed better correlation with pathology than mammography findings. Fourth, we considered only 3+ staining on IHC as positive for HER-2/neu because we did not perform fluorescence in situ hybridization (FISH) in all cases. Recently, many laboratories have undertaken to perform FISH assays because only 10–15% of 2+ tumours and less than 5% of 1+ tumours have gene amplification [35]. Therefore, 10–15% of 2+ tumours with gene amplification might be missed in our study, although 3+ staining is generally accepted as true overexpression for clinical purposes. Thus, in designing a retrospective study, we decided to set the criteria for positive for HER-2/neu as 3+ staining on IHC. Fifth, the statistical analysis had limitations. There was potential for sample bias because we included only patients who underwent both mammography and sonography. There were small numbers of tumours in some categories, which may have reduced the power of the statistical analysis. We used univariate analysis to assess the association between types of lesion on mammogram and sonogram and prognostic factors, and therefore there was no adjustment for other covariates (Tables 1 and 3). However, multivariate analysis was also used to study the association between the imaging features of masses described according to the BI-RADS lexicon and prognostic factors (Tables 2 and 4). Last, we used prognostic factors as a surrogate for outcomes. In addition, causality could not be inferred because our study was an observational study.
Conclusion
Certain sonographic and mammographic features are considered to have prognostic value in the subsequent treatment of patients with node-negative invasive breast cancer. Specifically, we suggest that radiologists should pay attention to masses that are associated with calcifications because associated calcifications on both mammography and sonography were predictors of positive EIC and HER-2/neu overexpression. Our results showed that for tumours with LVI or EIC, both mammographic and sonographic features showed worse correlation with pathology than those in tumours without LVI and EIC. Therefore, tumours suspected of having LVI or EIC, which were associated with calcifications, should be given more careful attention, and further evaluation with another modality such as MRI might be helpful in evaluating the accurate extent of disease. Further studies should be designed and performed to confirm this conclusion, eliminating the limitations of the present study.
Acknowledgments
We thank Seon-Ok Kim in the Division of Biostatistics, Center for Medical Research and Information, University of Ulsan College of Medicine, for her assistance in the statistical analysis of this manuscript.
References
- 1.Carter C, Allen C, Henson D. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181–7 [DOI] [PubMed] [Google Scholar]
- 2.Sundquist M, Thorstenson S, Brudin L, Nordenskjold B. Applying the Nottingham Prognostic Index to a Swedish breast cancer population. South East Swedish Breast Cancer Study Group. Breast Cancer Res Treat 1999;53:1–8 [DOI] [PubMed] [Google Scholar]
- 3.Elston C, Ellis I. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10 [DOI] [PubMed] [Google Scholar]
- 4.Neville A, Bettelheim R, Gelber R, Save-Soderbergh J, Davis B, Reed R, et al. Factors predicting treatment responsiveness and prognosis in node-negative breast cancer. The International (Ludwig) Breast Cancer Study Group. J Clin Oncol 1992;10:696–705 [DOI] [PubMed] [Google Scholar]
- 5.Nasser I, Lee A, Bosari S, Saganich R, Heatley G, Silverman M. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum Pathol 1993;24:950–7 [DOI] [PubMed] [Google Scholar]
- 6.Blamey R, Ellis I, Pinder S, Lee A, Macmillan R, Morgan D, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–1999. Eur J Cancer 2007;43:1548–55 [DOI] [PubMed] [Google Scholar]
- 7.Balslev I, Axelsson C, Zedeler K, Rasmussen B, Carstensen B, Mouridsen H. The Nottingham Prognostic Index applied to 9,149 patients from the studies of the Danish Breast Cancer Cooperative Group (DBCG). Breast Cancer Res Treat 1994;32:281–90 [DOI] [PubMed] [Google Scholar]
- 8.Volpi A, De PF, Nanni O, Granato A, Bajorko P, Becciolini A, et al. Prognostic significance of biologic markers in node-negative breast cancer patients: a prospective study. Breast Cancer Res Treat 2000;63:181–92 [DOI] [PubMed] [Google Scholar]
- 9.Hery M, Delozier T, Ramaioli A, Julien J, de Lafontan B, Petit T, et al. Natural history of node-negative breast cancer: are conventional prognostic factors predictors of time to relapse? Breast 2002;11:442–8 [DOI] [PubMed] [Google Scholar]
- 10.Lauria R, Perrone F, Carlomagno C, De Laurentiis M, Morabito A, Gallo C, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer 1995;76:1772–8 [DOI] [PubMed] [Google Scholar]
- 11.Lee A, Pinder S, Macmillan R, Mitchell M, Ellis I, Elston C, et al. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer 2006;42:357–62 [DOI] [PubMed] [Google Scholar]
- 12.Sundquist M, Thorstenson S, Klintenberg C, Brudin L, Nordenskjold B. Indicators of loco-regional recurrence in breast cancer. The South East Swedish Breast Cancer Group. Eur J Surg Oncol 2000;26:357–62 [DOI] [PubMed] [Google Scholar]
- 13.Voogd A, Nielsen M, Peterse J, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol 2001;19:1688–97 [DOI] [PubMed] [Google Scholar]
- 14.Rosen P, Groshen S, Saigo P, Kinne D, Hellman S. Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: a study of 644 patients with median follow-up of 18 years. J Clin Oncol 1989;7:1239–51 [DOI] [PubMed] [Google Scholar]
- 15.de Mascarel I, Bonichon F, Durand M, Mauriac L, MacGrogan G, Soubeyran I, et al. Obvious peritumoral emboli: an elusive prognostic factor reappraised. Multivariate analysis of 1320 node-negative breast cancers. Eur J Cancer 1998;34:58–65 [DOI] [PubMed] [Google Scholar]
- 16.Lindley R, Bulman A, Parsons P, Phillips R, Henry K, Ellis H. Histologic features predictive of an increased risk of early local recurrence after treatment of breast cancer by local tumor excision and radical radiotherapy. Surgery 1989;105:13–20 [PubMed] [Google Scholar]
- 17.Holland R, Connolly J, Gelman R, Mravunac M, Hendriks J, Verbeek A, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol 1990;8:113–18 [DOI] [PubMed] [Google Scholar]
- 18.Satake H, Shimamoto K, Sawaki A, Niimi R, Ando Y, Ishiguchi T, et al. Role of ultrasonography in the detection of intraductal spread of breast cancer: correlation with pathologic findings, mammography and MR imaging. Eur Radiol 2000;10:1726–32 [DOI] [PubMed] [Google Scholar]
- 19.Volpi A, Nanni O, De Poalo F, Granato A, Mangia A, Monti F, et al. HER-2 expression and cell proliferation: prognostic markers in patients with node-negative breast cancer. J Clin Oncol 2003;21:2708–12 [DOI] [PubMed] [Google Scholar]
- 20.American Cancer Society (ACS)/National Comprehensive Cancer Network (NCCN) Breast Cancer: Treatment Guidelines for Patients, version VIII. ACS/NCCN; 2006. [Google Scholar]
- 21.Lamb P, Perry N, Vinnicombe S, Wells C. Correlation between ultrasound characteristics, mammographic findings and histological grade in patients with invasive ductal carcinoma of the breast. Clin Radiol 2000;55:40–4 [DOI] [PubMed] [Google Scholar]
- 22.Thurfjell M, Lindgren A, Thurfjell E. Nonpalpable breast cancer: mammographic appearance as predictor of histologic type. Radiology 2002;222:165–70 [DOI] [PubMed] [Google Scholar]
- 23.Gajdos C, Tartter P, Bleiweiss I, Hermann G, de Csepel J, Estabrook A, et al. Mammographic appearance of nonpalpable breast cancer reflects pathologic characteristics. Ann Surg 2002;235:246–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stomper P, Geradts J, Edge S, Levine E. Mammographic predictors of the presence and size of invasive carcinomas associated with malignant microcalcification lesions without a mass. AJR Am J Roentgenol 2003;181:1679–84 [DOI] [PubMed] [Google Scholar]
- 25.Tabar L, Tony CH, Amy YM, Tot T, Tung T, Chen L, et al. Mammographic tumor features can predict long-term outcomes reliably in women with 1–14-mm invasive breast carcinoma. Cancer 2004;101:1745–59 [DOI] [PubMed] [Google Scholar]
- 26.Rotstein A, Neerhut P. Ultrasound characteristics of histologically proven grade 3 invasive ductal breast carcinoma. Australas Radiol 2005;49:476–9 [DOI] [PubMed] [Google Scholar]
- 27.Evans A, Pinder S, James J, Ellis I, Cornford E. Is mammographic spiculation an independent, good prognostic factor in screening-detected invasive breast cancer? AJR Am J Roentgenol 2006;187:1377–80 [DOI] [PubMed] [Google Scholar]
- 28.Bassett LW, Berg WA, Feig SA. Breast Imaging Reporting and Data System, BI-RADS: Mammography. 4th edn. Reston, VA: American College of Radiology, 2003 [Google Scholar]
- 29.Mendelson EB, Baum JK, Berg WA, Merritt CRB, Rubin E. Breast Imaging Reporting and Data. System, BI-RADS: Ultrasound. Reston, VA: American College of Radiology, 2003 [Google Scholar]
- 30.Reiner A, Reiner G, Spona J, Schemper M, Holzner J. Histopathologic characterization of human breast cancer in correlation with estrogen receptor status. A comparison of immunocytochemical and biochemical analysis. Cancer 1988;61:1149–54 [DOI] [PubMed] [Google Scholar]
- 31.Oken S, Mercado C, Memeo L, Hibshoosh H. Invasive ductal carcinoma with fibrotic focus: mammographic and sonographic findings with histopathologic correlation. AJR Am J Roentgenol 2005;185:490–4 [DOI] [PubMed] [Google Scholar]
- 32.Orel S, Mendonca M, Reynolds C, Schnall M, Solin L, Sullivan D. MR imaging of ductal carcinoma in situ. Radiology 1997;202:413–20 [DOI] [PubMed] [Google Scholar]
- 33.Gilles R, Zafrani B, Guinebretiere J, Meunier M, Lucidarme O, Tardivon A, et al. Ductal carcinoma in situ: MR imaging–histopathologic correlation. Radiology 1995;196:415–19 [DOI] [PubMed] [Google Scholar]
- 34.Berg W, Gilbreath P. Multicentric and multifocal cancer: whole-breast US in preoperative evaluation. Radiology 2000;214:59–66 [DOI] [PubMed] [Google Scholar]
- 35.Thomson T, Hayes M, Spinelli J, Hilland E, Sawrenko C, Phillips D, et al. HER-2/neu in breast cancer: interobserver variability and performance of immunohistochemistry with 4 antibodies compared with fluorescent in situ hybridization. Mod Pathol 2001;14:1079–86 [DOI] [PubMed] [Google Scholar]