Abstract
Objective
In the management of epithelial ovarian cancer (EOC), the identification of peritoneal deposits is the most important prognostic factor. We conducted a prospective study to evaluate the role of multidetector CT (MDCT) in identifying peritoneal deposits pre-operatively.
Methods
38 previously untreated patients (median age 50 years; range 26–70 years) were evaluated with contrast-enhanced MDCT of the abdomen and pelvis. All CT scans were performed on a four-slice MDCT scanner with thin-slice image acquisition. Multiplanar coronal, sagittal or oblique images were constructed and all images were reviewed by at least two radiologists. The extent of disease was determined and mapped for all areas of the abdomen and pelvis. CT scans were reviewed and compared with surgical findings. Peritoneal deposits and thickening were separately noted for each of the nine segments of the abdomen and pelvis (i.e. bilateral hypochondria, bilateral lumbar, bilateral iliac fossa, epigastrium, umbilical region and hypogastrium) and were mainly used to determine the accuracy of MDCT in the depiction of peritoneal carcinomatosis.
Results
Sensitivity, specificity, positive and negative predictive values and accuracy of CT in the detection of peritoneal deposits were similar to those reported in the literature. The most common anatomical sites to have peritoneal deposits were the pouch of Douglas (18 cases) and the right subdiaphragmatic region (18 cases).
Conclusion
Despite the improved scanning technology, image reconstruction and viewing ability of MDCT, its overall accuracy for the detection of peritoneal deposits is not significantly improved when compared with conventional CT; however, MDCT is useful in the assessment of disease at specific locations in the abdomen and pelvis.
Epithelial ovarian cancer (EOC) is the sixth most common cancer and the seventh cause of death from cancer in women (4.0% of cases and 4.2% of deaths) [1]. Incidence rates are highest in developed countries, with rates in these areas exceeding 9 per 100 000 (except for Japan where rates are 6.4 per 100 000). Incidence rates have been slowly increasing in many Western countries and Japan. The risk of ovarian cancer is reduced by high parity and the use of oral contraceptives. In India, age-adjusted incidence of ovarian cancer ranges from 5 to 8.3 cases per 100 000 women, according to various hospital-based cancer registries [2]. In India, the median age at the time of diagnosis is 45 years, which is about a decade less than developed countries. Most patients present with advanced disease (Stage 3 or 4).
EOC usually spreads to the abdomen via the transcoelomic route and, less commonly, to other distant sites via lymphatic and haematogenous routes. Patients are surgically staged according to the Federation of Gynaecology and Obstetrics (FIGO) system. Apart from staging, surgery also allows simultaneous tumour debulking, which has a therapeutic and prognostic significance [3]. CT has been the mainstay of pre-operative evaluation of EOC and is used to stage and assess large tumours at different locations in the pelvis and abdomen. One of the important prognostic factors in EOC is the degree of peritoneal disease; if CT could potentially identify peritoneal deposits this would help to achieve adequate cytoreduction. The demonstration of gastrointestinal tract and urinary tract involvement can also help to modify surgical plans [4]. In patients with unresectable disease, such as those with large deposits at the porta hepatis, root of mesentery, diaphragm and retroperitoneum, surgery can be avoided as optimal debulking is unlikely to be achieved in these patients [5, 6]. However, it is well known that pre-treatment prognostic stratification has little influence on clinical management. Indeed, the combination of cytoreduction and chemotherapy has only a modest effect on survival [7].
For evaluating the extent of the disease, and especially the detection of peritoneal deposits, CT has traditionally been considered unsatisfactory. Variable sensitivities of CT scans have been reported in the literature for the detection of peritoneal deposits; however, most of these studies were performed with older generation and incremental CT scanners and few used helical and multidetector CT (MDCT) scanners. We therefore conducted this prospective study to evaluate peritoneal deposits pre-operatively in ovarian cancer using MDCT.
Methods and materials
Patients with advanced EOC attending the gynaecology tumour clinic of our hospital were included in this study. Institutional ethics committee clearance was obtained and the patients were informed about this prospective study. Over a period of 23 months, from December 2004 to October 2006, 50 patients with EOC were included in the study. The diagnosis of advanced EOC was based on clinical, laboratory and/or radiological features. Patients with EOC who were considered for primary cytoreductive and staging surgery were included. Patients with recurrent ovarian cancer and distant organ metastasis were excluded.
All CT scans were performed on a four-slice MDCT scanner (Siemens Volume Zoom, Siemens, Erlangen, Germany). Approximately 750–1000 ml of diluted oral contrast was given 45 min to 1 h before the scan. Scanning was performed with 2.5 mm tube collimation after an iv bolus injection of 100 ml of non-ionic contrast material. The area of the abdomen and pelvis extending from the dome of the diaphragm to the pelvic floor was included. Delayed images of the pelvis were obtained if required. Image reconstruction was carried out with a 3 mm slice thickness at 2 mm increments. Additional contiguous images of 5 mm slice thickness were obtained for hard copy documentation. Multiplanar 5 mm slice thickness coronal images were also reconstructed using 3 mm axial images. Sagittal or oblique images were reconstructed if required on an individual basis. All images were reviewed interactively on the workstation (Magic View 300, Siemens). All CT examinations were reviewed by at least two radiologists and consensus was arrived at for subtle or doubtful abnormalities.
Contrast-enhanced MDCT scans were performed in 50 patients who fulfilled our inclusion criteria. Of these 50 patients, 11 did not undergo surgery: a few refused surgery and the remainder were lost to follow-up. On post-operative histological examination, one patient was diagnosed as having complicated dermoid cyst and was excluded from the study. Hence, 38 patients were included in the analysis. All cases were confirmed histologically to be EOC. The age of the patients ranged from 26 to 70 years (median age 50 years). CT findings obtained for the 38 patients with EOC were compared with the results of surgical findings. The duration between CT scan and surgery ranged from 1 to 26 days (mean 11 days). All the information obtained on CT was communicated to the operating surgeon before surgery.
CT and surgical findings were recorded. Peritoneal deposits and thickening were separately noted for each of the nine segments of the abdomen and pelvis (i.e. bilateral hypochondria, bilateral lumbar, bilateral iliac fossa, epigastrium, umbilical region and hypogastrium and pelvis). CT and surgical findings were noted in each patient with the main objective of assessing the accuracy of MDCT in identifying peritoneal deposits. The statistical analysis was performed using SPSS version 11.0 (Chicago, IL) and EpiInfo6 software (CDC, Atlanta, GA).
Results
The mean age of patients at presentation was 48.3 years (range 26–70 years). The majority of patients (22; 57.9%) were in the sixth and seventh decades of their lives. Serum CA-125 values ranged from 33.4–3578 units ml–1 (mean value 536.84 units ml–1). Serum CEA values ranged from 0.5–3.2 ng ml–1 (mean 1.45 ng ml–1). Serous cystadenocarcinoma was the most common histopathological subtype (31 patients; 81.5%). Other histopathological subtypes were undifferentiated carcinoma (4 patients; 10.52%) and mucinous cystadenocarcinoma (3 patients; 7.89%). The majority of patients had Stage 3 disease (76.3%); both ovaries were involved in all patients.
The detection of peritoneal deposits at different locations on CT and surgery is shown in Table 1. The accuracy of CT to demonstrate surgically confirmed peritoneal deposits at different locations is shown in Table 2. The sensitivity of CT in the detection of peritoneal deposits ranged from 33.3% to 88.9% (mean 61.58%). The sensitivity varied significantly in different areas of the abdomen: sensitivity was low (33.3%) in the umbilical and left lumbar region and high in the pelvis (80%) and epigastrium (88.9%).
Table 1. Comparison of CT with intra-operative findings in the detection of peritoneal deposits (n = 38).
| Location of peritoneal deposits | Number of patients, n (%) |
|
| CT | Surgery | |
| Right hypochondrium | 18 (47.4) | 23 (60.5) |
| Right lumbar | 7 (18.4) | 8 (21.1) |
| Right iliac | 9 (23.7) | 12 (31.6) |
| Epigastrium | 9 (23.7) | 10 (26.3) |
| Umbilical | 4 (10.5) | 6 (15.8) |
| Hypogastrium and pelvis | 18 (47.4) | 20 (52.6) |
| Left hypochondrium | 10 (26.3) | 14 (36.8) |
| Left lumbar | 2 (5.2) | 3 (7.89) |
| Left iliac | 5 (13.2) | 8 (21.1) |
Table 2. Accuracy of CT in the detection of peritoneal deposits (n = 38).
| Site of deposit or thickening | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
| Peritoneal deposits | |||||
| Right hypochondrium | 73.9 | 93.3 | 94.4 | 70.0 | 81.5 |
| Right lumbar | 75 | 96.7 | 85.7 | 93.7 | 92.1 |
| Right iliac | 58.3 | 92.3 | 77.8 | 82.8 | 81.5 |
| Epigastrium | 88.9 | 96.6 | 88.9 | 96.6 | 94.7 |
| Umbilical | 33.3 | 93.8 | 50.0 | 88.2 | 84.2 |
| Hypogastrium and pelvis | 80 | 88.9 | 88.9 | 80.0 | 84.2 |
| Left hypochondrium | 61.5 | 92.0 | 80.0 | 82.1 | 81.6 |
| Left lumbar | 33.3 | 97.1 | 50.0 | 94.4 | 92.2 |
| Left iliac | 50 | 96.7 | 80.0 | 87.9 | 86.8 |
| Peritoneal thickening/enhancement | |||||
| Right hypochondrium | 76.2 | 94.1 | 94.1 | 76.2 | 84.2 |
| Right lumbar | 50 | 91.2 | 40.0 | 93.9 | 86.8 |
| Right iliac | 66.7 | 93.1 | 66.7 | 94.3 | 86.8 |
| Epigastrium | 83.3 | 90.6 | 62.5 | 96.7 | 89.4 |
| Umbilical | 40.0 | 93.9 | 50.0 | 91.2 | 86.8 |
| Hypogastrium and pelvis | 83.3 | 95.0 | 93.8 | 86.4 | 89.4 |
| Left hypochondrium | 63.6 | 92.6 | 77.8 | 86.2 | 84.2 |
| Left lumbar | 50.0 | 97.1 | 66.7 | 94.3 | 92.1 |
| Left iliac | 33.3 | 90.6 | 40.0 | 87.9 | 81.6 |
PPV, positive predictive value; NPV, negative predictive value.
The sensitivity of CT was low for the detection of peritoneal deposits in the right lumbar umbilical and left iliac regions. The specificity for all findings was quite high, ranging from 88.9% to 97.1%. In one patient, CT demonstrated peritoneal deposits at various sites that were not seen at surgery. Morphologically, most of the lesions depicted on CT were plaque-like thickenings or showed diffuse peritoneal enhancement.
Discussion
Spiral CT is accurate in the depiction of peritoneal metastases from EOC, although sensitivity is reduced for tumour implants of 1 cm or less in diameter and for ancillary signs of peritoneal malignancy [8]. CT is also unable to detect microscopic disease (i.e. Stage 3A disease cannot be identified). Hence, CT is not a suitable modality for the accurate staging of EOC, especially in Stages 3A and 3B. Most peritoneal deposits are seen as solid nodules on CT. Diffuse thickening or enhancement of the parietal peritoneum and small bowel wall thickening or distortions are also indicators of peritoneal metastases [8]. Many factors govern the efficiency of CT in the detection of peritoneal deposits, such as diameter and density of the nodule, the presence or absence of ascites, opacification of the bowel with contrast, slice thickness of the CT scan and the technology and resolution of the CT scanner. The major limitation of CT has been its reduced sensitivity in the detection of deposits less than 1 cm in diameter [8].
To evaluate the accuracy of CT in the detection of peritoneal metastases, peritoneal deposits and thickening were separately noted for each of the nine segments of the abdomen and pelvis. The pelvis was included in the lower abdominal segments for this purpose. An alternative approach would have been to document the peritoneal nodules or deposits in specific anatomical compartments, such as the pouch of Douglas, paracolic gutters, subdiaphragmatic spaces, and so on. We chose not to adopt this method because deposits at unusual sites could have been difficult to accurately localise on CT and to confirm surgically.
It was technically difficult to count the number of peritoneal deposits on CT scans and during surgery. Hence, while tabulating the MDCT and surgical findings, the presence or absence of peritoneal deposits in the respective segments of the peritoneal cavity was considered irrespective of their actual number; for example, the presence of peritoneal deposits in the right hypochondrium on CT was considered correct when single or multiple deposit(s) were seen intra-operatively in the same compartment.
The low sensitivity of CT for the detection of peritoneal deposits in the right lumbar umbilical and left iliac regions relates, at least in part, to the small size of the metastases; in some cases they were manifested as tumour “seedlings” measuring only a few millimetres in diameter. Morphologically, most of the lesions depicted on CT were plaque-like thickenings or showed diffuse peritoneal enhancement. In other cases, the false-positive CT demonstration of some peritoneal nodules could represent unopacified bowel loops.
The sensitivity of conventional incremental or single-detector helical CT for detecting small peritoneal metastases is not very high, primarily owing to technical considerations. If section thickness exceeds lesion size, the lesion might not be optimally seen owing to partial volume averaging. With conventional and single-detector spiral CT scanners, it was not technically possible to obtain thin sections through the relatively large volume of the abdomen and pelvis. In most earlier studies, 10 mm thick sections were obtained either contiguously or at 10 mm intervals, and reported sensitivities of CT for the detection of peritoneal deposits were 40–79% [8–10]. With the advent of new improved spiral CT scanners it is possible to obtain thin slices in a single breath-hold, thereby eliminating errors of respiratory misregistrations. Using spiral CT, the Radiology Diagnostic Oncology Group has reported a CT sensitivity of 92% for the detection of peritoneal metastases [11].
MDCT has further improved the efficacy of CT. With MDCT thin sections can be obtained over a large volume of tissue, which improves the sensitivity of CT in detecting peritoneal carcinomatosis and could allow the detection of subcentimetre implants [12]. In addition, thin sections can be used to generate multiplanar sagittal and coronal images, which can depict the disease as it would appear at surgery and show the relationship of masses to viscera and blood vessels. Interactive review on a workstation can be used to distinguish bowel from implants. Axial oblique and coronal images can be used to evaluate the region of the uterosacral ligaments and pelvic sidewall, as has been described previously [10, 13].
For the detection of peritoneal deposits on CT, most investigators have reported sensitivities ranging from 0% to 93% and specificities of 62–100% (Table 3). The comparatively lower sensitivities in earlier reports can be attributed to older generation conventional (incremental) CT scanners, which had long imaging times, were unable to operate with thin slices and had inferior resolution. In a quest to improve the sensitivity of CT for the detection of small deposits, investigators have used various techniques such as the creation of pneumoperitonium or instillation of iodinated contrast before the CT scan [14, 15]; however, these approaches were too invasive to find use in routine clinical practice.
Table 3. Comparison of published reports for the detection of peritoneal deposits.
| Study | Number of patients | Sensitivity (%) | Specificity (%) | PPV | NPV | Accuracy |
| Buy et al (1988) [16] | 38 | 63 | 100 | 100 | 52 | 74 |
| Jacquet et al (1993) [12] | 45 | 79 | – | – | – | – |
| Kurtz et al (1999) [11] | 280 | 92 | – | – | – | – |
| Coakley et al (2002) [8] | 64 | 25–93 | 78–96 | 88–97 | 18–88 | – |
| Pannu et al (2003) [10] | 17 | 50–78.5 (excluding stomach and spleen) | 90–100 | – | – | >80 |
| Present study | 38 | 33.3–88.9 | 92–97.1 | 75.2 | 88.2 | 87.15 |
PPV, positive predictive value; NPV, negative predictive value.
The mean sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for CT in our study were 61.6%, 94.3%, 75.2% and 88.2%, respectively. The overall accuracy of CT for the detection of peritoneal deposits was high (87.15%). The most common sites of abdominal deposits in our series were the right subdiaphragmatic space (18 patients), greater omentum (20 patients) and the pouch of Douglas (18 patients), as seen on CT. In a study of 38 patients, Buy et al [16] also reported these three sites as the most common locations for peritoneal deposits.
Only Pannu et al [10] have specifically assessed the sensitivity of MDCT in the assessment of peritoneal deposits in EOC patients; these authors reported the sensitivity of MDCT to be in the range of 50–78.5% (the sensitivity was 100% for stomach and spleen involvement in two patients). Both this study and ours show no significant increase in the sensitivity of CT when MDCT is used for this purpose, as compared with other studies using conventional or helical CT scanners. It appears that the very small peritoneal deposits that were frequently encountered in our patients will continue to elude detection by CT.
Nevertheless, we found MDCT to be advantageous in specific locations: the sensitivity in certain sites, such as the diaphragm, paracolic gutters and pelvis, was high in our study. In the pelvis, sagittal images allow assessment of the vaginal cuff, cul-de-sac, peritoneal surface of the bladder and rectosigmoid. Axial oblique and coronal images can be used to evaluate the region of the uterosacral ligaments and pelvic side wall. The number and location of surface lesions of the liver and spleen can be mapped and the extent of omental disease demonstrated on coronal images (Figure 1). The paracolic gutters can also be evaluated on coronal images for small implants near the bowel surface (Figure 2). The entire length of the diaphragm can be displayed on sagittal and coronal images to evaluate for minimal nodularity and plaque-like thickening (Figure 3). Review of both the axial and multiplanar images contributed to the sensitivity and specificity of the study.
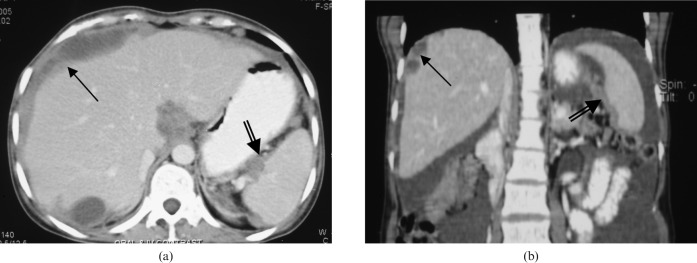
Figure 1.
Subdiaphragmatic peritoneal deposits and splenic hilum deposits. Subdiaphragmatic deposits in a 37-year-old woman with epithelial ovarian cancer. (a) Diffuse right subdiaphragmatic plaque-like peritoneal deposits (single black arrow) and splenic hilum deposits (double black arrow) seen on axial section. (b) These deposits are seen to better advantage in coronal section.
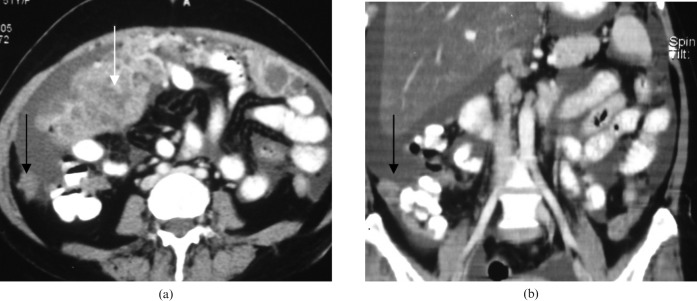
Figure 2.
Peritoneal deposits in the paracolic gutter. Peritoneal deposits in the paracolic gutter in a 47-year-old woman with epithelial ovarian cancer. (a) Axial CT image showing peritoneal deposits at the right paracolic gutter (black arrow); omental deposits are also seen (white arrow). (b) Coronal images showing the same.
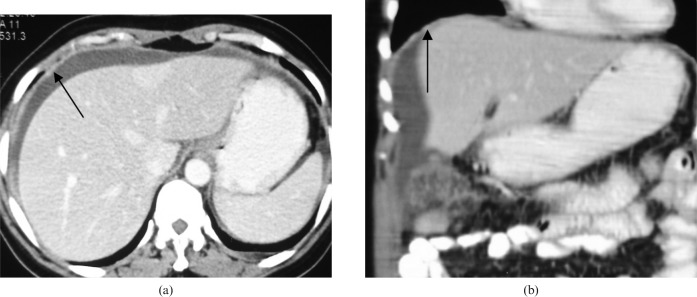
Figure 3.
Diffuse plaque-like peritoneal deposits. A 55-year-old woman with a known case of epithelial ovarian cancer showing diffuse deposits. (a) Axial scan showing diffuse subdiaphragmatic peritoneal thickening and enhancement (black arrow). (b) The coronal image shows better delineation of the whole area.
Metastases can become implants on either the diaphragmatic surface of the peritoneum or the peritoneum overlying the surface of the liver. Because it is difficult to directly visualise the subdiaphragmatic space through the standard midline incision, CT demonstration of deposits at these sites in EOC patients should prompt a thorough examination of the diaphragmatic surface during surgery. The greater omentum, mainly the infracolic omentum, is one of the sites most commonly affected by ovarian cancer; in our study, this location was shown to be affected in 20 patients on CT scan. Intra-operatively, omental deposits were seen in 24 patients. Apart from the microscopic nature of omental disease, other causes for false-positive CT findings in our study included the presence of omental deposits close to the primary tumour and an inability to differentiate the deposits from unopacified bowel loops. In most cases in our series, the lesions in the pouch of Douglas were 1 cm or more in diameter, which could explain the high rate of detection with CT. Lesions in some parts of the peritoneum, such as the serosal surface of the small bowel, and deposits in the left lumbar and bilateral iliac regions were difficult to identify.
Although the detection of peritoneal implants will not change the therapeutic approach, CT is useful in the pre-operative diagnosis of peritoneal carcinomatosis and for the detection of lesions not seen during surgery. Buy et al [16] observed a high specificity and PPV of CT for the diagnosis of peritoneal metastases.
Like other investigators, we also observed a high specificity (94.3%) and PPV (75.2%) for the diagnosis of peritoneal metastases. This finding presumably reflects the fact that non-cancerous mimics of peritoneal malignancy are rare. Despite this, two CT examinations were interpreted by all readers as highly suggestive of peritoneal metastases; however, no peritoneal metastases were found at surgery. The basis of these false-positive CT findings is unknown; however, it is theoretically possible that small-volume disease might be overlooked at surgery. Patient outcome rather than surgical findings may ultimately be a more desirable standard of reference for imaging studies [14]. Experienced gynaecological oncologists operated on all of the patients in our study and CT features were correlated with intra-operative as well as pathological findings, when available.
There are certain limitations of our study, as the number of patients included (n = 38) is relatively small to make strong recommendations. Although we used recent technology, MDCT for the pre-operative evaluation of patients with EOC showed no statistically significant improvement in sensitivity of CT for the detection of peritoneal disease. However, as for the limited experience reported by others, MDCT was found to be useful for the better depiction and demarcation of the disease, especially at complex anatomical sites. Recently, four-slice MDCT technology has been superseded by 64-slice (and above) technology; thus, future studies could shed further light in this area.
Conclusions
The sensitivity, specificity, PPV, NPV and accuracy of CT for the detection of peritoneal deposits were found to be similar to those reported previously. Despite the improved scanning, image reconstruction and viewing ability of MDCT, the overall accuracy of CT for the detection of peritoneal deposits is not significantly improved. MDCT is useful, however, for the assessment of disease at specific locations in the abdomen and pelvis. Further studies with more recent versions of MDCT might give the final word on this issue.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin 2005;55:74–108 [DOI] [PubMed] [Google Scholar]
- 2.Mathur SR, Verma K. Epidemiology of ovarian cancer. Ind J Med Paed Oncology 2003;24:15–23 [Google Scholar]
- 3.Ascher SM, Imaoka I, Jha RC. Cancer of the adnexal organs. In: Bragg DG, Rubin P, Hricak H, editors. Oncologic imaging, 2nd edition. Philadelphia: WB Saunders Company, 2002: 549–74 [Google Scholar]
- 4.Shamsunder S. Clinical evaluation of a patient with suspected epithelial ovarian cancer. Ind J Med Paed Oncology 2004;25:10–12 [Google Scholar]
- 5.Cookley FV. Staging of ovarian cancer: role of imaging. Radiol Clin N Am 2002;40:609–36 [DOI] [PubMed] [Google Scholar]
- 6.Shanta V. Perspective in malignant ovarian tumors. Ind J Med Paed Oncology 2004;25:4–14 [Google Scholar]
- 7.Harris M, Gore M. Part I: Chemotherapy for epithelial ovarian cancer-treatment at first diagnosis. Lancet Oncol 2002;3:529–36 [DOI] [PubMed] [Google Scholar]
- 8.Coakley FV, Choi PH, Gougoutas CA, Pothuri B, Venkatraman E, Chi D., et al Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology 2002;223:495–9 [DOI] [PubMed] [Google Scholar]
- 9.Pannu HK, Bristow RE, Montz FJ, Fishman EK. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics 2003;23:687–701 [DOI] [PubMed] [Google Scholar]
- 10.Pannu HK, Horton KM, Fishman EK. Thin section dual phase multidetector row computed tomography detection of peritoneal metastases in gynecological cancers. J Comput Assist Tomogr 2003;27:333–40 [DOI] [PubMed] [Google Scholar]
- 11.Kurtz AB, Tsimikas JV, Tempany CMC, Hamper UM, Arger PH, Bree RL, et al. Diagnosis and staging of ovarian cancer: comparative values of Doppler and conventional US, CT and MR imaging correlated with surgery and histopathological analysis – report of the radiology diagnostic oncology group. Radiology 1999;212:19–27 [DOI] [PubMed] [Google Scholar]
- 12.Jacquet P, Jelink JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 1993;72:1631–6 [DOI] [PubMed] [Google Scholar]
- 13.Halvorsen RA, Panushka C, Oakley GJ, Letourneau JG, Adcock LL. Intraperitoneal contrast material improves the CT detection of peritoneal metastases. AJR 1991;157:37–40 [DOI] [PubMed] [Google Scholar]
- 14.Bristow RE, Duska LR, Lambrou LR, Fishman EK, O'Neill MJ, Trimble EL, et al. A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer 2000;89:1532–40 [DOI] [PubMed] [Google Scholar]
- 15.Alves FC, Goncalo M, Abraul E, Pinto E, Oliveira C, Ramos VI. Induced pneumoperitoneum in CT evaluation of peritoneal carcinomatosis. Abdom Imaging 1995;20:52–5 [DOI] [PubMed] [Google Scholar]
- 16.Buy JN, Moss AA, Ghossain MA, Sciot C, Malbec L, Vadrot D, et al. Peritoneal implants from ovarian tumours: CT findings. Radiology 1988;169:691–4 [DOI] [PubMed] [Google Scholar]