Abstract
We have previously shown that stimulation of the preganglionic cervical sympathetic trunk leads to an acute increase in tyrosine hydroxylase (TyrOHase) activity in the rat superior cervical ganglion. This increase appears to be mediated in part by acetylcholine and in part by a second neurotransmitter. As a first step in an attempt to determine the identity of this noncholinergic transmitter, we have examined the ability of a number of neuropeptides to increase ganglionic TyrOHase activity in vitro. Secretin and vasoactive intestinal peptide (VIP) both stimulated TyrOHase activity, whereas angiotensin II, bombesin, bradykinin, cholecystokinin octapeptide, glucagon, insulin, luteinizing hormone-releasing hormone, [D-Ala2, Met5]enkephalinamide, motilin, neurotensin, somatostatin, and substance P produced no effects. Secretin produced a significant increase in TyrOHase activity at 1 nM and a maximal elevation at 0.1 μM. VIP produced a significant increase at 0.1 μM and a near maximal effect at 10 μM. Although secretin was about 2 orders of magnitude more potent than VIP, it produced a significantly smaller maximal increase in enzyme activity. Incubation of ganglia with both secretin (10 μM) and VIP (10 μM) produced an increase in TyrOHase activity that was not significantly different from that produced by VIP alone. The stimulatory effects of secretin and VIP were reversible within minutes after removal of the peptides. Neither incubation of intact ganglia with the cholinergic antagonists hexamethonium and atropine nor prior decentralization of ganglia altered the response to the peptides. Thus, the data demonstrate that secretin and VIP acutely increase TyrOHase activity in the superior cervical ganglion and suggest that they produce this effect by acting directly on ganglionic neurons. It remains to be determined whether secretin or VIP or a related peptide is released during preganglionic nerve firing and whether one or more of these peptides is responsible for the noncholinergic elevation of TyrOHase activity produced by preganglionic nerve stimulation.
Keywords: neuropeptide, catecholamine, sympathetic nervous system, gastrointestinal hormone
Full text
PDF
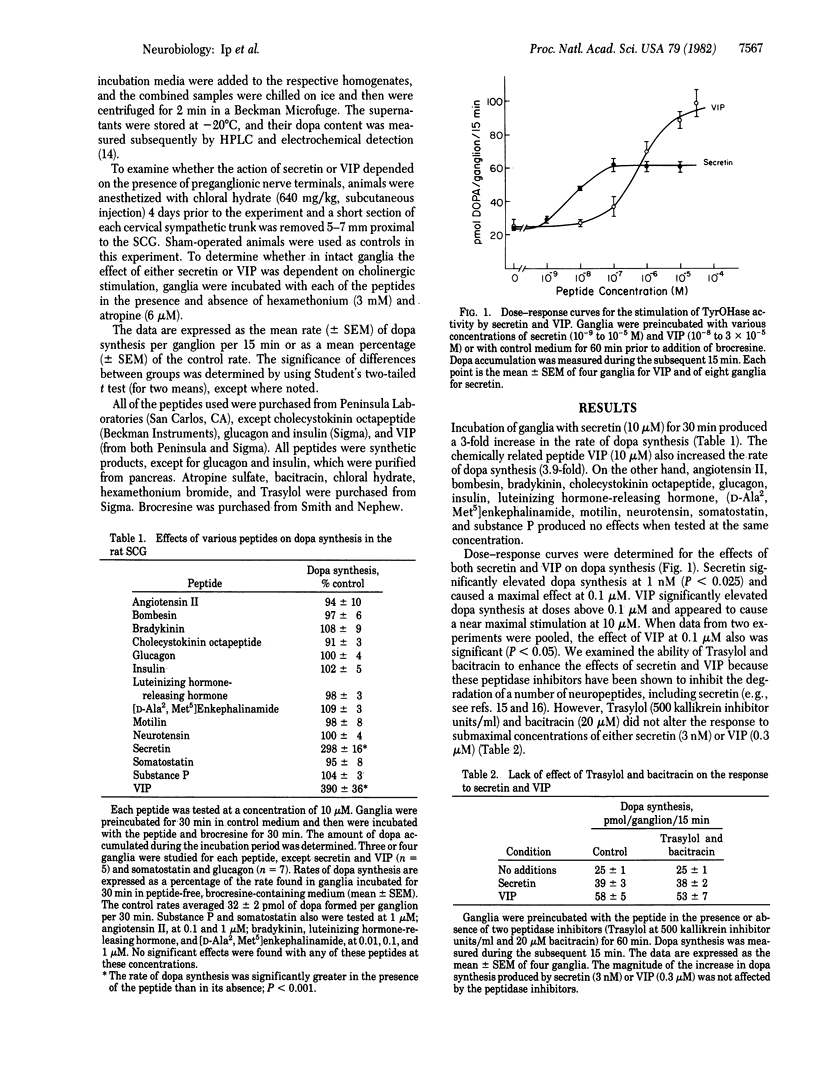
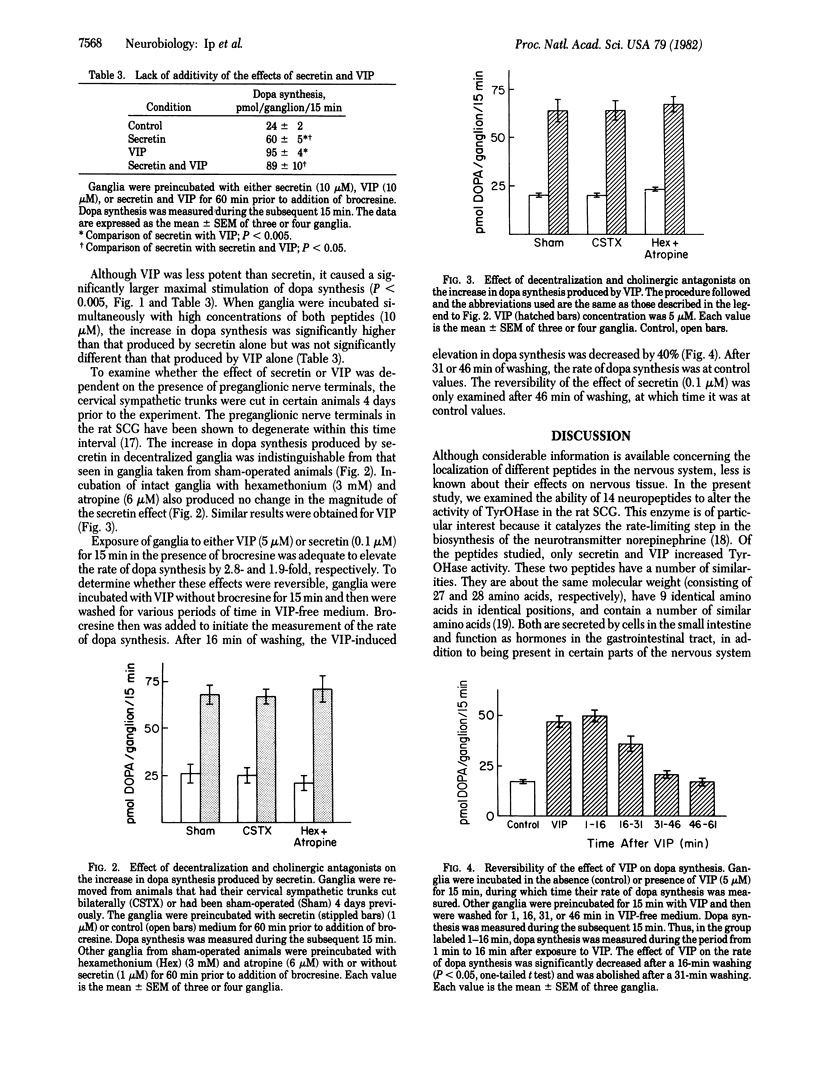

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe J. H., Libet B. Orthodromic production of non-cholinergic slow depolarizing response in the superior cervical ganglion of the rabbit. J Physiol. 1981 Nov;320:333–346. doi: 10.1113/jphysiol.1981.sp013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe J. P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. J Biol Chem. 1976 Aug 10;251(15):4629–4634. [PubMed] [Google Scholar]
- Desbuquois B., Krug F., Cuatrecasas P. Inhibitors of glucagon inactivation. Effect on glucagon--receptor interactions and glucagon-stimulated adenylate cyclase activity in liver cell membranes. Biochim Biophys Acta. 1974 Mar 20;343(1):101–120. doi: 10.1016/0304-4165(74)90242-6. [DOI] [PubMed] [Google Scholar]
- Erny R. E., Berezo M. W., Perlman R. L. Activation of tyrosine 3-monooxygenase in pheochromocytoma cells by adenosine. J Biol Chem. 1981 Feb 10;256(3):1335–1339. [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Elde R., Schultzberg M., Goldstein M., Luft R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3587–3591. doi: 10.1073/pnas.74.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Schultzberg M., Goldstein M., Nilsson G. On the occurrence of substance P-containing fibers in sympathetic ganglia: immunohistochemical evidence. Brain Res. 1977 Aug 19;132(1):29–41. doi: 10.1016/0006-8993(77)90704-1. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Ikeno T., Dickens G., Lloyd T., Guroff G. The receptor-mediated activation of tyrosine hydroxylation in the superior cervical ganglion of the rat. J Neurochem. 1981 May;36(5):1632–1640. doi: 10.1111/j.1471-4159.1981.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. Further evidence for peptidergic transmission in sympathetic ganglia. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5008–5012. doi: 10.1073/pnas.77.8.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Mutt V., Carlquist M., Tatemoto K. Secretin-like bioactivity in extracts of porcine brain. Life Sci. 1979 Nov 12;25(20):1703–1707. doi: 10.1016/0024-3205(79)90472-7. [DOI] [PubMed] [Google Scholar]
- Nishi S., Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J Neurophysiol. 1968 Jan;31(1):109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- O'Donohue T. L., Charlton C. G., Miller R. L., Boden G., Jacobowitz D. M. Identification, characterization, and distribution of secretin immunoreactivity in rat and pig brain. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5221–5224. doi: 10.1073/pnas.78.8.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G., Field P. M., Ostberg A. J., Iversen L. L., Zigmond R. E. A quantitative ultrastructural and biochemical analysis of the process of reinnervation of the superior cervical ganglion in the adult rat. Brain Res. 1974 May 10;71(1):1–16. doi: 10.1016/0006-8993(74)90187-5. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Terenius L., Elfvin L. G., Lundberg J. M., Brandt J., Elde R. P., Goldstein M. Enkephalin immunoreactive nerve fibres and cell bodies in sympathetic ganglia of the guinea-pig and rat. Neuroscience. 1979;4(2):249–270. doi: 10.1016/0306-4522(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Bowers C. W. Influence of nerve activity of the macromolecular content of neurons and their effector organs. Annu Rev Physiol. 1981;43:673–687. doi: 10.1146/annurev.ph.43.030181.003325. [DOI] [PubMed] [Google Scholar]



