Abstract
Objective
To assess the accuracy contrast-enhanced ultrasound (CEUS) in bladder cancer detection using transurethral biopsy in conventional cystoscopy as the reference standard and to determine whether CEUS improves the bladder cancer detection rate of baseline ultrasound.
Methods
43 patients with suspected bladder cancer underwent conventional cystoscopy with transurethral biopsy of the suspicious lesions. 64 bladder cancers were confirmed in 33 out of 43 patients. Baseline ultrasound and CEUS were performed the day before surgery and the accuracy of both techniques for bladder cancer detection and number of detected tumours were analysed and compared with the final diagnosis.
Results
CEUS was significantly more accurate than ultrasound in determining presence or absence of bladder cancer: 88.37% vs 72.09%. Seven of eight uncertain baseline ultrasound results were correctly diagnosed using CEUS. CEUS sensitivity was also better than that of baseline ultrasound per number of tumours: 65.62% vs 60.93%. CEUS sensitivity for bladder cancer detection was very high for tumours larger than 5 mm (94.7%) but very low for tumours <5 mm (20%) and also had a very low negative predictive value (28.57%) in tumours <5 mm.
Conclusion
CEUS provided higher accuracy than baseline ultrasound for bladder cancer detection, being especially useful in non-conclusive baseline ultrasound studies.
Carcinoma of the urinary bladder is the most common malignancy of the urinary tract that must be ruled out in patients with haematuria with negative upper urinary tract findings [1]. Cystoscopy remains the most sensitive method of detecting bladder cancer, but has several limitations: it is an invasive procedure; it is uncomfortable in some patients and it requires sedation or anaesthesia. Conventional ultrasound (US) is one of the imaging techniques used to screen for bladder cancer, but with variable accuracy. The best results are obtained using the latest equipment and new imaging tools such as three-dimensional (3D) ultrasound [2-5]. Angiogenesis is essential to allow growth of malignancies, and the detection of tumoural neovascularisation is one of the keys of imaging modalities to achieve a definite diagnosis. CT and MRI are accurate techniques for bladder cancer detection when they are performed with the injection of intravascular contrast agents. Detection relies on the identification of bladder cancer neovascularisation and recent studies have shown high accuracy with both techniques [6,7]. The introduction of microbubble contrast agents and the development of contrast-specific software have increased the value of ultrasound in the field of oncology [8,9]. Ultrasound contrast agents are strictly intravascular and are very sensitive in revealing tumour microvascularisation, helping in the detection and characterisation of malignancies [10-13]. Recently, the behaviour of bladder cancer has been described after the administration of ultrasound contrast agent, and its diagnosis relies on the detection of hypervascular wall bladder thickening [14].
The aim of our study was to retrospectively assess the value of contrast-enhanced ultrasound (CEUS) in bladder cancer detection in a selected high-risk group of patients using transurethral biopsy in conventional cystoscopy as the reference standard and to determine whether CEUS improves the bladder cancer detection rate of baseline ultrasound.
Methods
Patients
The study was approved by the Ethical Committee of our institution and informed consent was obtained from all study participants. From September 2007 to April 2008, about 160 bladder tumours were diagnosed at our hospital. From those patients with bladder tumours, we selected a subgroup who were referred to the operating theatre of the Department of Urology for conventional cystoscopy under general anaesthesia. The selection of this subgroup was at random, since it depended only on the availability of the radiologists of the Radiology Department to perform the ultrasound study on the day prior to the surgical procedure. 43 non-consecutive patients (ranging in age from 29 to 89 years, mean age 71.4±12.5 years) were included in this study. Flexible cystoscopy was indicated because of the presence of macroscopic haematuria (25 cases, 58.1%), surveillance after transurethral resection (14 cases, 32.6%) and incidental sonographic findings (4 cases, 9.3%). When cystoscopically suspicious lesions were detected, transurethral biopsies were performed and the final diagnoses were obtained by histology.
All patients underwent blind baseline ultrasound and CEUS the day before surgery. The urologists who performed the cystoscopy did not know the results of the previous ultrasound studies.
Imaging techniques
All ultrasound studies were performed by one of the radiologists of the urogenital department who had at least 8 years of experience in ultrasound using Sequoia 512 equipment (Siemens Sequoia, Mountain View, CA). First of all, a baseline ultrasound of the total bladder in fundamental mode, using greyscale, was performed with a multifrequency 4C1 convex array probe in order to identify bladder wall thickening or bladder lesions. Patients were instructed not to void for at least 1 h before the ultrasound study and were not scanned until they had the sensation of a full bladder in order to ensure good bladder distension. After the baseline study, dynamic CEUS of the whole bladder was performed using the specific contrast software Cadence contrast pulse sequencing technology (CPS; Siemens, Erlangen, Germany), which allows real-time evaluation of contrast agents with minimum bubble destruction at low mechanical index power levels. CPS was performed with the same convex array probe, with a double focus in the area of interest, using the following settings: insonating frequency, 3 MHz; acoustic power, −75 to −90 dB; frame rate, 17–20. A low mechanical index (<0.2) was used in order to avoid microbubble disruption. CEUS studies were performed after the administration of 2.4 ml of Sonovue as a bolus using a 21-gauge peripheral intravenous cannula followed by a 5-ml saline flush.
Bladder wall enhancement was studied for up to 3 min. Images and cineloops of baseline, arterial and venous phases were selected and stored on digital cineloops by the same radiologists who performed the ultrasound studies for the off-line analysis after changing the names of patients using a correlative code.
Imaging analysis
Two radiologists, each with at least 5 years of experience in interpreting CEUS studies, independently interpreted the acquired images and cineloops of the bladder off-line at least 3 weeks after the recruitment of patients. Both radiologists were blinded to the final diagnosis, but they knew that all patients were referred to cystoscopy because of a diagnosis of bladder cancer by flexible cystoscopy. All bladders were reviewed before and after contrast agent injection. Reviewers were asked to identify, record the presence, number and size and classify the location of bladder tumours using the same classification of five segments used by the urologists on cystoscopy in our centre (right lateral wall, left lateral wall, anterior wall–domus, fundus, posterior wall–floor). Ultrasound diagnoses of all discrepant studies were established by consensus. In the baseline study, bladder cancer was defined as the presence of focal bladder wall thickening or the presence of focal masses protruding into the bladder lumen. From the CEUS study, bladder cancer was defined as the presence of focal hyperenhanced wall thickening or enhancing masses that protrude into the bladder lumen. A 3-point scale was used for lesion detection using baseline ultrasound and CEUS (1, absence of tumour; 2, presence of bladder cancer; 3, results uncertain). The results of baseline ultrasound and CEUS were compared with the findings of conventional cystoscopy and biopsy, which were considered the reference standard.
Statistical analysis
SPSS version 14.0 (SPSS, Chicago, IL) was used for statistical analysis. Baseline characteristics of the patients and bladder tumours are expressed as mean±SE. The relationship between the classification of cancer or absence of cancer at baseline, CEUS and the final diagnosis were analysed with the Fisher exact test. The overall sensitivity, specificity, accuracy, positive predictive value and negative predictive value for bladder cancer detection using ultrasound and CEUS were analysed by number of patients and number of lesions. For the estimation of sensitivity, uncertain results were classified as negative for cancer. For the estimation of specificity, uncertain results were classified as positive for cancer. A value of p<0.05 was considered statistically significant.
Results
43 non-consecutive patients (36 men, 7 women) ranging in age from 29 to 89 years (mean age 71.4±12.5 years) sent to the operating theatre to undergo cystoscopy owing to suspected bladder cancer were finally included in the study. Reference standard tests (cystoscopy and biopsy) revealed 64 bladder cancers in 33 out of 43 patients. All cancers except one were transitional cell carcinomas. The other was an undifferentiated carcinoma. Of the 10 patients without bladder cancer, absence of bladder disease was confirmed by cystoscopy and biopsies in 7. Biopsy of the other three patients showed chronic inflammatory bladder wall changes (two cases) and a papillary hyperplasia (one case). Of the 33 patients with bladder cancer, the number of lesions in each bladder detected by conventional cystoscopy was 1 in 22 patients, and multifocal in 11 patients (range 2–7 lesions), with a total of 64 bladder lesions. The size of the bladder cancer lesions ranged from 0.3 to 7 cm, with a mean size of 1.34±0.35 cm (1 tumour was not measured because of diffuse occupation of the bladder). 39 tumours were larger than 5 mm and the other 25 were 5 mm or less.
US/CEUS tumour detection
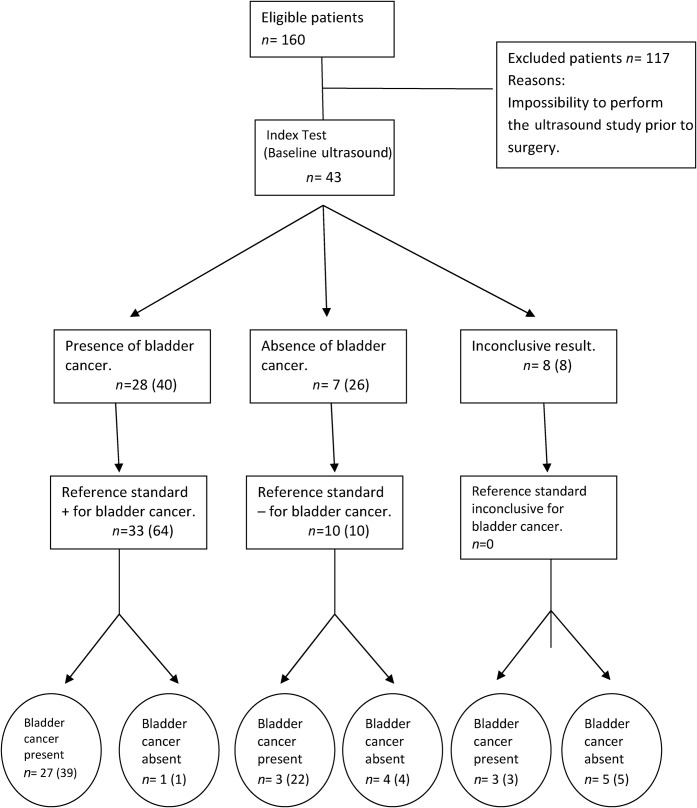
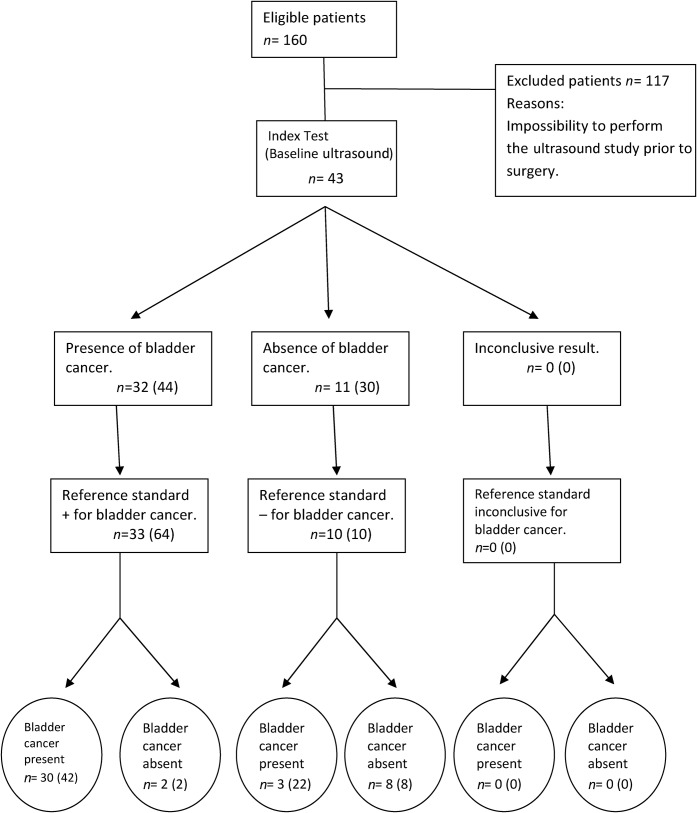
All US/CEUS studies were technically good and considered suitable for diagnosis. Only one dose of contrast agent was necessary in all patients. Figures 1 and 2 summarise the results in the format of a Standards for Reporting of Diagnostic Accuracy (STARD) flow diagram [15]. Baseline ultrasound and CEUS were able to detect tumours from 5 mm in size (Figure 3). Following the established criteria, the baseline ultrasound suspicion was of absence of tumour in 7 patients, presence of bladder cancer in 28 patients and uncertain results in 8 patients. Causes of uncertain ultrasound results were presence of bladder clots that could not be differentiated from bladder cancer (two cases) and doubtful irregularity of the bladder wall without polypoid mass (six cases) (Figure 4). The final diagnosis of uncertain cases was absence of cancer in five patients (included as ultrasound false positives in our analysis) and presence of cancer in three patients (included as false negatives in our analysis). CEUS suspicion was of absence of tumour in 11 patients, presence of bladder cancer in 32 patients and uncertain results in 0 patients. All bladder cancers identified with baseline ultrasound were also identified with CEUS. The accuracy of baseline ultrasound in bladder cancer detection per patient was 72.09% (31/43 patients), with a sensitivity of 81.81% (27/33), specificity of 40% (4/10), positive predictive value of 81.81% (27/33) and negative predictive value of 40% (4/10) (Figure 1). By contrast, the accuracy of CEUS to detect bladder cancer per patient significantly increased to 88.37% (38/43 patients), with a sensitivity of 90.9% (30/33), specificity of 80% (8/10), positive predictive value of 93.75% (30/32) and negative predictive value of 72.72% (8/11) (Figures 2 and 5). All bladder cancers identified with baseline ultrasound were identified with CEUS. Furthermore, CEUS was able to rule out the presence or absence of bladder cancer in 87.5% (7/8) of uncertain baseline US cases, with the detection of 3 bladder cancers not correctly identified by baseline ultrasound. On analysing the accuracy of the technique according to the number of bladder cancers detected, the accuracy of baseline ultrasound was 58.1% (43/74) with a sensitivity of 60.93% (39/64), specificity of 40% (4/10), positive predictive value of 86.6% (39/45) and negative predictive value of 13.79% (4/29) (Figure 1). By contrast, the accuracy of CEUS according to the number of bladder cancers increased to 67.56% (50/74) with a sensitivity of 65.62% (42/64), specificity of 80% (8/10), positive predictive value of 95.45% (42/44) and negative predictive value of 26.66% (8/30) (Figure 2).
Figure 1.
Flow diagram of baseline ultrasound based on Standards for Reporting of Diagnostic Accuracy. Two parameters (presence or absence of bladder cancer and presence or absence with respect the total number of bladder cancers (in parentheses) were evaluated in all patients.
Figure 2.
Flow diagram of contrast-enhanced ultrasound based on Standards for Reporting of Diagnostic Accuracy. Two parameters (presence or absence of bladder cancer and presence or absence with respect to the total number of bladder cancers (in parentheses) were evaluated in all patients.
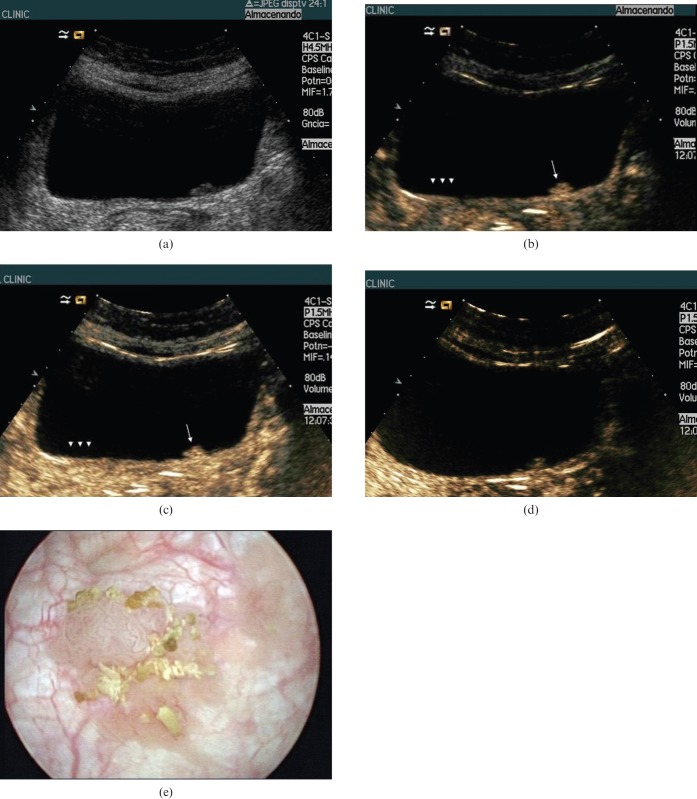
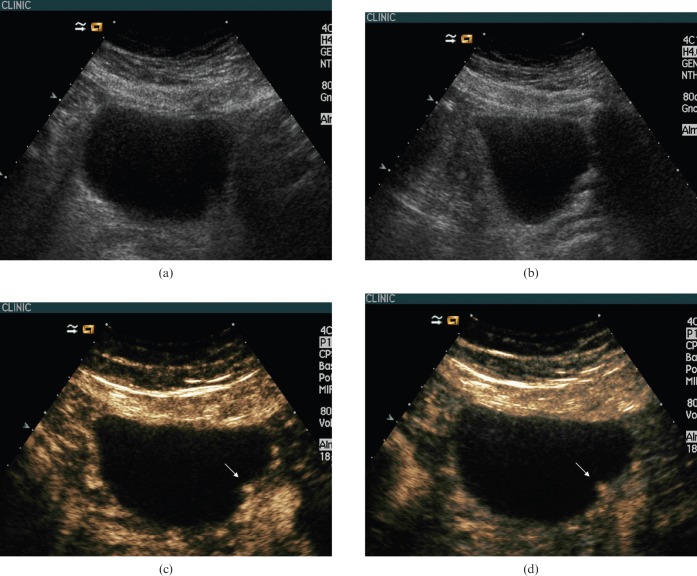
Figure 3.
62-year-old man with bladder cancer. (a) Greyscale baseline ultasound (US) of the bladder showed a 1 cm polypoid mass on the left posterior wall of the bladder. (b) Contrast-enhanced (CE) ultrasound at 22 s showed early enhancement of the polypoid lesion (arrow). Enhancement of the normal wall bladder (cap arrows) is almost imperceptible in the early arterial phase. (c) CEUS at 40 s showed homogeneous enhancement of the polypoid lesion (arrow). Enhancement of the normal wall bladder (cap arrows) is very tiny throughout the arterial and venous phases (Figure 1d) and cannot be differentiated from the microvascularisation of the perivesical tissue. (d) CEUS at 120 s showed faint enhancement of the polypoid lesion. Enhancement of the bladder wall is also faint at this time. (e) Cystoscopy confirmed the existence of a bladder wall tumour. Diagnosis by biopsy (not shown) was urothelial bladder cancer.
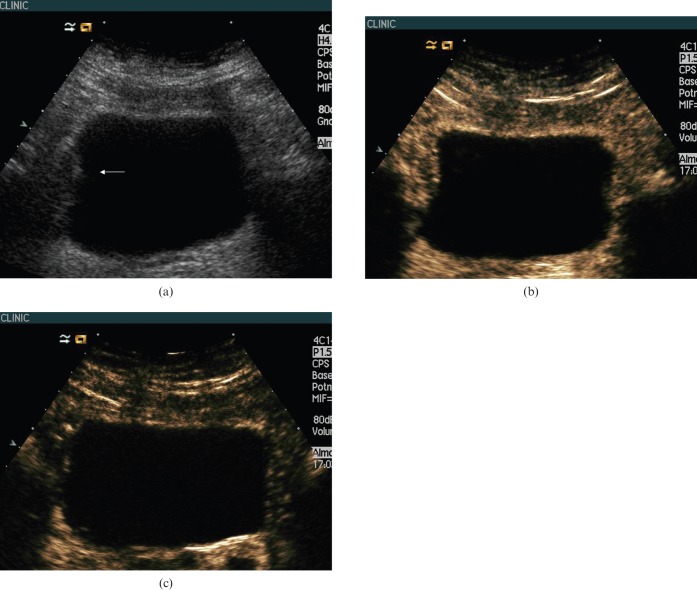
Figure 4.
69-year-old man with suspicion of bladder cancer. (a) Baseline ultrasound (US) showed irregularity of the right bladder wall that was suggestive but not conclusive of bladder cancer and classified as uncertain. (b) Contrast-enhanced (CE) ultrasound at 42 s showed homogeneous enhancement of the bladder wall without focal masses, suggesting the absence of a bladder tumour. (c) CEUS at 135 s did not show focal enhancing masses. Cystoscopy and bladder wall biopsies confirmed the absence of bladder tumour.
Figure 5.
74-year-old man with bladder cancer. (a) Axial and (b) sagittal images of baseline ultrasound (US) that did not detect bladder wall abnormalities. (c) Contrast-enhanced (CE) ultrasound at 90 s showed a homogeneous enhancing mass of 5 mm of the left bladder wall (arrow). (d) Persistent enhancement of the small tumour was detected at 140 s (arrow). Urothelial bladder cancer was confirmed by cystoscopy and resection.
There were only two false positive CEUS findings for bladder cancer. The first was a patient with a growth of the prostate mimicking a bladder tumour and the other was a papillary hyperplasia, considered to be a precursor lesion of bladder neoplasm by the World Health Organization and the International Society of Urological Pathologists [16], which was indistinguishable from bladder cancer by both CEUS and conventional cystoscopy. The false negative CEUS findings for bladder cancer were 22 bladder cancers detected on cystoscopy. 20 of these 22 were <5 mm and were located at the left lateral wall (n = 3), right lateral wall (n = 3), domus–anterior wall (n = 3), fundus (n = 5), or posterior bladder wall–floor (n = 7), 2 of them at the bladder neck. Only 2 sessile tumours larger than 5 mm (a 15-mm tumour at the bladder fundus and a 10-mm tumour at the left lateral wall) were missed. Therefore, CEUS sensitivity for bladder cancer detection was significantly higher for tumours larger than 5 mm, with a very low sensitivity for bladder cancer ≤5 mm of 20% (5/25) and a negative predictive value of 28.57% (8/28), which rose to a sensitivity of 94.87% (37/39) and a negative predictive value of 80% (8/10) for bladder cancer >5 mm (Figure 6).
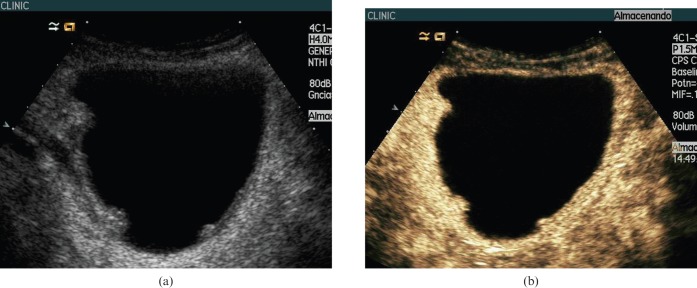
Figure 6.
62-year-old man with multiple bladder cancers. (a) Baseline ultasound (US) showed three polypoid lesions on the lateral bladder walls larger than 5 mm. (b) Contrast-enhanced (CE) ultrasound at 19 s showed the same number of polypoid lesions of different sizes extending into the bladder lumen. At cystoscopy two more lesions smaller than 5 mm, not identified using ultrasound or CEUS, were detected.
Discussion
This study demonstrates the high bladder cancer detection rate of CEUS, which had a very high sensitivity for the presence of bladder cancer per patient (90.9%). Tumoural neovascularisation can be evaluated using imaging modalities after the administration of contrast agents and bladder cancer is a hypervascular tumour that shows strong enhancement in the arterial phase, followed by a plateau of enhancement and a slow washout when evaluated with imaging modalities after the administration of contrast agents [14,17-19]. Despite conventional ultrasound currently being the preferred non-invasive imaging technique for the screening of bladder cancers, its accuracy depends on several factors, including distension of the bladder, tumour size, morphology and location of the lesions [2]. The main limitation of ultrasound, which was found to be a factor in the present study, is its low sensitivity in detecting lesions smaller than 1 cm [3,20]. CEUS sensitivity for the number of detected bladder cancers was 65.5%, which may be explained by the high number of tumours ≤5 mm detected by cystoscopy (n = 25). These very small tumours are usually flat and do not produce focal bladder wall thickening or abnormal enhancement owing to the absence of considerable neovascularisation, as has been hypothesised in studies using other imaging techniques [20]. Our results are slightly worse than those obtained using CT or MRI with contrast agents [18,21-23], or using multidetector CT (MDCT) urography [24-26]. In the study by Kim et al [18], using MDCT, the detection rate was 97% for all bladder cancers and 85% for bladder cancers smaller than 1 cm. However, all cancers detected in that study were invasive and only 13 out of 77 were smaller than 1 cm. In a more recent study using dynamic contrast-enhanced MDCT with multiplanar reconstructions, Jinzaki et al [22] obtained an overall sensitivity of 90% and a sensitivity of 25% for bladder lesions 5 mm or smaller when using 5-mm axial sections as the reconstruction protocol or a sensitivity of 58% using 2.5-mm axial sections with a 1.25-mm overlap. With the use of MDCT urography, Turney et al [25] and Sadow et al [26] found a sensitivity of 93% and 79%, respectively in detecting bladder cancer but with a high number of equivocal (20% in the study by Turney) or misinterpreted (9% in the study by Sadow) studies.
With respect to location, we missed lesions from all segments but especially on the bladder floor and fundus. In the literature, there is no agreement regarding the most difficult places to detect bladder cancers using ultrasound. Those suggested include the anterior and posterior bladder wall [3], the bladder neck or dome areas [20] and the anterior wall [27]. Detection of small tumours in the bladder floor can be a challenge in male patients with benign prostatic hyperplasia (BPH) [28-30]. A large prostatic central gland protruding into the bladder lumen can be difficult to differentiate from bladder cancer. In our experience, bladder tumours enhance faster than the normal prostate gland owing to its arterial neovascularisation, but intense enhancement in areas of benign prostatic hyperplasia can be found using CEUS, mimicking tumoural enhancement [31]. Another difficulty when evaluating patients with BPH is the possibility of bladder wall thickening with trabeculations, which in some cases may mimic urothelial cancers.
When compared with baseline ultrasound, in our study CEUS did not significantly improve the sensitivity in detecting more bladder cancers, detecting only three lesions more. However, CEUS significantly increased the accuracy of baseline ultrasound according to the presence or absence of bladder tumour, especially in uncertain baseline ultrasound cases. In clinical practice, several reasons such as the presence of bladder clots, surgical wall changes, bladder wall trabeculation or the impossibility to retain urine to totally distend the bladder may hamper good visualisation of the bladder using baseline ultrasound. In our experience, CEUS is helpful in differentiating these entities from bladder cancer, detecting bladder cancer neovascularisation enhancement [14].
One limitation of this study is the recruitment of patients with high suspicion of bladder cancer obtained by flexible cystoscopy who were to undergo rigid cystoscopy and the absence of inclusion of healthy patients. However, radiologists who carried out the ultrasound and CEUS studies and who reviewed the ultrasound and CEUS images did not know the cystoscopic and histological results. With the introduction of this recruitment bias, the real value of CEUS as a screening for bladder cancer in patients with haematuria cannot be provided.
In conclusion, CEUS improves the bladder cancer detection rate of ultrasound, especially in ultrasound studies that are non-conclusive.
References
- 1.Sutton JM. Evaluation of haematuria in adults. JAMA 1990;263:2475–80 [PubMed] [Google Scholar]
- 2.Francica G, Bellini SA, Scarano F, Miragliuolo A, De Marino FA, Maniscalco M. Correlation of transabdominal sonographic and cystoscopic findings in the diagnosis of focal abnormalities of the urinary bladder wall: a prospective study. J Ultrasound Med 2008;27:887–94 [DOI] [PubMed] [Google Scholar]
- 3.Lopes RI, Nogueira L, Albertotti CJ, Takahashi DY, Lopes RN. Comparison of virtual cystoscopy and transabdominal ultrasonography with conventional cystoscopy for bladder tumour detection. J Endourol 2008;22:1725–9 [DOI] [PubMed] [Google Scholar]
- 4.Kocakoc E, Kiris A, Orhan I, Poyraz AK, Artas H, Firdolas F. Detection of bladder tumours with 3-dimensional sonography and virtual sonographic cystoscopy. J Ultrasound Med 2008;27:45–53 [DOI] [PubMed] [Google Scholar]
- 5.Mitterberger M, Pinggera GM, Neuwirt H, Maier E, Akkad T, Strasser H, et al. Three-dimensional ultrasonography of the urinary bladder: preliminary experience of assessment in patients with haematuria. BJU Int 2007;99:111–16 [DOI] [PubMed] [Google Scholar]
- 6.Tuncbilek N, Kaplan M, Altaner S, Atakan IH, Süt N, Inci O, et al. Value of dynamic contrast-enhanced MRI and correlation with tumour angiogenesis in bladder cancer. AJR Am J Roentgenol 2009;192:949–55 [DOI] [PubMed] [Google Scholar]
- 7.Park SB, Kim JK, Lee HJ, Choi HJ, Cho KS. Haematuria: portal venous phase multi detector row CT of the bladder—a prospective study. Radiology 2007;245:798–805 [DOI] [PubMed] [Google Scholar]
- 8.Lencioni R, Della Pina C, Crocetti L, Bozzi E, Cioni D. Clinical management of focal liver lesions: the key role of real-time contrast-enhanced ultrasound. Eur Radiol 2007;17 (Suppl 6):F73–9 [DOI] [PubMed] [Google Scholar]
- 9.Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol 2007;17:1995–2008 [DOI] [PubMed] [Google Scholar]
- 10.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—update 2008. Ultraschall Med 2008;29:28–44 [DOI] [PubMed] [Google Scholar]
- 11.Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, et al. Characterisation of focal liver lesions with contrast-specific ultrasound modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology 2004;232:420–30 [DOI] [PubMed] [Google Scholar]
- 12.Albrecht T, Hohmann J, Oldenburg A, Skrok J, Wolf KJ. Detection and characterisation of liver metastases. Eur Radiol 2004;14 Suppl 8:P25–33 [DOI] [PubMed] [Google Scholar]
- 13.Nicolau C, Vilana R, Catala V, Bianchi L, Gilabert R, Garcia A, et al. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol 2006;186:158–67 [DOI] [PubMed] [Google Scholar]
- 14.Nicolau C, Bunesch L, Sebastia C, Salvador R. Diagnosis of bladder cancer: contrast-enhanced ultrasound. Abdom Imaging 2010;35:494–503 [DOI] [PubMed] [Google Scholar]
- 15.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Radiology 2003;226:24–8 [DOI] [PubMed] [Google Scholar]
- 16.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998;22:1435–48 [DOI] [PubMed] [Google Scholar]
- 17.Mac Vicar AD. Bladder cancer staging. BJU Int 2000;86:111–22 [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Park SY, Ahn HJ, Kim CS, Cho KS. Bladder cancer: analysis of multi-detector row helical CT enhancement pattern and accuracy in tumour detection and perivesical staging. Radiology 2004;231:725–31 [DOI] [PubMed] [Google Scholar]
- 19.Caruso G, Salvaggio G, Campisi A, Melloni D, Midiri M, Bertolotto M, et al. Bladder tumor staging: comparison of contrast-enhanced and gray-scale ultrasound. AJR Am J Roentgenol 2010;194:151–6 [DOI] [PubMed] [Google Scholar]
- 20.Itzchak Y, Singer D, Fischelovitch Y. Ultrasonographic assessment of bladder tumours. I. Tumour detection. J Urol 1981;126:31–3 [DOI] [PubMed] [Google Scholar]
- 21.Barentsz JO, Jager GJ, van Vierzen PB. Staging urinary bladder cancer after transurethral biopsy: value of fast dynamic contrast-enhanced MR imaging. Radiology 1996;201:185–93 [DOI] [PubMed] [Google Scholar]
- 22.Jinzaki M, Tanimoto A, Shinmoto H, Horiguchi Y, Sato K, Kuribayashi S, et al. Detection of bladder tumours with dynamic contrast-enhanced MDCT. AJR Am J Roentgenol 2007;188:913–918 [DOI] [PubMed] [Google Scholar]
- 23.Kim B, Semelka RC, Ascher SM, Chalpin DB, Carroll PR, Hricak H. Bladder tumour staging: comparison of contrast-enhanced CT, T1- and T2-weighted MR imaging, dynamic gadolinium-enhanced imaging, and late gadolinium-enhanced imaging. Radiology 1994;193:239–245 [DOI] [PubMed] [Google Scholar]
- 24.Cohan RH, Caoili EM, Cowan NC, Weizer AZ, Ellis JH. MDCT Urography: Exploring a new paradigm for imaging of bladder cancer. AJR Am J Roentgenol 2009;192:1501–8 [DOI] [PubMed] [Google Scholar]
- 25.Turney BW, Willatt JM, Nixon D, Crew JP, Cowan NC. Computed tomography urography for diagnosing bladder cancer. BJU Int 2006;98:345–8 [DOI] [PubMed] [Google Scholar]
- 26.Sadow CA, Silverman SG, O'Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an Academic Medical Center. Radiology 2008;249:195–202 [DOI] [PubMed] [Google Scholar]
- 27.Ozden E, Turgut AT, Turkolmez K, Resorlu B, Safak M. Effect of bladder carcinoma location on detection rates by ultrasonography and computed tomography. Urology 2004;69:889–92 [DOI] [PubMed] [Google Scholar]
- 28.Wasserman NF. Benign prostatic hyperplasia: a review and ultrasound classification. Radiol Clin North Am 2006;44:689–710 [DOI] [PubMed] [Google Scholar]
- 29.Hsu TH, Matin SF. Benign prostatic hyperplasia mimicking bladder tumour. Urology 2001;57:1166. [DOI] [PubMed] [Google Scholar]
- 30.Wong JT, Wasserman NF, Padurean AM. Bladder squamous cell carcinoma. Radiographics 2004;24:855–860 [DOI] [PubMed] [Google Scholar]
- 31.Halpern EJ. Contrast-enhanced ultrasound imaging of prostate cancer. Rev Urol 2006;8(Suppl 1):S29–S37 [PMC free article] [PubMed] [Google Scholar]