Abstract
Objectives
The aim of this study was to evaluate the relationships between the severity of appendicitis as depicted on CT and blood inflammatory markers of serum white blood cell (WBC) count and C-reactive protein (CRP).
Methods
CT images in 128 patients (109 surgically proven and 19 with clinically excluded appendicitis) were retrospectively reviewed. Two radiologists by consensus evaluated and scored (using a 0, 1 or 2 point scale) severities based on CT-determined appendiceal diameters, appendiceal wall changes, caecal changes, periappendiceal inflammatory stranding and phlegmon or abscess formation. We investigated whether CT findings were significantly related to elevated WBC counts or CRP levels and performed the correlations of WBC counts and CRP levels with CT severity scores. Patients were also subjectively classified using four grades from normal (Grade I) to perforated appendicitis (Grade IV) on the basis of CT findings to evaluate differences in WBC counts and CRP levels between grades.
Results
Only appendiceal wall changes and the phlegmon or abscess formation were related to elevated WBC counts and CRP levels, respectively (p<0.05). CT severity scores were found to be more strongly correlated with CRP levels (r = 0.669) than with WBC counts (r = 0.222). On the basis of CT grades, the WBC counts in Grade I were significantly lower than in other grades (p<0.001), whereas CRP levels in Grade IV were significantly higher than in other grades (p<0.001).
Conclusion
CRP levels were found to correlate with CT-determined acute appendicitis severity and could be a useful predictor for perforated appendicitis, whereas WBC counts might be useful to detect early acute appendicitis.
Acute appendicitis is one of the most common surgical conditions in patients with right lower quadrant pain. Acute appendicitis is usually diagnosed on the basis of clinical findings such as fever, right lower quadrant pain and tenderness and muscle guarding [1]. However, the accuracy of clinically based diagnoses depends on clinician experience and has been reported to range from 71% to 97% [2]. By contrast, ultrasonography and CT have substantially increased the accuracy of diagnosing acute appendicitis. In particular, multidetector row CT (MDCT) has been reported to be highly accurate and effective at diagnosing acute appendicitis [3,4].
We have observed that in many institutions blood inflammatory markers such as white blood cell (WBC) counts and C-reactive protein (CRP) levels are performed in patients suspected of having acute appendicitis. In fact, some investigators have stressed the importance of these blood inflammatory markers in the context of deciding upon discharge or admission for further investigation [5-10]. However, some reports show that these inflammatory markers have low diagnostic accuracy in acute appendicitis [11-13]. As a result of these disparate results, the importance of WBC counts and CRP levels during the diagnostic stage remains controversial.
Some articles conclude that WBC counts and CRP levels are reliable indicators of disease severity and that they are significantly correlated with pathological findings [5,9]. In addition, a small number of studies have reported that CT findings are significantly correlated with surgical–pathological severity [14,15]. However, to the best of our knowledge, no study has been performed on the relationship between the CT findings of acute appendicitis and WBC counts or CRP levels. Accordingly, we undertook this retrospective study to evaluate these relationships in patients with acute appendicitis.
Methods and materials
Patients
Institutional review board approval was obtained for this study, but the requirement for informed consent was waived because of its retrospective nature. During a 15-month period, 162 adult patients (aged 15 years and above) suspected to have acute appendicitis underwent an abdominal CT scan. After initial CT interpretations, 34 patients were excluded because they had another inflammatory focus such as diverticulitis (n = 12), pelvic inflammatory disease (n = 15) or non-specific enterocolitis (n = 7). Thus, 128 patients (63 men, 65 women; age range 15–85; mean age 38 years) were enrolled in this study and one investigator reviewed the emergency medical charts of these selected patients. Of these 128 patients, 109 underwent appendectomy within 6 h of CT scanning and 106 were pathologically proven to have appendicitis. Despite a normal appendix by initial CT scan, three patients underwent appendectomy because of unexplained abdominal pain; pathological findings later showed they had a normal appendix. The other 19 patients who did not undergo appendectomy were considered to have a normal appendix; this was confirmed by clinical follow-up at least 6 months after the initial CT scan.
Imaging techniques
CT scans were performed using an MDCT scanner (Brilliance 64; Philips Medical Systems, Cleveland, OH) without oral contrast administration. At our institution, the use of oral contrast was discontinued after the introduction of MDCT. CT technical parameters were as follows: collimation 0.625 mm; table speed 50.8 mm rotation–1; pitch 1.014; rotation time 0.5 s; and voltage 120 kV (peak). Post-contrast scans of entire abdomens were performed with a 70 s delay after starting the infusion of 120 ml of non-ionic contrast material (Iomeprol; Bracco SpA, Milan, Italy) through an antecubital vein at 4 ml s–1. The axial section data were reconstructed at a thickness of 5 mm with 5-mm increments and at a thickness of 2 mm with 1-mm increments. The second data set was reformatted coronally at a thickness of 3 mm with 3-mm increments.
Laboratory analyses
The peripheral blood samples used to determine blood inflammatory marker levels were obtained in routine emergency department practice in all patients suspected of having appendicitis. All 128 patients enrolled in this study underwent WBC and CRP determinations less than 2 h before CT scanning. In our laboratory, the upper WBC count and CRP level limits are 10×103 µl–1 and 0.05 mg dl–1, respectively.
CT image analysis
All 128 CT scans were retrospectively evaluated by consensus between two radiologists with 8 and 14 years of dedicated abdominal imaging experience, respectively. The two reviewers were unaware of medical records (including laboratory results and clinical and surgical–pathological diagnoses), although they knew that all examinations involved patients with suspected acute appendicitis owing to right lower quadrant pain. These two reviewers evaluated both axial reconstructions and coronally reformatted images and subjectively assessed the five CT findings of appendicitis: appendiceal diameter, appendiceal wall changes, caecal changes, periappendiceal inflammatory stranding and periappendiceal phlegmon or abscess formation. The reviewers assigned a severity score from 0 to 2 to each of these five findings (Table 1). Appendiceal diameter was defined as the maximum diameter in full magnification view. Appendiceal wall changes were classified as absent, enhancing wall thickening or defect in enhancing wall thickening. Appendiceal wall thickening was defined as a wall thickness of >2 mm. Caecal changes were classified as absent, caecal wall thickening or caecal wall thickening with pericaecal fluid. Caecal wall thickening was determined by comparison with the normal wall thickness of the ascending colon immediately distal to the caecum. The degree of periappendiceal inflammatory stranding was subjectively classified as absent, mild or moderate to severe by consensus between the two reviewers. Mild stranding was defined as perceptible haziness or increased attenuation in the mesoappendix or in retroperitoneal fat. Phlegmon was defined as diffuse and substantial inflammation of the periappendiceal fat with ill-defined fluid collections and an abscess was defined as a discrete fluid collection surrounded by a wall.
Table 1. CT severity scores based on CT findings.
| Scores | Appendiceal diameter | Appendiceal wall changes | Caecal changes | Periappendiceal inflammatory stranding | Phlegmon or abscess formation |
| 0 | <6 mm | Absent | Absent | Absent | None |
| 1 | 6–10 mm | Enhancing wall thickening | Wall thickening | Milda | Phlegmon |
| 2 | >10 mm | Defect in enhancing wall thickening | Wall thickening with surrounding fluid | Moderate to severeb | Abscess |
aPerceptible haziness or increased attenuation in the mesoappendix or retroperitoneal fat.
bMore severe change than score 1.
The two reviewers also subjectively classified patients using four grades, again by consensus, based on the CT findings: Grade I, normal; Grade II, mild appendicitis; Grade III, appendicitis with localised peritonitis and Grade IV, perforated appendicitis (Table 2) (Figure 1). This grading was arrived at by modifying a previously described grading system [14]. When grades allocated by the reviewers differed, a decision was arrived at by consensus after considering surgical–pathological findings.
Table 2. CT grades of acute appendicitis.
| Grade | CT definition | CT findings |
| I | Normal | Normal appendix |
| II | Mild appendicitis | Fluid-filled appendix >6 mm in diameter and enhancing wall thickening with/without subtle periappendiceal stranding |
| III | Appendicitis with localised peritonitis | Grade II definition plus moderate to severe periappendiceal stranding without defect in enhancing appendiceal wall |
| IV | Perforated appendicitis | Grade III definition plus defect in enhancing appendiceal wall with/without phlegmon or abscess |
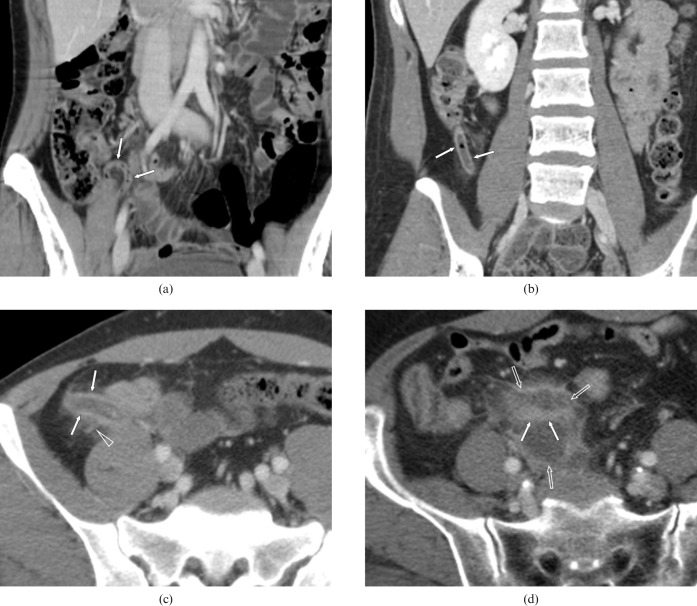
Figure 1.
The four-point grade CT scale. (a) Example of Grade I showing a 28-year-old woman with a normal appendix. The coronal reformation image shows the entire length of the normal appendix (arrows). (b) Example of Grade II showing a 20-year-old woman with appendicitis. This coronal reformation image shows a fluid-filled appendix (arrows) of diameter 8 mm. The appendiceal wall shows enhancement without periappendiceal stranding. (c) Example of Grade III showing a 47-year-old man with appendicitis. This axial image shows a fluid-filled appendix (arrows) of diameter 12 mm with moderate periappendiceal stranding (open arrowhead). (d) Example of Grade IV showing a 69-year-old woman with a perforated appendix with periappendiceal abscesses. This axial image shows the abscess (open arrows) and an inner tubular area of increased enhancement (arrows), which represents an inflamed appendix.
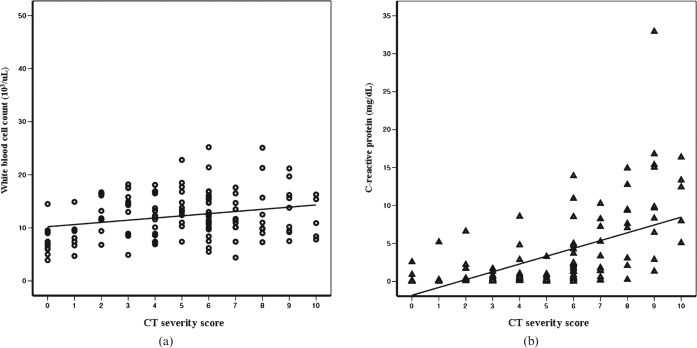
Figure 2.
Scatter plots of correlations between the CT severity score and (a) white blood cell (WBC) count and (b) C-reactive protein (CRP) levels. CT severity scores were found to be more strongly correlated with CRP levels (r = 0.669, p<0.01) than with WBC count (r = 0.222, p = 0.012).
Statistical analysis
All WBC and CRP data are presented as the mean ± standard deviation. A multiple regression model was used to determine whether CT findings were related to WBC counts and CRP levels. Correlations between CT severity scores (0 to 10) and WBC counts and CRP levels were analysed using Spearman's correlation coefficients. A p-value of <0.05 was considered statistically significant. Differences in the WBC counts and CRP levels between the grades were analysed by one-way analyses of variance and then verified by the post hoc test. A p-value <0.008 (0.05/6 times multiple comparisons) was considered statistically significant.
Results
In the results of multiple regression analysis regarding the significance of relationships between five CT findings and an elevated WBC count or CRP level, only appendiceal wall change was found to be significantly related to an elevated WBC count (p<0.05). Only periappendiceal phlegmon or abscess were found to be significantly related to an elevated CRP level (p<0.01) (Table 3).
Table 3. Multiple regression analysis of the relationships between CT findings and WBC counts and CRP levels.
| CT findings | WBC |
CRP |
||||
| Coefficient (β) | Standard error | p-Value | Coefficient (β) | Standard error | p-Value | |
| Appendiceal diameter | 0.142 | 1.746 | 0.252 | 0.049 | 1.619 | 0.625 |
| Appendiceal wall changes | 0.286 | 1.586 | 0.043 | −0.005 | 1.471 | 0.967 |
| Caecal changes | −0.094 | 0.979 | 0.380 | −0.004 | 0.908 | 0.961 |
| Periappendiceal inflammatory stranding | 0.084 | 1.263 | 0.498 | 0.107 | 1.171 | 0.292 |
| Phlegmon or abscess formation | −0.076 | 1.065 | 0.386 | 0.621 | 0.988 | 0.000 |
CRP, C-reactive protein; WBC, white blood cell.
Table 4 lists the mean and standard deviation of WBC counts and CRP levels and numbers of patients corresponding to CT severity scores. CT severity scores were found to be highly correlated with CRP levels (r = 0.669, p<0.01), but poorly correlated with WBC counts (r = 0.222, p = 0.012).
Table 4. Blood inflammatory markers according to CT severity scores.
| Score | No. | Mean WBC count ×103 (µl)a | Mean CRP level (mg dl–1)a |
| 0 | 13 | 7.75 ± 2.69 | 0.30 ± 0.72 |
| 1 | 7 | 8.70 ± 3.21 | 0.82 ± 1.92 |
| 2 | 9 | 13.16 ± 3.57 | 1.48 ± 2.09 |
| 3 | 11 | 13.46 ± 4.29 | 0.49 ± 0.56 |
| 4 | 15 | 12.03 ± 3.81 | 1.42 ± 2.35 |
| 5 | 12 | 14.32 ± 4.15 | 0.52 ± 0.92 |
| 6 | 26 | 13.16 ± 4.41 | 2.73 ± 3.49 |
| 7 | 11 | 11.68 ± 3.84 | 3.63 ± 3.57 |
| 8 | 9 | 13.51 ± 6.05 | 7.39 ± 4.90 |
| 9 | 10 | 13.22 ± 4.80 | 11.85 ± 9.02 |
| 10 | 5 | 11.76 ± 3.92 | 11.04 ± 4.49 |
| Total | 128 | 12.22 ± 4.44 | 3.18 ± 5.02 |
aData are mean ± standard deviation.
CRP, C-reactive protein; No., number of patients; WBC, white blood cell.
Of the 128 patients in our study population, 118 were classified into four grades on the basis of CT findings alone. The two reviewers disagreed regarding grades in the other 10 patients, thus these were classified after considering CT findings and surgical–pathological results. Of the 128 patients, 22, 30, 43 and 33 were classified as Grade I, II, III and IV, respectively. The mean, standard deviation and 95% confidence interval of WBC counts and CRP levels according to grade are summarised in Table 5. A one-way analysis of variance showed that both WBC count (F = 7.68, p<0.01) and CRP level (F = 23.1, p<0.01) had significant effects on the four CT grades. Bonferroni's multiple comparison test showed that the mean WBC count for Grade I was significantly lower than for the other grades (p<0.001), but no significant differences were observed for the other grades. By contrast, the mean CRP level in Grade IV was significantly higher than in other grades (p<0.001). Although no significant differences were found between CRP levels in the grades, CRP level was found to increase by grade.
Table 5. Blood inflammatory markers according to CT grade.
| Grade | No. | Mean WBC count ×103 (µl–1)a | Mean CRP (mg dl–1)a |
| I | 22 | 8.38 ± 2.96 (7.10–9.67)b | 0.32 ± 0.70 (0.02–0.63) |
| II | 30 | 12.97 ± 3.73 (11.6–14.4) | 0.99 ± 1.63 (0.38–1.60) |
| III | 43 | 13.17 ± 3.88 (11.97–14.36) | 2.39 ± 3.25 (1.39–3.39) |
| IV | 33 | 12.85 ± 5.28 (10.97–14.72) | 8.10 ± 6.90 (5.65–10.54)c |
| Total | 128 | 12.22 ± 4.44 (11.44–12.99) | 3.18 ± 5.02 (2.30–4.06) |
aData are mean ± standard deviation; data in parentheses are 95% confidence intervals.
bMean Grade I values were significantly lower than those of other grades (p<0.001, Bonferroni's multiple comparisons test).
cMean Grade IV values were significantly higher than those of other grades (p<0.001, Bonferroni's multiple comparisons test).
CRP, C-reactive protein; No., number of patients; WBC, white blood cell.
Among the 106 patients with pathologically proven appendicitis in our study population, 27 patients had a normal WBC count and 9 had a normal CRP level. No patients with appendicitis had normal levels of both WBCs and CRP. Of the 22 patients with a pathologically or clinically proven normal appendix, 3 had elevated WBC and CRP levels and 9 had an elevated CRP level.
Discussion
Blood inflammatory markers, such as WBCs and CRP, cannot be relied upon to make a specific diagnosis. However, these inflammatory markers have contributory values and can aid clinical judgements. Furthermore, when applied to cases with acute right lower quadrant pain, as determined by clinical examination, these inflammatory markers can aid diagnosis. In this setting, elevated levels of inflammatory markers have been reported to increase the probability of acute appendicitis by some investigators [5,7,9], whereas others have concluded that patients with right lower quadrant pain with a normal WBC count and CRP level are unlikely to have acute appendicitis [5-10].
To the best of our knowledge, this is the first study to evaluate relationships between blood inflammatory markers and CT findings in a single patient population. In terms of the CT findings examined, only the presence of acute appendicitis and appendiceal wall changes were found to be significantly related with an elevated WBC count. This finding suggests that an increased WBC count is related to mural inflammation of the appendix, which is a feature of early appendicitis [1]. By contrast, elevated CRP levels were found to be related to periappendiceal phlegmon and abscess formation, which are features of advanced disease.
Some have reported that WBC count is correlated with the severity of appendicitis [9,16]. However, in the present study only a poor correlation (r = 0.222) was found between WBC count and disease severity, as determined by CT findings. In addition, we found no significant differences between Grades II, III and IV in terms of WBC count. Furthermore, if we did not consider the standard deviation, mean WBC count in Grade III (13 170 µl–1) was slightly higher than in Grade IV (12 850 µl–1).
CRP is synthesised by hepatocytes during the acute response phase to a variety of infectious or inflammatory disease processes [9]. The reported predictive values of CRP in appendicitis vary widely: reported sensitivities range from 40% to 99% and specificities from 27% to 90% [17]. Amalesh et al [11] reported that the accuracy of CRP for diagnosing acute appendicitis is low and added that CRP levels are not useful when deciding on surgery. However, Ortega-Deballon et al [5] recently concluded that CRP level is the most useful laboratory parameter in terms of diagnosing acute appendicitis and that CRP levels are strongly correlated with inflammation severity. In the present study, we also found a high correlation (r = 0.669) between CRP level and disease severity based on CT findings. Furthermore, although no significant differences were found between the CRP levels of patients with Grades I, II and III, CRP values were found to be proportional to grade.
In the present study, WBC count was found to better differentiate normal and inflamed appendices than CRP level, whereas CRP level was found to be better at detecting perforated appendicitis. Accordingly, the present study shows that an elevated WBC count better detects early appendiceal inflammation, whereas an elevated CRP level better detects protracted inflammation such as that encountered 2 or 3 days after symptom onset [18]. Furthermore, the results of the present study, in which disease severities were mainly determined using CT findings, concur with those of previous studies that were based on surgical–pathological results [7,19].
It has been reported that although WBC count and CRP level are helpful in terms of diagnosing appendicitis, they are inferior to imaging studies such as ultrasonography and CT in terms of confirming the presence of acute appendicitis [12,13]. Accordingly, it was concluded that these inflammatory markers could not be usefully incorporated into an algorithm designed to restrict or recommend further imaging studies. However, when the appendix is not visualised by imaging studies or when imaging results are inconclusive, inflammatory markers could provide contributory diagnostic information. Therefore, we suggest that additional studies in patients who have equivocal imaging results are needed to determine the potential roles of inflammatory markers.
Several limitations of the present study should be considered. Firstly, the patient selection procedure excluded cases other than those of acute appendicitis. This was done because the inclusion of patients with other inflammatory focuses might have influenced relationships between CT-based severity and inflammatory markers. Secondly, the criteria used to determine CT severity scores were relatively subjective and, furthermore, the presence of appendicolith was not included; however, we suggested that appendicolith per se does not influence inflammatory marker levels. In addition, less than 10% of the normal population had an appendicolith [20,21]. Accordingly, we did not include appendicolith as a CT criterion for scoring purposes. Thirdly, we did not evaluate the correlation between CT findings and surgical–pathological findings, other than when reviewers disagreed on CT grade. Finally, our study population contained a relatively high number of patients with a perforated appendix, which would have contributed to the strong correlation found between CT-determined appendicitis severity and CRP level.
In summary, this study shows that CT-determined acute appendicitis severity is better correlated with CRP level than with WBC count. Our findings suggest that an elevated CRP level supports a diagnosis of perforated appendicitis, whereas an elevated WBC count could help differentiate normal and inflamed appendices. Accordingly, the findings of the present study suggest that blood inflammatory markers might be useful tools in acute appendicitis patients with an equivocal diagnosis or disease stage after CT.
References
- 1.Birnbaum BA, Wilson SR. Appendicitis at the millennium. Radiology 2000;215:337–48 [DOI] [PubMed] [Google Scholar]
- 2.John H, Neff U, Kelemen M. Appendicitis diagnosis today: clinical and ultrasonic deductions. World J Surg 1993;17:243–9 [DOI] [PubMed] [Google Scholar]
- 3.Paulson EK, Harris JP, Jaffe TA, Haugan PA, Nelson RC. Acute appendicitis: added diagnostic value of coronal reformations from isotropic voxels at multi-detector row CT. Radiology 2005;235:879–85 [DOI] [PubMed] [Google Scholar]
- 4.Miki T, Ogata S, Uto M, Nakazono T, Urata M, Ishibe R, et al. Enhanced multidetector-row computed tomography (MDCT) in the diagnosis of acute appendicitis and its severity. Radiat Med 2005;23:242–55 [PubMed] [Google Scholar]
- 5.Ortega-Deballon P, Ruiz deAdana-Belbel JC, Hernández-Matías A, García-Septiem J, Moreno-Azcoita M. Usefulness of laboratory data in the management of right iliac fossa pain in adults. Dis Colon Rectum 2008;51:1093–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan MN, Davie E, Irshad K. The role of white cell count and C-reactive protein in the diagnosis of acute appendicitis. J Ayub Med Coll Abbottabad 2004;16:17–19 [PubMed] [Google Scholar]
- 7.Grönroos JM, Grönroos P. Leucocyte count and C-reactive protein in the diagnosis of acute appendicitis. Br J Surg 1999;86:501–4 [DOI] [PubMed] [Google Scholar]
- 8.Sack U, Biereder B, Elouahidi T, Bauer K, Keller T, Tröbs RB. Diagnostic value of blood inflammatory markers for detection of acute appendicitis in children. BMC Surg 2006;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang HR, Wang YC, Chung PK, Chen WK, Jeng LB, Chen RJ. Laboratory tests in patients with acute appendicitis. ANZ J Surg 2006;76:71–4 [DOI] [PubMed] [Google Scholar]
- 10.Nordback I, Harju E. Inflammation parameters in the diagnosis of acute appendicitis. Acta Chir Scand 1988;154:43–8 [PubMed] [Google Scholar]
- 11.Amalesh T, Shankar M, Shankar R. CRP in acute appendicitis — is it a necessary investigation? Int J Surg 2004;2:88–9 [DOI] [PubMed] [Google Scholar]
- 12.Johansson EP, Rydh A, Riklund KA. Ultrasound, computed tomography, and laboratory findings in the diagnosis of appendicitis. Acta Radiol 2007;48:267–73 [DOI] [PubMed] [Google Scholar]
- 13.Kessler N, Cyteval C, Gallix B, Lesnik A, Blayac PM, Pujol J, et al. Appendicitis: evaluation of sensitivity, specificity, and predictive values of US, Doppler US, and laboratory findings. Radiology 2004;230:472–8 [DOI] [PubMed] [Google Scholar]
- 14.Raptopoulos V, Katsou G, Rosen MP, Siewert B, Goldberg SN, Kruskal JB. Acute appendicitis: effect of increased use of CT on selecting patients earlier. Radiology 2003;226:521–6 [DOI] [PubMed] [Google Scholar]
- 15.Hansen AJ, Young SW, De Petris G, Tessier DJ, Hernandez JL, Johnson DJ. Histologic severity of appendicitis can be predicted by computed tomography. Arch Surg 2004;139:1304–8 [DOI] [PubMed] [Google Scholar]
- 16.Guraya SY, Al-Tuwaijri TA, Khairy GA, Murshid KR. Validity of leukocyte count to predict the severity of acute appendicitis. Saudi Med J 2005;26:1945–7 [PubMed] [Google Scholar]
- 17.Hallan S, Asberg A. The accuracy of C-reactive protein in diagnosing acute appendicitis — a meta-analysis. Scand J Clin Lab Invest 1997;57:373–80 [DOI] [PubMed] [Google Scholar]
- 18.Wu HP, Lin CY, Chang CF, Chang YJ, Huang CY. Predictive value of C-reactive protein at different cutoff levels in acute appendicitis. Am J Emerg Med 2005;23:449–53 [DOI] [PubMed] [Google Scholar]
- 19.Marchand A, Van Lente F, Galen RS. The assessment of laboratory tests in the diagnosis of acute appendicitis. Am J Clin Pathol 1983;80:369–74 [DOI] [PubMed] [Google Scholar]
- 20.Benjaminov O, Atri M, Hamilton P, Rappaport D. Frequency of visualization and thickness of normal appendix at nonenhanced helical CT. Radiology 2002;225:400–6 [DOI] [PubMed] [Google Scholar]
- 21.Jan YT, Yang FS, Huang JK. Visualization rate and pattern of normal appendix on multidetector computed tomography by using multiplanar reformation display. J Comput Assist Tomogr 2005;29:446–51 [DOI] [PubMed] [Google Scholar]