Abstract
A variety of techniques are now available to directly or indirectly detect signal from tissues, fluids and materials that have short, ultrashort or supershort T2 or T2* components. There are also methods of developing image contrast between tissues and fluids in the short T2 or T2* range that can provide visualisation of anatomy, which has not been previously seen with MRI. Magnetisation transfer methods can now be applied to previously invisible tissues, providing indirect access to supershort T2 components. Particular methods have been developed to target susceptibility effects and quantify them after correcting for anatomical distortion. Specific methods have also been developed to image the effects of magnetic iron oxide particles with positive contrast. Major advances have been made in techniques designed to correct for loss of signal and gross image distortion near metal. These methods are likely to substantially increase the range of application for MRI.
It is a pleasure to thank the president and members of the council of the British Institute of Radiology for the opportunity to honour the memory of Professor Mayneord, who had a pivotal role in founding medical physics in the UK [1,2]. He was prescient in suggesting in 1945, a time when the use of magnetism in medicine was in disrepute, that the study of magnetic susceptibility could yield both useful and interesting information. This was published in the immediate aftermath of World War II in an issue of the British Medical Bulletin celebrating the 50th anniversary of Roentgen's discovery of X-rays [3]. It was also a year before the discovery of nuclear magnetic resonance (NMR), 28 years before the discovery of MRI, and over 40 years before the general use of susceptibility-weighted imaging (SWI) [4,5] and the observation of the variation in bulk magnetic susceptibility of tendons, ligaments and menisci with orientation to the static magnetic field [6,7].
It is also a pleasure to acknowledge the critical role of Gordon Higson of the Department of Health in helping to fund the early development of X-ray CT by Sir Godfrey Houndsfield and others at EMI and in supporting the early development of MRI partly from royalties derived from CT [8]. This was a major contribution to the work done by MR groups based in the UK in the late 1970s and led to clinical imaging in 1980–1 [9-14]. A particular regret is the death of Brian Worthington, a close collaborator with both Bill Moore and Sir Peter Mansfield, and author of the first MRI study on a series of patients [13]. Worthington wrote extensively on neuroradiology, obstetrics and gynaecology, as well as image perception.
During the first year of clinical MRI, only steady-state free precession (SSFP), mobile proton density (ρm) and T1 weighted clinical images were available. Clinical heavily T2 weighted spin-echo (SE) images arrived suddenly in February 1982 and transformed the practice of MRI [15-17]. These images showed abnormalities with high signal and contrast, and they rapidly became the mainstay of clinical diagnosis in the brain. Even with the subsequent development of new types of sequences, such as fast spin echo [18], clinical diffusion weighted imaging [19] and fluid attenuated inversion recovery [20], detection of signal from longer mean T2 relaxation components still remains the dominant form of MRI for diagnosis of parenchymal disease in the brain and much of the rest of the body.
However, even in 1981, low- or zero-level signals were recognised in cortical bone by Smith [21] and Edelstein et al [22]. The appearance was attributed to short mean T2 components in this tissue leading to undetectable signal levels at the time of data acquisition. The lack of signal from normal tissue was useful in providing a dark background against which abnormalities in cortical bone, with mean T2s sufficiently increased to provide detectable signal, could be recognised; however, the absence of signal meant that there was no possibility of measuring normal values of ρm, T1 or T2, nor of studying normal perfusion. In addition, there was no opportunity for active contrast manipulation, little or no distinction between adjacent short T2 tissues and no normal contrast enhancement or effects from molecular imaging agents. As a result, the study of cortical bone and other MR “invisible” short T2 tissues, such as tendons, ligaments and menisci, has been more limited than that of other tissues, such as brain, liver and muscle, where MR signals are readily detectable with clinical systems.
In spite of these difficulties, there has been a proliferation of new approaches to imaging short T2 tissue components, including options for developing tissue contrast in the short T2 and T2* range, as well as methods of imaging in the presence of metal. This has included solutions and partial solutions to technical problems, some of which have appeared intractable for 20 years or more.
The theme of this paper is clinical MRI of “dark matter” (i.e. tissues, fluids and materials that show little or no signal with conventional imaging techniques). It includes direct and indirect imaging as well as spectroscopy. As an initial step, some general principles underlying this type of imaging are reviewed.
General principles
The protons in rigid crystals or solids typically have very short T2s due to fixed field effects; however, in solution, motion of molecules leads to averaging of spin interactions over time and much longer T2s. This gives rise to the concept of ρm, representing more mobile tissue components with T2s that are long compared with those of immobile components. The term “visible” can also be applied to the longer T2 components since they produce detectable signal, and “invisible” can be applied to short T2 components, which do not result in detectable signal.
It is important to distinguish between the T2 of the tissue or fluid that reflects effects such as dipolar–dipolar interactions and chemical exchange, and the observed T2 (T2*) of tissues or fluids that also reflects local susceptibility effects, chemical shift and J-coupling, as well as flow, magic angle and other effects. The dominant effect among these is often from susceptibility; this results in a shortening of T2* relative to T2 due to inhomogeneous magnetic fields within voxels and intravoxel dephasing.
It is often useful to consider relaxivity, R2 or R2*, which is the reciprocal of T2 or T2*, i.e.
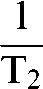 or
or 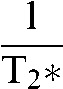 , rather than the transverse relaxation times. This is because relaxivities are additive so that, for example, within a voxel
, rather than the transverse relaxation times. This is because relaxivities are additive so that, for example, within a voxel
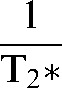 =
=  + γΔB, or R2* = R2 + γΔB
+ γΔB, or R2* = R2 + γΔB
Thus, the observed relaxivity is the sum of the tissue relaxivity and γ times the inhomogeneity in B (i.e. ΔB) within the voxel. Other relaxivities (owing to chemical shift, contrast agents, etc.) can be added in the same way.
When there is a majority of short T2/T2* components in a tissue, fluid or material, it typically appears low-signal or dark with clinical imaging techniques. A minority of short T2/T2* components is common in many tissues. In this situation, signal is usually apparent from longer T2/T2* components, but little or no contribution to the signal comes from the short T2/T2* components. All tissues have some short T2/T2* components from protons in large molecules, including those in membranes.
The focus in this paper is on tissue and fluids, but materials may also have short T2/T2*s and/or low or zero mobile proton densities. This includes relaxation agents (such as gadolinium chelates) and susceptibility agents (such as magnetic iron oxide particles, MIOPs). These materials may produce very large susceptibility differences in tissues and fluids, and can result in very short T2*s. Many materials, including most plastics, also have short T2s. Other materials, such as contrast agents and metals, may have no significant ρm but can produce strong effects on surrounding tissues.
There is no precise definition of what constitutes a short echo time (TE) and what is an ultrashort TE (UTE), and there is argument about how TE should be measured for tissues with short T2s [23-25]. For simplicity, a short TE is taken to be less than 10 ms, and an ultrashort one less than 1 ms. It is also possible to define short T2/T2*s as less than 10 ms, ultrashort as less than 1 ms and supershort as less than 0.1 ms. This reflects the fact that, with older MR systems and conventional SE sequences, tissues with T2s or T2*s less than 10 ms produced little or no signal and were “invisible”. With more recent systems and gradient echo sequences, the cut-off is closer to 1 ms. Ultrashort pulse sequences can often directly detect signal in the 1–0.1 ms range, but indirect methods are usually required to image supershort T2 (<0.1 ms) tissues.
MR signals are usually spatially encoded using frequency and phase effects produced by linear applied gradient fields. Susceptibility effects also include changes in the local field, and these may result in errors in locating the position of the signal. In fact, the local susceptibility differences may be greater than those of the encoding gradient magnetic field and result in image distortion. This means that, in addition to shortening of T2 owing to susceptibility effects, resulting in low signal, image distortion may be present with both loss of signal and local “pile up” (i.e. increase in signal where signals from different regions are incorrectly superimposed on one another). In general terms, phase encoding tolerates gross field distortion much better than frequency encoding both for slice selection and spatial localization.
Quantitation of tissue or fluid T2*s is made difficult by the addition of other effects; this results in measured values (T2*s) that include effects from local susceptibility and other effects. Measurements may also be confounded by distortion of the spatial encoding process by susceptibility effects. It may also be difficult to assign susceptibility effects to a particular tissue and to distinguish them from inhomogeneity in B0. There are also difficulties in accurate measurement of both T2/T2* values and the relative proportions of two or more different components with the signal-to-noise levels attainable in reasonable times on clinical systems.
Imaging may be regarded as direct when it detects signal from the tissues, fluid or materials of interest, and indirect when the signals are detected from other species that are affected by the short T2/T2* tissues, fluids or materials of interest. For short T2/T2* tissues, the most common way of imaging directly is to use a short TE or UTE to detect short T2/T2* signals before they have decayed to zero or the noise floor. It is also possible to place highly ordered collagen-rich tissues at the magic angle to prolong their T2/T2s* to make the signal detectable. In other situations it is possible to increase the TE so protons in fat and water, which are out of phase, can become in phase, and the combined signal can then become detectable. Likewise, spin echo sequences can be used to increase T2* by reversing the effects of local field inhomogeneities.
Indirect forms of imaging short T2/T2* components include visualisation of the extent of invisible short T2 tissues when they are surrounded by a longer T2 tissue with detectable signal. Another indirect method is to observe the effect of short T2 tissue's susceptibility or relaxation on the surrounding or adjacent longer T2 tissues. An example of this is to assess trabecular bone by the effect this tissue has on adjacent longer T2* of red or yellow bone marrow. Relaxation agents typically work in this way, with no signal directly detectable from them, but the effects detectable through relaxation or susceptibility effects produced on the protons in associated water or other tissues. A third indirect method is magnetisation transfer (MT), which typically magnetically saturates invisible short T2 components and results in a change in the magnetisation transferred to the longer T2 components. This usually results in a shortening of T1 and a reduction in detectable signal in the detected longer T2 component.
At present, several different approaches are being used to image short and ultrashort T2 and T2* tissues, fluids and materials. These may involve both direct and indirect approaches, as well as situations where the primary objective is detection or correction of image distortion owing to susceptibility effects rather than detection of short T2/T2* signal, although undetectable signals may become detectable as a consequence of this correction.
The first approach is direct and regards the problem as essentially one of imaging short T2 components. This can be addressed by using a short TE/UTE and/or a method of increasing T2/T2* so that signal can be detected from the tissue or fluid. This includes a variety of techniques. This approach is frequently associated with methods of reducing or suppressing the signal from longer T2 components to isolate the short T2 components and improve conspicuity or assist with quantitation.
The second approach is MT that is indirect. This may be extended to invisible tissues by using short TE approaches. The definition of free and bound pools then changes, and there may be increased problems in isolating MT effects because of direct saturation of the newly visible pool.
The third approach has been termed SWI, where magnitude and/or phase data from a gradient echo sequence are typically used to recognise loss of signal from the tissue itself and/or surrounding tissues if the T2* levels of the tissue are too short to be detectable with the TE in use. This technique can be direct, indirect or both. The TE necessary to produce useful contrast between normal and abnormal tissues in an organ may result in loss of signal in other areas of the image where greater susceptibility differences are present. The basic approach is qualitative and may involve empirical combinations of magnitude and phase data. The technique has limitations in situations where the signal becomes undetectable so that it is not possible to calculate magnitude or phase data, and in situations where the image becomes distorted because of problems in slice selection and/or frequency encoding. A development from this is quantitative susceptibility imaging (QSI) or susceptibility SWI mapping (SWIM), where approaches are used to assess and correct for the effect of static field perturbations on spatial encoding of the signal and, therefore, to try to avoid compromising signal values. This typically requires the solution to a complex inverse problem, but it is now an area of considerable interest.
The fourth is positive contrast or white matter imaging, which assesses the effect of MIOPs. These particles typically reduce the signal from tissues as a result of a decrease in T2 and susceptibility effects. This tends to produce a loss of signal in the area of the image that is of most interest. This can be a particular problem when it occurs in tissues with pre-existing very low signals, so that the reduction is undetectable; however, it is also a problem in other areas where the loss of signal may lead to loss of anatomical detail and poor localisation of the site of contrast agent accumulation. One approach to this problem is to correct the field distortion induced by the MIOPs and allow signal to be detected where these particles accumulate. Different techniques have been used, but the observed result may still reflect both contrast accumulation and field distortions produced by the agent.
The fifth group of techniques is targeted at imaging in the presence of metal. Metals may show very large susceptibility differences from those of tissues and can produce very large susceptibility effects, with loss of signal due to T2* shortening and gross image distortion. The primary objective in this situation is to deal with the image distortion and restore image integrity to a sufficient degree to make the images clinically useful. In the process, short T2* tissues and fluids may become detectable.
There is an overlap between these approaches, and they may be combined. In some situations, it may be appropriate to ignore the effects of susceptibility differences in producing image distortion and regard the problem as one of detecting short T2 signals, whereas in others, image distortion due to susceptibility is the primary problem that needs to be addressed.
Tissue, fluid and material properties
The tissues of the human body can be divided into those that are “visible” in the sense that they provide detectable signal with clinical MR systems and those that are “invisible” because their mean T2s or T2*s are too short to provide a detectable signal. All tissues have multicomponent T2s. This means that they contain a mixture of short and long T2 components. The invisible tissues have a majority of short T2 components and a minority of long T2 components. The latter components typically do not provide enough signal to be detectable in relation to image noise levels. The “visible” tissues of the body (such as brain, liver and muscle) have a majority of long T2 components that produce signal with conventional techniques. They also have a minority of short T2 components, which do not contribute significantly to the detectable signal.
Within the invisible group of tissues (mean T2<10 ms) it is possible to differentiate a first group (including tendons, ligaments and menisci) with short mean T2s of approximately 1–10 ms and a second group (including cortical bone and dentine) with ultrashort mean T2s of 0.1–1 ms. There is also a third group (including dental enamel, protons in membranes and molecules, as well as crystalline bone) with supershort mean T2s of less than 0.1 ms. Materials can also be classified in a similar way.
An important factor in this context is the magic angle effect [26,27], because it can greatly increase the T2 of short T2 tissues, such as tendons, ligaments and menisci. When the orientation of tissues that contain highly ordered collagen is changed, their T2 varies from a minimum at θ = 0°, where dipolar interactions are greatest, to a maximum at 3 cos2 θ – 1≈0 and θ = 55°, where θ is the orientation of the fibres to B0. The increase can be large, for example from 0.6 ms to 21 ms [26] or from 7 to 23 ms [27] in the Achilles tendon.
A recently described phenomenon is directional susceptibility in tendons, whereby their bulk magnetic susceptibility varies with orientation to B0, with signals at the water end of the proton spectrum when fibres are parallel to B0 and at the fat end of the spectrum (lower frequency) when fibres are perpendicular to B0 [6]. The difference is relatively large (of the order of three parts per million).
The ρm of tissues also varies markedly; bone has a ρm of 15–20%, and semisolid tissues (such as tendons and ligaments) have values of 60–70%. ρm is generally a more important factor in generating contrast with short T2 tissues than it is with longer T2 tissues. The low ρm for bone places a limit on the maximum signal that can be obtained from it. Both the low ρm and the short T2* of cortical bone contribute to its low signal intensity.
The mean T1s of some tissues with a majority of short T2 components is short, with cortical bone having a particularly short T1; in fact, less than that of fat [28]. The relative differences in mean T2 or T2* between normal and abnormal tissues are often much greater than those in mean T1.
Relative to air, soft tissues generally show a susceptibility difference of approximately −9 ppm, and bone and calcified tissue approximately −11 ppm. By comparison, the principal peak of fat resonates at approximately −12 ppm. Paramagnetic materials show small positive frequency shifts and superparamagnetic materials show greater positive shifts. Metals (for example titanium), metal alloys and some types of stainless steel may show very large positive shifts of 10 s to 1000 s of parts per million. The changes in field may be considerably greater than machine gradient fields used to encode MR signals, and therefore may cause image distortion.
In disease, increases in T2 are frequently seen, but decreases in T2 may be seen with increased iron content and in other disease processes. Loss of magic angle effect may be seen in degeneration and fibrosis.
Acquisition methods for short T2/T2* components
Some of the techniques now being used to directly detect signal from tissues on clinical systems have been used in material science and tissue studies using small-bore high-field spectrometers for many years. The methods are now in use on clinical systems that are usually lower performance in terms of B0, gradient strength, slew rate and peak B1 field (Table 1). The prototype sequence for imaging short T2 tissues is single-point imaging (SPI), where a single point in k-space is acquired with a UTE. This is typically used with three-dimensional (3D) phase encoding, which tolerates field distortions well, but unfortunately makes the technique time-consuming even with optimised k-space sampling [29].
Table 1. Short and ultrashort echo time (TE) imaging techniques.
| Technique | Radiofrequency pulses and gradient | k-space trajectory |
| Single point [29] | Non-selective hard pulse with gradient applied | 3D point-by-point |
| Multipoint [30] | Hard pulse with gradient applied | 3D partial lines |
| Several points | ||
| UTE [31,159-161] | 2D two half pulses | Radial from centre out |
| BLAST [162] | 3D hard pulse | FID acquisition |
| PETRA [163] | No gradient applied during radiofrequency | |
| ZTE [164] | ||
| BLAST | ||
| PETRA | ||
| ZTE | ||
| WASPI [32] | 3D hard pulse with gradient on. Preparation pulses with water and fat signal suppression | Radial from centre out, FID acquisition |
| Gradient echo | 2D, 3D | Radial rephasing gradients |
| Cones [165] | 3D | Spiral, from centre out, FID data collection |
| Spiral [166] | ||
| Stack of spirals [33] | ||
| Echo planar imaging [167] | ||
| Twisted radial projection [168] | ||
| bSSFP [116,169] | ||
| bUTE [170] | ||
| VIPR-ATR [171] | ||
| bUTE | ||
| VIPR-ATR | ||
| SWIFT, SEA [34-37] | 3D radiofrequency sub-pulses | Radial, centre out |
3D, three-dimensional; 2D, two-dimensional; UTE, ultrashort TE; BLAST, back projection low angle shot; PETRA, pointwise encoding time reduction with radial acquisition; ZTE, zero TE; WASPI, water- and fat-suppressed proton projection imaging; FID, free induction decay; bSSFP, balanced steady-state free precession; bUTE, balanced UTE; VIPR-ATR, vastly undersampled isotropic projection reconstruction-alternating length repetition times; SWIFT, sweep imaging with Fourier transformation; SEA, simultaneous excitation and acquisition.
It is possible to acquire several points at a time, which makes the sequences more time-efficient, but results in longer TEs for the additional points [30]. There are also free induction decay (FID)-based techniques, where a radial line of k-space is acquired from the centre out [31]. This can be coupled with long T2 water and fat suppression to selectively image short T2 components as water- and fat-suppressed proton projection imaging (WASPI) [32]. Other trajectories in k-space are possible, including a stack of spirals (SOS) [33] and echo planar imaging (EPI).
A particularly innovative method of imaging short T2 components is to divide the excitation pulse into sub-pulses and acquire data after each of these pulses. This is known as sweep imaging with Fourier transformation (SWIFT) or simultaneous excitation and acquisition (SEA). The acquired data need to be deconvolved with the excitation pulse, but the end result is a much more time-efficient acquisition than with typical 3D acquisitions [34-37]. Other techniques that have only been used in the pre-clinical phase include methods in which radiofrequency (rf) absorption is assessed rather than signal detection [38]. The methods borrow from continuous wave spectroscopy and electron spin resonance, where electronic T2s are extremely short and may be of the order of a microsecond.
Magnetisation preparation signal suppression techniques and pulse sequences
Traditional contrast mechanisms exploiting differences in ρm, chemical shift and other tissue properties can be used in ways that are already well known from conventional imaging.
There are also numerous old contrast mechanisms operating in new ways, as well as new contrast mechanisms that are of interest in imaging short/ultrashort T2/T2* components in tissue. They are typically used in conjunction with the acquisition techniques mentioned in the previous section. These provide a wide range of possible ways of effecting magnetisation. For example, 90°, 180°, fat saturation and magnetisation transfer pulses can all be used to suppress unwanted long T2 signals and to produce T2 contrast in the short T2 range. There are some fairly new potential mechanisms (as far as clinical imaging is concerned) that involve reductions in dipolar coupling [39,40] and double quantum filters [41]. These techniques are usually used in conjunction with one of the acquisition methods previously described.
Magnetisation transfer
When used for short T2/T2* tissues, this differs from conventional clinical approaches because short/UTE acquisitions make it possible to study MT in tendons, ligaments, menisci and cortical bone [42], and tendon [43]. The definition of the bound (short T2) and free (long T2) pools may change because previously undetected signals are included in the free (detectable) pool. Direct saturation is a greater problem. There may be a greater degree of magnetisation exchange present in short mean T2 tissues. The technique provides indirect access to supershort T2 relaxation components in tissues with T2s of approximately 5–15 μs, which are not directly accessible with most UTE techniques.
Susceptibility-weighted imaging
SWI has been in use for a considerable amount of time. It usually exploits reductions in T2* to develop contrast; the imaging may use both magnitude and phase data [44,45]. The T2* may be so short that it becomes, in effect, an indirect form of imaging using the reduction in signal of adjacent longer T2 components. The applicability of the technique and related methods can be expanded by using forms of data collection with short TEs or UTEs that can detect signal from very short T2* components [46,47]. Quantitative methods of imaging susceptibility changes need to account for errors in spatial encoding, which may require solutions to complex inverse problems [48,49]. To date, the techniques have mainly been applied to brain imaging. Phase and frequency changes can be detected in ordered fibrous structures even with UTE sequences [47].
Positive contrast and white marker imaging
These forms of imaging have been used to describe particular situations with MIOPs that may not only reduce T2 and T2*, but produce local field distortions. A variety of different methods are available. It is possible to selectively excite only off-resonance spins. It is also possible to apply an additional gradient so that only the magnetisation of spins in regions affected by MIOPs is refocused. The inhomogeneities from the particles induce echo shifts, and these can be used to calculate and correct for the field distortion. The images reflect both tissue MIOP concentration and deviations of the local magnetic field produced by the particles [50-54]. Techniques using SWIFT [55] and UTE [56-58] have also been successful for imaging MIOPs.
Imaging in the presence of metal
When forms of metal are implanted in the body, an extreme situation may arise in which there is very marked T2* shortening, but the image distortion is so great that images of regions adjacent to the metal cannot be interpreted. This has been a longstanding problem. A variety of solutions have been proposed in the past, but these have had relatively little clinical impact. The recent development of multi-acquisition variable-resonance image combination (MAVRIC) [59] and slice encoding for metal artefact correction (SEMAC) [60] has resulted in a remarkable degree of restoration of images that are grossly degraded by metallic artefact when imaged using conventional approaches [61]. The use of MAVRIC irradiation and detection of signal at the same off-resonance frequency can image signals for which the resonant frequency has been shifted by metal. The results from different off-resonance frequencies are then combined. SEMAC uses phase encoding during slice selection to relocate signals that are improperly located by the slice selection process. View angle tilting (VAT) [62] is then used with this technique to correct for errors with in-plane spatial encoding. Faster versions [63,64] and a MAVRIC–SEMAC hybrid [65] have also been implemented; the term multispectral imaging (MSI) has been applied to these approaches. UTE alone shows an improvement over conventional techniques [66], but this technique may be more effective in combination with MAVRIC [67]. It may also be used in the form of radial sampling with off-resonance recognition (RASOR) [68]. There is continued technical progress in mapping gradient and B1 distortion [69,70].
Imaging of boundaries involving short T2/T2* tissues
Structures of interest in the short T2 range include thin layers (such as those in entheses, periosteum and the deep layers of articular cartilage where there are short T2 tissues), susceptibility effects between the soft (or semisolid) tissues and bone, as well as partial volume effects between these tissues that are present over curved surfaces. In this situation, high-resolution 3D isotropic UTE imaging often has a distinct advantage since it can detect short T2/T2* signals as well as reduce the impact of susceptibility differences and partial volume effects. Imaging of ordered fibrous structures, such as tendons and ligaments, include some of the above issues, but loss of contrast of the fibre structure or “blurred” appearance may arise from obliquity of the fibres relative to the imaging slice. This effect may simulate changes due to disease. There are also distinctive artefacts at boundaries from chemical shift effects, including those associated with radial acquisitions.
Clinical proton applications
There are now two-dimensional (2D) and 3D UTE sequences available with imaging times of 5–6 min and clinically acceptable spatial resolutions [33,71]. In general, the difficulty of acquiring short/ultrashort T2/T2* signals means that invisible tissues are imaged at lower spatial resolution, but with signal levels and contrast that are not attainable with conventional techniques. There is a balance necessary to obtain qualitative and/or quantitative information that is novel with spatial resolutions that are sufficient to show anatomical features with acceptable clarity.
Cortical bone
Cortical bone can be demonstrated with high signal [28]. Its T2 is about 0.4 ms and T1 250–350 ms at 1.5 T, which is shorter than typical values for fat. Its mobile proton density is about 15–20%. Detectable signal can be used for both quantitative and qualitative studies [72-77] (Figures 1 and 2), as well as for comparison with spectroscopic studies [78]. UTE measurements of bone may be of value for attenuation corrections in PET/MRI [79,80].
Figure 1.
Ultrashort echo time subtraction MRI of the skull. The inner and outer tables are seen in a manner similar to X-ray computed tomography displayed with bone windows.
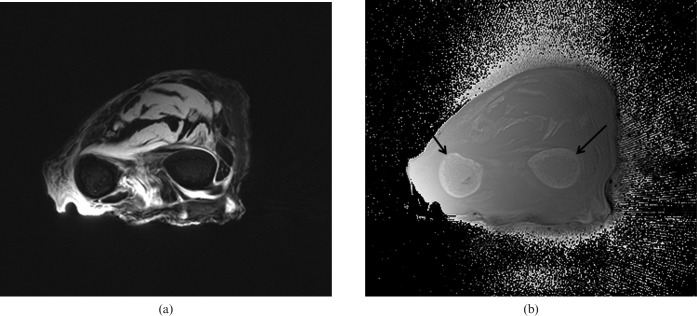
Figure 2.
(a) Transverse magnitude and (b) phase images of the forearm with a ultrasound echo time (TE; −12 μs) sequence. Differences in phase are seen between the cortical bone of the radius and ulnar (arrows) and the surrounding soft tissues, as well as between muscle and tendon in (b).
Tendons, ligaments and entheses
With conventional sequences, the signal from tendons, ligaments and entheses is very low or zero. Entheses are the attachment sites of tendons, ligaments and capsules to bone. They typically contain calcified and uncalcified fibrocartilage, which both have short T2s. These tissues have a major role in dispersing mechanical stress at the junction between flexible tendons or ligaments and rigid bone.
Tendons and ligaments contain endotenon and endoligament, which have longer T2s than the fibrous components (although they are still in the short T2 range) and less magic angle effect. Uncalcified fibrocartilage has a longer T2 than the tensile components of tendons, as well as an increase in T2 due to the magic angle effect, although this may be present over a wider range of angles and reflects the more dispersed arrangement of the fibres within it.
Tendons and ligaments can be seen readily with UTE sequences and entheses have been studied in detail [81-83]. Off-resonance fat suppression pulses reduce the signal from short T2 fibres (which have a broad linewidth) more than endotenon or enthesis fibrocartilage (which have longer T2s and narrower linewidths), and this can be an effective contrast mechanism. Inversion pulses may be used to selectively invert and null enthesis fibrocartilage (exploiting its longer T2) and so visualise this tissue with high contrast. It is also possible to visualise oblique and transverse fibres in tendons using a combination of fat-suppressed UTE sequences to reduce short T2 tissue water components and magic angle imaging to lengthen the T2 of the fibres at particular angles to B0 (Figure 3).
Figure 3.

Sagittal short echo time image of the Achilles tendon. Oblique fibres at the magic angle are seen within the tendon (white arrows). Fibrocartilage of the tendon enthesis is also seen as a high-signal area (black arrow).
Entheses are selectively involved in the seronegative spondyloarthropathies, such as ankylosing spondylitis and psoriatic arthropathy. The differential diagnosis is of a loss or reduction in fascicular pattern, and includes normal sesamoid fibrocartilage, partial volume effects with a loss of fascicular pattern due to partial volume effects, magic angle effects and disease.
The menisci of the knee
The central region of the adult meniscus has no blood supply (the white zone), while the more peripheral region (the red zone) does have one. Healing of tears in the white zone is often unsatisfactory and the preferred surgical strategy is usually resection of the torn tissue. Suture and repair is more successful in the red zone. Distinction between the two zones has not previously been possible with MRI using conventional sequences, in spite of repeated attempts [84]. Using UTE sequences and gadolinium-based contrast enhancement, the two zones can be distinguished [79] and provide a basis for surgical planning.
Anatomical descriptions of the meniscus include circumferential, radial, lamella, vertical and meshwork fibre groups. With conventional imaging, some radial fibres may be distinguishable from the majority of circumferential fibres [84], but with UTE and magic angle imaging, each of these fibre groups can be identified (Figure 4) [85-90]. It is also possible to distinguish the internal structure of the meniscus from that of the root ligaments (Figure 5), and the more central cartilaginous region from the peripheral, more fibrous region of the meniscus.
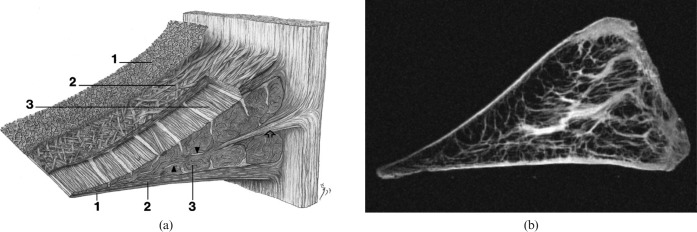
Figure 4.
(a) Diagram of the fibre structure of the meniscus from Petersen and Tillmann [172] (with permission), and (b) short echo time image of the meniscus. In (a) a very thin (30 nm) layer is shown (1) with the lamella (2) and circumferential fibres (3). In (b) layer (1) is not seen, but the external lamella fibres are seen as high-signal on the surface of the meniscus and extensive radial fibres are seen within the meniscus.
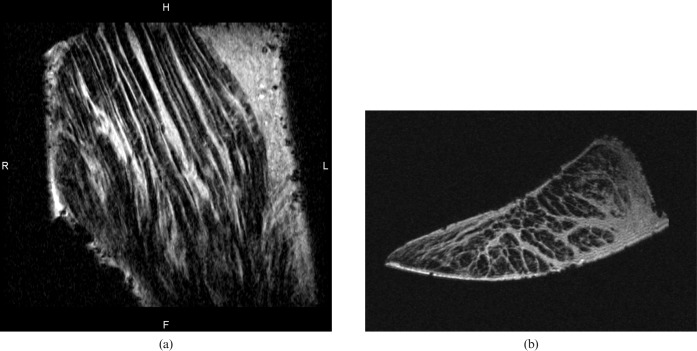
Figure 5.
(a) Longitudinal and (b) transverse short echo time images of the root ligament of the meniscus. Linear high-signal endoligament and fine transverse fibres are seen in (a). High-signal endoligament extending across the ligament is seen in (b).
The fibre structure provides a basis for understanding the biomechanics of the knee and the various patterns of tears in the meniscus. It also helps in distinguishing magic angle effects within fibre groups from degenerative changes. Quantitative studies of T1ρ and T2 may be informative [91-93]. Quantitative studies including repair have also been of value [94].
The temporomandibular joint disc shows some of the characteristics of the meniscus of the knee. Fibre structure can be seen. Lamella, circumferential anteroposterior and superoinferior fibres are identifiable (Figure 6).
Figure 6.
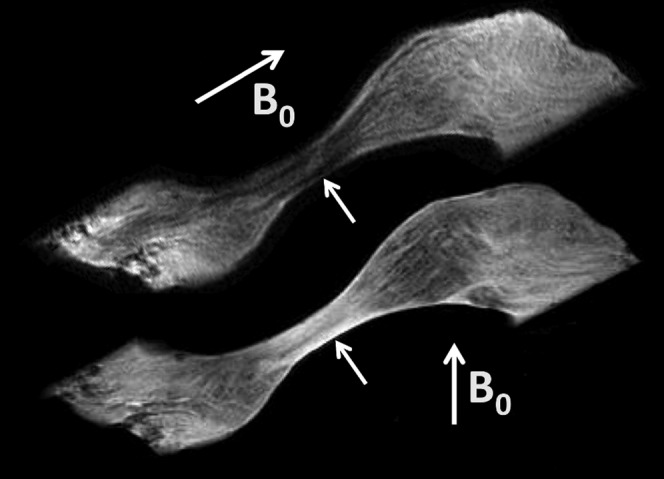
Sagittal short echo time image of the temporomandibular disc at different relations to B0 (arrows). The intermediate zone is low-signal in the upper image with anteroposterior and lamella fibres parallel to B0, and high-signal when these fibres are at the magic angle (lower image).
Articular cartilage
Articular cartilage has a range of T2s from approximately 1 to 30–40 ms, from deep to superficial. When using conventional imaging, the deep radial and calcified layers, as well as the adjacent subchondral bone, are invisible. In UTE imaging, the signal is detectable from the deeper layers of cartilage, allowing more superficial cartilage and subchondral bone to be distinguished [95-99]. This provides a basis for study of the junction between the cartilage and bone, which may be important in the pathogenesis of osteoarthritis. Complex magic angle effects are seen because of the fibrous architecture of articular cartilage.
In disease, there may be loss of signal from the deep layer and increased extent of the short T2 associated with deep layers. There is electron microscopic evidence of thinning of the deep layers in osteoarthritis, but preservation in osteomalacia.
Spine
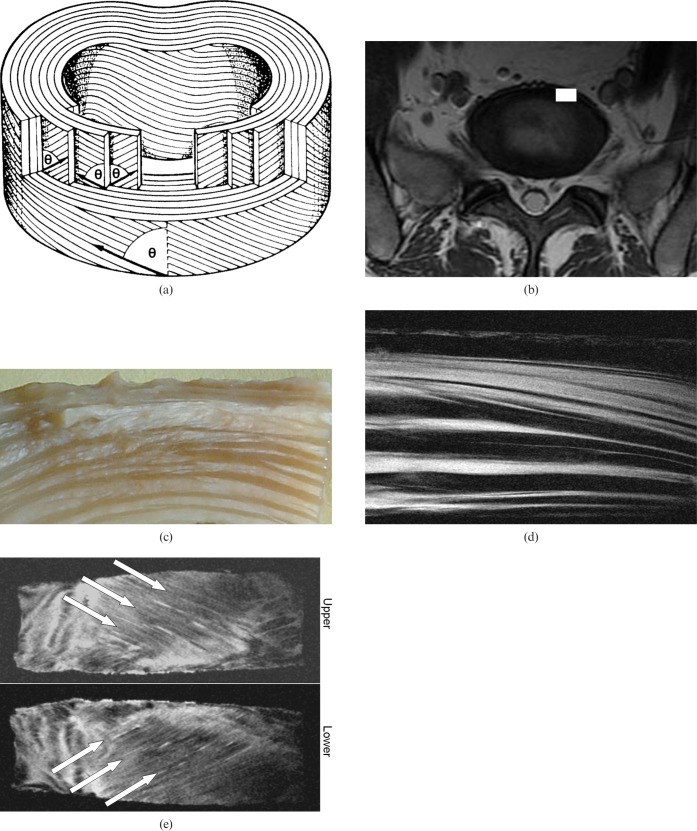
Imaging of the spine includes many visible tissues, which means attention to date has focused on invisible structures such as enthuses, the end plate of the disc [100,101], and short T2 components in the intervertebral discs and red bone marrow. Fibrocartilage has also been demonstrated in the functional entheses of the transverse ligament of the atlas and the alar ligament. The dorsal capsules of the facet joints of the lumbar spine are also subject to cartilagenous metaplasia. Evidence of iron deposition can be seen in intervertebral discs in thalassaemia [102]. Sclerotic metastases and cement after vertebroplasty have been identified [103,104]. The structure of the annulus fibrosus can be demonstrated (Figure 7). The pattern of alternating fibres between lamellae is well demonstrated.
Figure 7.
(a) Diagram of the annulus of the intervertebral disc from Bogduk [173] (with permission), (b) axial image of the L5/S1 disc, (c) photograph of a segment of an annulus of the disc, (d) the corresponding fibre structure seen with a short echo time (TE) sequence and (e) oblique coronal views of adjacent lamella. The lamella structure of the disc is shown in (a) with alternating layers of fibres at angle θ to the plane of the disc. An L5/S1 disc is shown in (b) with the white rectangle showing a section of the annulus as seen in (c). A short TE image (d) shows high signal from some lamellae and extracellular matrix, and low signal from other lamellae, following a generally alternating pattern. (e) Arrows show the fibre directions in alternate lamella at θ = 25° to the plane of the disc.
Central nervous system
There are significant short T2 components in many tissues of the body with longer mean T2s, including brain, spinal cord and peripheral nerve. These components can be specifically detected using UTE and other acquisition methods coupled with techniques that suppress long T2 signals [105-107]. It is possible to specifically image short T2 components in myelin and use these to map white matter and identify disease [108-113].
Lung and heart
Imaging of the lung was the first application of UTE imaging [31]. More recent studies have identified emphysema, cystic fibrosis and other conditions [114-120].
Fibrosis has been identified in the heart [121].
Liver
The liver contains a relatively high proportion of short T2 components. The T2*s of these may be prolonged in fibrosis [122]. Fibrosis in this situation is often of a relatively open structure and includes free water.
Pelvis
UTE sequences have found application in studying the effects of cryosurgery in carcinoma of the prostate [123]. Freezing of tissues results in a substantial reduction in T2* [124].
Atherosclerotic plaque
Short T2 components and calcification have been identified and characterised in atherosclerotic plaque [125-127].
Other proton applications
Contrast enhancement with gadolinium chelates may be seen within previously invisible tissues using UTE sequences [128], as well as with MIOPs [50-58]. Dental imaging has successfully identified caries [129-131]. Mummified tissue has also been studied [132].
Other nuclei
Sodium imaging has a long history, with the principal applications in the brain [133-138], heart [139], kidney [140,141] and musculoskeletal system [142-144]. Phosphorus imaging has also been performed with UTE sequences [145,146]. Oxygen-17 studies have been performed in the brain [135,137], and fluorine studies have begun [147].
Quantitative approaches
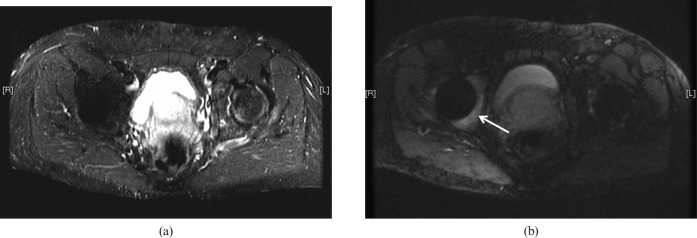
Quantitation may include specific MR properties, particularly T2 and T2* [148-152], the properties of the remaining signal after long T2 components are suppressed and the ratio of short T2 to long T2 components. There are other features (such as the magic angle effect and dipolar contrast) that can be characterised [153,154], as well as susceptibility effects (Figure 8).
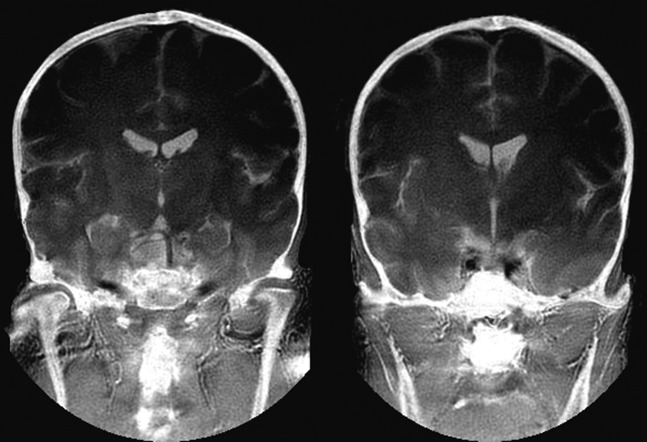
Figure 8.
(a) Conventional short tau inversion recovery (STIR) image of a prosthesis of the right hip and (b) slice encoding for metal artefact correction STIR image of the same region. The bone marrow of the acetabulum shows an increased signal in (b) (arrow). This area is not seen in (a).
There are issues regarding measuring T2 and T2* in the correct range, characterising different T2 components (e.g. long and short), including their relative proportions, and dealing with artefacts from various sources. Quantitation may be confounded by slice selection, problems with eddy currents and by contamination of short T2 components with long T2 components that are present in higher concentration.
Artefacts
Short time-constant eddy currents and gradient timing errors may result in artefacts and errors in measurement that can be corrected [155-158].
Conclusion
Imaging of short T2 and T2* components is an expanding area of application for MRI, which has seen a convergence of methods primarily targeted at tissues with short T2 components, SWI, MIOP imaging and metal artefact control. The methods have borrowed from solid-state imaging, spectroscopy (including continuous wave methods), electron spin resonance and MR microscopy. The much lower technical performance of clinical systems compared with small-bore spectrometers is a major limitation, but innovative methods for overcoming this problem are now being developed.
The tissues of interest have mainly been in the musculoskeletal system, but all tissues of the body have some short T2 components, and study of these may prove to be of diagnostic importance. Some techniques, such as imaging in the presence of metal (Figure 8), are likely to be immediately useful in the clinical domain, while others will probably require validation and comparative assessment to establish their role. Quantitative approaches may be useful given the large fractional changes in short T2 and T2* components that may be seen in disease. The techniques used for imaging often require high-gradient performance with control of short-term eddy currents to a level not previously thought necessary in clinical MR systems. In spite of these and other technical difficulties, application of the study for short T2 and T2* tissues appears to be an area of MRI that will be of considerable importance in the near future.
Acknowledgments
The author has received grant support from the National Institutes of Health and General Electric Healthcare.
References
- 1.Spiers FW. William Valentine Mayneord. Biogr Mems Fell R Soc 1991;37:343–64 [Google Scholar]
- 2.Lamerton LF. Obituary Professor W V Mayneord CBE, DSc, FInstP, FRS. Br J Radiol 1988;61:1093–4 [DOI] [PubMed] [Google Scholar]
- 3.Mayneord WV. Physics in medicine. Br Med Bull 1945;3:129–32 [Google Scholar]
- 4.Young IR, Khenia S, Thomas DG, Davis CH, Gadian DG, Cox IJ, et al. Clinical magnetic susceptibility mapping of the brain. J Comput Assist Tomogr 1987;11:2–6 [DOI] [PubMed] [Google Scholar]
- 5.Haacke EM, Reichenbach JR, (eds). Susceptibility weighted imaging in MRI: basic concepts and clinical applications. Hoboken, NJ: Wiley-Blackwell, 2011 [Google Scholar]
- 6.Krasnosselskaia LV, Fullerton GD, Dodd SJ, Cameron IL. Water in tendon: orientational analysis of the free induction decay. Magn Reson Med 2005;54:280–88 [DOI] [PubMed] [Google Scholar]
- 7.Du J, Chiang AJ, Chung CB, Statum S, Znamirowski R, Takahashi A, et al. Orientational analysis of the Achilles tendon and enthesis using an ultrashort echo time spectroscopic imaging sequence. Magn Reson Imag 2010;28:178–84 [DOI] [PubMed] [Google Scholar]
- 8.Higson GR. Seeing things more clearly. Br J Radiol 1987;60:1049–57 [DOI] [PubMed] [Google Scholar]
- 9.Mallard JR. Magnetic resonance imaging—the Aberdeen perspective on developments in the early years. Phys Med Biol 2006;51:R45–60 [DOI] [PubMed] [Google Scholar]
- 10.Smith FW, Mallard JR, Hutchison JM, Reid A, Johnson G, Redpath TW, et al. Clinical application of nuclear magnetic resonance. Lancet 1981;1:78–9 [DOI] [PubMed] [Google Scholar]
- 11.Mansfield P. Snapshot magnetic resonance imaging (Nobel Lecture). Angew Chem Int Ed Engl 2004;43:5456–64 [DOI] [PubMed] [Google Scholar]
- 12.Hinshaw WS, Andrew ER, Bottomley PA, Holland GN, Moore WS. Display of cross sectional anatomy by nuclear magnetic resonance imaging. Br J Radiol 1978;68:173–81 [DOI] [PubMed] [Google Scholar]
- 13.Hawkes RC, Holland GN, Moore WS, Worthington BS. Nuclear magnetic resonance (NMR) tomography of the brain: a preliminary clinical assessment with demonstration of pathology. J Comput Assist Tomogr 1980;4:577–86 [DOI] [PubMed] [Google Scholar]
- 14.Young IR, Hall AS, Pallis CA, Legg NJ, Bydder GM, Steiner RE. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet 1981;2:1063–6 [DOI] [PubMed] [Google Scholar]
- 15.Bailes DR, Young IR, Thomas DJ, Straughan K, Bydder GM, Steiner RE. NMR imaging of the brain using spin-echo sequences. Clin Radiol 1982;33:395–414 [DOI] [PubMed] [Google Scholar]
- 16.Bydder GM, Steiner RE, Young IR, Hall AS, Thomas DJ, Marshall J, et al. Clinical NMR imaging of the brain: 140 cases. AJR Am J Roentgenol 1982;139:215–36 [DOI] [PubMed] [Google Scholar]
- 17.Crooks LE, Mills CM, Davis PL, Brant-Zawadzki M, Hoenninger J, Arakawa M, et al. Visualization of cerebral and vascular abnormalities by NMR imaging. The effects of imaging parameters on contrast. Radiology 1982;144:843–52 [DOI] [PubMed] [Google Scholar]
- 18.Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med 1986;3:823–33 [DOI] [PubMed] [Google Scholar]
- 19.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401–7 [DOI] [PubMed] [Google Scholar]
- 20.De Coene B, Hajnal JV, Gatehouse P, Longmore DB, White SJ, Oatridge A, et al. MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. AJNR Am J Neuroradiol 1992;13:1555–64 [PMC free article] [PubMed] [Google Scholar]
- 21.Smith FW. Clinical application of NMR tomographic imaging. Witcofski RL, Karstaedt N, Partain CL. (eds). NMR imaging. Winston Salem, NC: Bowman Gray School of Medicine; 1982:125–32 [Google Scholar]
- 22.Edelstein WA, Bottomley PA, Hart HR, Leue WM, Schenck JF, Redington RW. NMR imaging at 5.1 MHz: work in progress. Witcofski RL, Karstaedt N, Partain CL. (eds). NMR imaging. Winston Salem, NC: Bowman Gray School of Medicine; 1982:139–45 [Google Scholar]
- 23.Robson MD, Gatehouse PD, Young IR, Bydder GM. Ultrashort TE (UTE) imaging of short T2 relaxation components: how should the T2 weighting be described? Proc Intl Soc Mag Reson Med 2004;11:636 [Google Scholar]
- 24.Robson MD, Gatehouse PD. Consequences of T2 relaxation during half-pulse slice selection for ultrashort TE imaging. Magn Reson Med 2010;64:610–5 [DOI] [PubMed] [Google Scholar]
- 25.Springer F, Steidle G, Martirosian P, Claussen CD, Schick F. Effects of in-pulse transverse relaxation in 3D ultrashort echo time sequences: analytical derivation, comparison to numerical simulation and experimental application at 3T. J Magn Reson 2010;206:88–96 [DOI] [PubMed] [Google Scholar]
- 26.Fullerton GD, Cameron IL, Ord VA. Orientation of tendons in the magnetic field and its effect on T2 relaxation times. Radiology 1985;155:433–5 [DOI] [PubMed] [Google Scholar]
- 27.Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ. Anisotropy of NMR properties of tissue. Magn Reson Med 1994;32:592–601 [DOI] [PubMed] [Google Scholar]
- 28.Reichert IL, Robson MD, Gatehouse PD, He T, Chappell KE, Holmes J, et al. Magnetic resonance imaging of cortical bone with ultrashort TE pulse sequences. Magn Reson Imaging 2005;23:611–18 [DOI] [PubMed] [Google Scholar]
- 29.Chen Q, Halse M, Balcom BJ. Centric scan SPRITE for spin density imaging of short relaxation time porous materials. Magn Reson Imaging 2005;23:263–6 [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Seara MA, Wehrli SL, Wehrli FW. Multipoint mapping for imaging of semi-solid materials. J Magn Reson 2003;160:144–50 [DOI] [PubMed] [Google Scholar]
- 31.Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology 1991;179:771–81 [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Ackerman JL, Chesler DA, Graham L, Wang Y, Glimcher MJ. Density of organic matrix of native mineralized bone measured by water-and-fat suppressed proton projection MRI. Magn Reson Med 2003;50:59–68 [DOI] [PubMed] [Google Scholar]
- 33.Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution three-dimensional ultra-short echo time MR imaging. Magn Reson Med 2008;60:135–45 [DOI] [PubMed] [Google Scholar]
- 34.Idiyatullin D, Corum C, Park JY, Garwood M. Fast and quiet MRI using a swept radiofrequency. J Magn Reson 2006;181:342–49 [DOI] [PubMed] [Google Scholar]
- 35.Idiyatullin D, Corum C, Moeller S, Garwood M. Gapped pulses for frequency-swept MRI. J Magn Reson 2008;193:267–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blümlich B, Gong Q, Byrne E, Greferath M. NMR with excitation modulated by Frank sequences. J Magn Reson 2009;199:18–24 [DOI] [PubMed] [Google Scholar]
- 37.Weiger M, Hennel F, Pruessmann KP. Sweep MRI with algebraic reconstruction. Magn Reson Med 2010;64:1685–95 [DOI] [PubMed] [Google Scholar]
- 38.Fagan AJ, Davies GR, Hutchison JM, Glasser FP, Lurie DJ. Development of a 3-D multi-nuclear continuous wave NMR imaging system. J Magn Reson 2005;176:140–50 [DOI] [PubMed] [Google Scholar]
- 39.Grenier D, Pascui O, Briguet A. Dipolar contrast for dense tissues imaging. J Magn Reson 2000;147:353–6 [DOI] [PubMed] [Google Scholar]
- 40.Regatte RR, Schweitzer ME, Jerschow A, Reddy R. Magic sandwich echo relaxation mapping of anisotropic systems. Magn Reson Imaging 2007;25:433–8 [DOI] [PubMed] [Google Scholar]
- 41.Navon G, Eliav U, Demco DE, Blümich B. Study of order and dynamic processes in tendon by NMR and MRI. J Magn Reson Imaging 2007;25:362–80 [DOI] [PubMed] [Google Scholar]
- 42.Springer F, Martirosian P, Machann J, Schwenzeer NF, Claussen CD, Schick F. Magnetization transfer contrast imaging in bovine and human cortical bone applying an ultrashort echo time sequence at 3 Tesla. Magn Reson Med 2009;61:1040–8 [DOI] [PubMed] [Google Scholar]
- 43.Hodgson RJ, Evans R, Wright P, Grainger AJ, O'Connor PJ, Helliwell P, et al. Quantitative magnetization transfer ultrashort echo time imaging of the Achilles tendon. Magn Reson Med 2011;65:1372–6 [DOI] [PubMed] [Google Scholar]
- 44.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC-N. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 2009;30:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 2009;30:232–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du J, Chiang AJ, Chung CB, Statum S, Znamirowski R, Takahashi A, et al. Orientational analysis of the Achilles tendon and enthesis using an ultrashort echo time spectroscopic imaging sequence. Magn Reson Imaging 2010;28:178–84 [DOI] [PubMed] [Google Scholar]
- 47.Du J, Carl M, Bydder GM. Ultrashort TE imaging: phase and frequency mapping of susceptibility effects in short T2 tissues of the musculoskeletal system. Reichenbach JR, Haacke EM. (eds). Susceptibility weighted imaging in MRI: basic concepts and clinical applications. Hoboken, NJ: Wiley-Blackwell; 2011:669-96 [Google Scholar]
- 48.Schäfer A, Wharton S, Gowland P, Bowtell R. Using magnetic field simulation to study susceptibility-related phase contrast in gradient echo MRI. Neuroimage 2009;48:126–37 [DOI] [PubMed] [Google Scholar]
- 49.de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, et al. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: validation and application to brain imaging. Magn Reson Med 2010;63:194–206 [DOI] [PubMed] [Google Scholar]
- 50.Cunningham CH, Arai T, Yang PC, McConnell MV, Pauly JM, Conolly SM. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med 2005;53:999–1005 [DOI] [PubMed] [Google Scholar]
- 51.Suzuki Y, Cunningham CH, Noguchi K, Chen IY, Weissman IL, Yeung AC, et al. In vivo serial evaluation of superparamagnetic iron-oxide labeled stem cells by off-resonance positive contrast. Mag Reson Med 2008;60:1269–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu W, Frank JA. Detection and quantification of magnetically labeled cells by cellular MRI. Eur J Radiol 2009;70:258–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahnke H, Liu W, Herzka D, Frank JA, Schaeffter T. Susceptibility gradient mapping (SGM): a new post-processing method for positive contrast generation applied to superparamagnetic iron oxide particle (SPIO)-labeled cells. Magn Reson Med 2008;60:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W, Dahnke H, Rahmer J, Jordan EK, Frank JA. Ultrashort T2* relaxometry for quantitation of highly concentrated superparamagnetic iron oxide (SPIO) nanoparticle labeled cells. Magn Reson Med 2009;61:761–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou R, Idiyatullin D, Moeller S, Corum C, Zhang H, Qiao H, et al. SWIFT detection of SPIO-labeled stem cells grafted in the myocardium. Magn Reson Med 2010;63:1154–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crowe LA, Wang Y-X, Gatehouse P, Tessier J, Waterton J, Robert P, et al. Ex vivo MR imaging of atherosclerotic rabbit aorta labeled with USPIO – enhancement of iron loaded regions in UTE imaging. Proc Intl Soc Mag Reson Med 2005;13:115 [Google Scholar]
- 57.Girard OM, Du J, Agemy L, Sugahara KN, Rotamraju VR, Ruoslahti E, et al. Optimization of iron oxide nanoparticle detection using ultrashort echo time pulse sequences: comparison of T1, T2*, and synergistic T1-T2* contrast mechanisms. Magn Reson Med 2011;65:1649–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Zhong X, Wang L, Chen H, Wang YA, Yeh J, et al. T1-weighted ultrashort echo time method for positive contrast imaging of magnetic nanoparticles and cancer cells bound with the targeted nanoparticles. J Magn Reson Imaging 2011;33:s194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med 2009;61:381–90 [DOI] [PubMed] [Google Scholar]
- 60.Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: slice encoding for metal artifact correction in MRI. Magn Reson Med 2009;62:66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koff MF, Hayter CL, Shah P, Koch KM, Miller TT, Potter HG. Magnetic resonance imaging of arthroplasty: comparison of MAVRIC and conventional fast spin echo techniques. Proc Intl Soc Mag Reson Med 2011;19:289. [DOI] [PubMed] [Google Scholar]
- 62.Cho ZH, Kim DJ, Kim YK. Total inhomogeneity correction including chemical shifts and susceptibility by view angle tilting. Med Phys 1988;15:7–11 [DOI] [PubMed] [Google Scholar]
- 63.Hargreaves BA, Chen W, Lu W, Alley MT, Gold GE, Brau AC, et al. Accelerated slice encoding for metal artifact correction. J Magn Reson Imaging 2010;31:987–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li G, Nittka M, Paul D, Lauer L. MSVAT-SPACE for fast metal implants imaging. Proc Intl Soc Mag Reson Med 2011;19:3171 [Google Scholar]
- 65.Koch KM, Brau AC, Chen W, Gold GE, Hargreaves BA, Koff M, et al. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med 2011;65:71–82 [DOI] [PubMed] [Google Scholar]
- 66.Rahmer J, Börnert P, Dries SP. Assessment of anterior cruciate ligament reconstruction using 3D ultrashort echo-time MR imaging. J Magn Reson Imaging 2009;29:443–8 [DOI] [PubMed] [Google Scholar]
- 67.Carl M, Du J, Koch K. Investigations on imaging near metal with combined 3D UTE-MAVRIC. Proc Intl Soc Mag Reson Med 2011;19:2668 [Google Scholar]
- 68.Seevinck PR, de Leeuw H, Bos C, Bakker CJG. Highly localized positive contrast of small paramagnetic objects using 3D center-out radial sampling with off-resonance reception. Magn Reson Med 2011;65:146–56 [DOI] [PubMed] [Google Scholar]
- 69.Koch KM, King KF, Chen W, Gold G, Hargreaves BA. Frequency encoding in the presence of extreme static field gradients. Proc Intl Soc Mag Reson Med 2011;19:293 [Google Scholar]
- 70.Monu UD, Worters PW, Sung K, Koch KM, Gold GE, Hargreaves BA. B1 mapping near metallic implants. Proc Intl Soc Mag Reson Med 2011;19:3175 [Google Scholar]
- 71.Wang K, Yu H, Brittain JH, Reeder SB, Du J. k-space water-fat decomposition with T2* estimation and multifrequency fat spectrum modeling for ultrashort echo time imaging. J Magn Reson Imaging 2010;31:1027–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology 2008;248:824–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anumula S, Wehrli SL, Magland J, Wright AC, Wehrli FW. Ultra-short echo-time MRI detects changes in bone mineralization and water content in OVX rat bone in response to alendronate treatment. Bone 2010;46:1391–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson 2010;207:304–11 [DOI] [PubMed] [Google Scholar]
- 75.Rad HS, Lam SC, Magland JF, Ong H, Li C, Song HK, et al. Quantifying cortical bone water in vivo by three-dimensional ultra-short echo-time MRI. NMR Biomed 2011;24:855–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kokabi N, Bae W, Diaz E, Chung CB, Bydder GM, Du J. Ultrashort TE MR imaging of bovine cortical bone: the effect of water loss on the T(1) and T(2)* relaxation times. Magn Reson Med 2011;18:1174–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horch RA, Gochberg DF, Nyman JS, Does MD. Selective imaging of bound and pore water in human cortical bone. Proc Intl Soc Mag Reson Med 2011;19:1117 [Google Scholar]
- 78.Horch RA, Nyman JS, Gochberg DF, Dortch RD, Does MD. Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging. Magn Reson Med 2010;64:680–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keereman V, Fierens Y, Broux T, De Deene Y, Lonneux M, Vandenberghe S. MRI-based attenuation correction for PET/MRI using ultrashort echo time sequences. J Nucl Med 2010;51:812–8 [DOI] [PubMed] [Google Scholar]
- 80.Catana C, van derKouwe A, Benner T, Michel CJ, Hamm M, Fenchel M, et al. Toward implementing an MRI-based PET attenuation-correction method for neurologic studies on the MR-PET brain prototype. J Nucl Med 2010;51:1431–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benjamin M, Milz S, Bydder GM. Magnetic resonance imaging of entheses. Part 1. Clin Radiol 2008;63:691–703 [DOI] [PubMed] [Google Scholar]
- 82.Benjamin M, Milz S, Bydder GM. Magnetic resonance imaging of entheses. Part 2. Clin Radiol 2008;63:704–11 [DOI] [PubMed] [Google Scholar]
- 83.Hodgson RJ, Grainger AJ, O'Connor PJ, Evans R, Coates L, Marzo-Ortega H, et al. Imaging of the Achilles tendon in spondyloarthritis: a comparison of ultrasound and conventional, short and ultrashort echo time MRI with and without intravenous contrast. Eur Radiol 2011;21:1144–52 [DOI] [PubMed] [Google Scholar]
- 84.Hauger O, Frank LR, Boutin RD, Lektrakul N, Chung CB, Haghighi P, et al. Characterization of the “red zone” of knee meniscus: MR imaging and histologic correlation. Radiology 2000;217:193–200 [DOI] [PubMed] [Google Scholar]
- 85.Gatehouse PD, He T, Puri BK, Thomas RD, Resnick D, Bydder GM. Contrast-enhanced MRI of the menisci of the knee using ultrashort echo time (UTE) pulse sequences: imaging of the red and white zones. Br J Radiol 2004;77:641–7 [DOI] [PubMed] [Google Scholar]
- 86.Bydder M, Rahal A, Fullerton GD, Bydder GM. The magic angle effect: a source of artifact, determinant of image contrast, and technique for imaging. J Magn Reson Imaging 2007;25:290–300 [DOI] [PubMed] [Google Scholar]
- 87.Bydder GM, Chung CB. Magnetic resonance imaging of short T2 relaxation components in the musculoskeletal system. Skeletal Radiol 2009;8:201–5 [DOI] [PubMed] [Google Scholar]
- 88.Bydder GM. Imaging of short and ultrashort T2 and T2* tissues using clinical MR systems. Imaging Med 2010;2:225–33 [Google Scholar]
- 89.Wang M, Radjenovic A, Stapleton TW, Venkatesh R, Williams S, Ingham E, et al. A novel and non-destructive method to examine meniscus architecture using 9.4 Tesla MRI. Osteoarthritis Cartilage 2010;18:1417–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsai P-H, Li C, Magland J, Huang T-Y, Wehrli FW, Chung H-W. Demonstration of meniscal fiber structure in vivo by radial imaging with minimal phase excitation and adiabatic fat suppression pulses at high field. Proc Intl Soc Mag Reson Med 2011;19:1115 [Google Scholar]
- 91.Du J, Carl M, Diaz E, Takahashi A, Han E, Szeverenyi NM, et al. Ultrashort TE T1rho (UTE T1rho) imaging of the Achilles tendon and meniscus. Magn Reson Med 2010;64:834–42 [DOI] [PubMed] [Google Scholar]
- 92.Stehling C, Luke A, Stahl R, Baum T, Joseph G, Pan J, et al. Meniscal T1rho and T2 measured with 3.0T MRI increases directly after running a marathon. Skeletal Radiol 2011;40:725–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams A, Qian Y, Chu CR. Clinical ultra-short TE-enhanced T2* mapping of meniscus. Proc Intl Soc Mag Reson Med 2011;19:562 [Google Scholar]
- 94.Koff MF, Fortier LA, Rodeo SA, Takahashi A, Maher S, Delos D. Temporal and regional changes of T2* in the repaired meniscus. Proc Intl Soc Mag Reson Med 2011;19:568 [Google Scholar]
- 95.Gold GE, Thedens DR, Pauly JM, Fechner KP, Bergman G, Beaulieu CF, et al. MR imaging of articular cartilage of the knee: new methods using ultrashort TEs. AJR Am J Roentgenol 1998;170:1223–6 [DOI] [PubMed] [Google Scholar]
- 96.Omoumi P, Teixeira P, Delgado G, Chung CB. Imaging of lower limb cartilage. Top Magn Reson Imaging 2009;20:189–201 [DOI] [PubMed] [Google Scholar]
- 97.Koff MF, Potter HG. Non-contrast MR techniques and imaging of cartilage. Radiol Clin North Am 2009;47:495–504 [DOI] [PubMed] [Google Scholar]
- 98.Nissi MJ, Rautiainen J, Lehto LJ, Tiitu V, Kiviranta O, Pulkkinen H, et al. SWIFT imaging of osteochondral repair in equine model with correlation to μCT. Proc Intl Soc Mag Reson Med 2011;19:564 [Google Scholar]
- 99.Salo E-N, Nissi MJ, Liimatainen T, Gröhn O, Mangia S, Michaeli S, et al. Multi-parametric MRI assessment of articular cartilage degeneration. Proc Intl Soc Mag Reson Med 2011;19:1108 [Google Scholar]
- 100.Law T, Samartzis D, Kim M, Chan Q, Khong P-L, Cheung MK, et al. Ultrashort time-to-echo MRI of the cartilaginous endplate and relationship to degenerative disc disease and Schmorl's nodes. Proc Intl Soc Mag Reson Med 2011;19:570 [Google Scholar]
- 101.Moon SM, Yoder JH, Elliott DM, Wehrli FW, Wright AC. In vivo MRI of the cartilaginous endplate of the intervertebral disc. Proc Intl Soc Mag Reson Med 2011;19:1120 [Google Scholar]
- 102.Hall-Craggs MA, Porter J, Gatehouse PD, Bydder GM. Ultrashort echo time (UTE) MRI of the spine in thalassaemia. Br J Radiol 2004;77:104–10 [DOI] [PubMed] [Google Scholar]
- 103.Messiou C, Collins DJ, Robson MD, Bydder GM, Morgan VA, deSouza NM. Quantifying sclerotic bone metastases with 2D ultra short TE MRI: a feasibility study. Cancer Biomarkers 2010;7:211–8 [DOI] [PubMed] [Google Scholar]
- 104.Hiwatashi A, Yoshiura T, Yamashita K, Kamano H, Honda H. Ultrashort TE MRI: usefulness after percutaneous vertebroplasty. AJR Am J Roentgenol 2010;195:W365–W368 [DOI] [PubMed] [Google Scholar]
- 105.Waldman A, Rees JH, Brock CS, Robson MD, Gatehouse PD, Bydder GM. MRI of the brain with ultra-short echo-time pulse sequences. Neuroradiology 2003;45:887–92 [DOI] [PubMed] [Google Scholar]
- 106.Portman O, Flemming S, Cox JP, Johnston DG, Bydder GM. Magnetic resonance imaging of the normal pituitary gland using ultrashort TE (UTE) pulse sequences. Neuroradiology 2008;50:213–20 [DOI] [PubMed] [Google Scholar]
- 107.Lehto LJ, Djaudat I, Corum CA, Garwood M, Gröhn OH. MRI detection of short T2 component in brain by SWIFT. Proc Int Soc Mag Reson Med 2010;17:3326 [Google Scholar]
- 108.Minty EP, Bjarnason TA, Laule C, MacKay AL. Myelin water measurement in the spinal cord. Magn Reson Med 2009;61:883–92 [DOI] [PubMed] [Google Scholar]
- 109.Kolind SH, Mädler B, Fischer S, Li DK, MacKay AL. Myelin water imaging: implementation and development at 3.0T and comparison to 1.5T measurements. Magn Reson Med 2009;62:106–15 [DOI] [PubMed] [Google Scholar]
- 110.Macmillan EL, Mädler B, Fichtner N, Dvorak MF, Li DK, Curt A, et al. Myelin water and T(2) relaxation measurements in the healthy cervical spinal cord at 3.0T: repeatability and changes with age. Neuroimage 2011;54:1083–90 [DOI] [PubMed] [Google Scholar]
- 111.Laule C, Vavasour IM, Leung E, Li DK, Kozlowski P, Traboulsee AL, et al. Pathological basis of diffusely abnormal white matter: insights from magnetic resonance imaging and histology. Mult Scler 2011;17:144–50 [DOI] [PubMed] [Google Scholar]
- 112.Horch RA, Gore JC, Does MD. Origins of the ultrashort T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med 2011;66:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilhelm MJ, Ong HH, Wehrli SL, Tsai P-H, Hackney DB, Wehrli FW. Prospects for quantitative imaging of myelin with dual-echo short inversion time 3D UTE MRI. Proc Intl Soc Mag Reson Med 2011;19:2460 [Google Scholar]
- 114.Takahashi M, Togao O, Obara M, van Cauteren M, Ohno Y, Doi S, et al. Ultra-short echo time (UTE) MR imaging of the lung: comparison between normal and emphysematous lungs in mutant mice. J Magn Reson Imaging 2010;32:326–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Togao O, Tsuji R, Ohno Y, Dimitrov I, Takahashi M. Ultrashort echo time (UTE) MRI of the lung: assessment of tissue density in the lung parenchyma. Magn Reson Med 2010;64:1491–8 [DOI] [PubMed] [Google Scholar]
- 116.Failo R, Wielopolski PA, Tiddens HA, Hop WC, Mucelli RP, Lequin MH. Lung morphology assessment using MRI: a robust ultra-short TR/TE 2D steady state free precession sequence used in cystic fibrosis patients. Magn Reson Med 2009;61:299–306 [DOI] [PubMed] [Google Scholar]
- 117.Corum CA, Idiyatullin D, Moeller S, Chamberlain R, Sachdev D, Garwood M. Lung imaging in the mouse with SWIFT. Proc Int Soc Mag Res Med 2010;17:204 [Google Scholar]
- 118.Ohno Y, Koyama H, Yoshikawa T, Aoyama N, Takenaka D, Matsumoto K, et al. T2* measurements of 3.0 T MRI with ultra-short TE: capabilities of pulmonary functional assessment and clinical stage classification in smokers. Proc Intl Soc Mag Reson Med 2011;19:3033 [Google Scholar]
- 119.Muradyan I, Hrovat M, Dabaghyan M, Butler J, Hatabu H, Patz S. Pulmonary T2* dependence on the lung volume: preliminary results. Proc Intl Soc Mag Reson Med 2011;19:933 [Google Scholar]
- 120.Rajaram S, Swift AJ, Capener D, Condliffe R, Elliot C, Hurdman J, et al. Comparative study of SSPF lung MRI at 1.5T with high resolution computed tomography in patients with interstitial lung fibrosis. Proc Intl Soc Mag Reson Med 2011;19:3036 [Google Scholar]
- 121.de Jong S, Zwanenburg JJ, Visser F, van derNagel R, van Rijen HV, Vos MA, et al. Direct detection of postinfarction myocardial fibrosis with ultrashort TE (UTE) MRI. Proc Intl Soc Mag Reson Med 2011;19:1352 [Google Scholar]
- 122.Chappell KE, Patel N, Gatehouse PD, Main J, Puri BK, Taylor-Robinson SD, et al. Magnetic resonance imaging of the liver with ultrashort TE (UTE) pulse sequences. J Magn Reson Imaging 2003;18:709–13 [DOI] [PubMed] [Google Scholar]
- 123.Wansapura JP, Daniel BL, Vigen KK, Butts K. In vivo MR thermometry of frozen tissue using R2* and signal intensity. Acad Radiol 2005;12:1080–4 [DOI] [PubMed] [Google Scholar]
- 124.Kaye EA, Josan S, Lu A, Rosenberg J, Daniel BL, Pauly KB. Consistency of signal intensity and T2* in frozen ex vivo heart muscle, kidney, and liver tissue. J Magn Reson Imaging 2010;31:719–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chan CF, Keenan NG, Nielles-Vallespin S, Gatehouse P, Sheppard MN, Boyle JJ, et al. Ultra-short echo time cardiovascular magnetic resonance of atherosclerotic carotid plaque. J Cardiovasc Magn Reson 2010;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma S, Boujraf S, Bornstedt A, Hombach V, Ignatius A, Oberhuber A, et al. Quantification of calcification in endarterectomy samples by means of high-resolution ultra-short echo time imaging. Invest Radiol 2010;45:109–13 [DOI] [PubMed] [Google Scholar]
- 127.Du J, Corbeil J, Znamirowski R, Angle N, Peterson M, Bydder GM, et al. Direct imaging and quantification of carotid plaque calcification. Magn Reson Med 2010;65:1013–20 [DOI] [PubMed] [Google Scholar]
- 128.Robson MD, Gatehouse PD, So PW, Bell JD, Bydder GM. Contrast enhancement of short T2 tissues using ultrashort TE (UTE) pulse sequences. Clin Radiol 2004;59:720–6 [DOI] [PubMed] [Google Scholar]
- 129.Tymofiyeva O, Boldt J, Rottner K, Schmid F, Richter EJ, Jakob PM. High-resolution 3D magnetic resonance imaging and quantification of carious lesions and dental pulp in vivo. MAGMA 2009;22:365–374 [DOI] [PubMed] [Google Scholar]
- 130.Djaudat I, Corum CA, Moeller S, Prasad HS, Garwood M, Nixdorf DR. SWIFT versus X-ray in dental imaging. Proc Int Soc Mag Reson Med 2010;17:543 [Google Scholar]
- 131.Bracher AK, Hofmann C, Bornstedt A, Boujraf S, Hell E, Ulrici J, et al. Feasibility of ultra-short echo time (UTE) magnetic resonance imaging for identification of carious lesions. Magn Reson Med 2011;66:538–45 [DOI] [PubMed] [Google Scholar]
- 132.Rühli FJ, von Waldburg H, Nielles-Vallespin S, Böni T, Speier P. Clinical magnetic resonance imaging of ancient dry human mummies without rehydration. JAMA 2007;298:2618–20 [DOI] [PubMed] [Google Scholar]
- 133.Ra JB, Hilal SK, Cho ZH. A method for in vivo MR imaging of the short T2 component of sodium-23. Magn Reson Med 1986;3:296–302 [DOI] [PubMed] [Google Scholar]
- 134.Nielles-Vallespin S, Weber MA, Bock M, Bongers A, Speier P, Combs SE, et al. 3D radial projection technique with ultrashort echo times sodium MRI: clinical applications in human brain and skeletal muscle. Magn Reson Med 2007;57:74–81 [DOI] [PubMed] [Google Scholar]
- 135.Atkinson IC, Sonstegaard R, Pliskin NH, Thulborn KR. Vital signs and cognitive function are not affected by 23-sodium and 17-oxygen magnetic resonance imaging of the human brain at 9.4T. J Magn Reson Imaging 2010;32:82–7 [DOI] [PubMed] [Google Scholar]
- 136.Lu A, Atkinson IC, Claiborne TC, Damen FC, Thulborn KR. Quantitative sodium imaging with a flexible twisted projection pulse sequence. Magn Reson Med 2010;63:1583–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Atkinson IC, Thornton KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage 2010;51:723–33 [DOI] [PubMed] [Google Scholar]
- 138.Lu A, Atkinson IC, Thulborn KR. In vivo brain sodium T2* mapping with a multiple-echo flexible TPI sequence. Proc Intl Soc Mag Reson Med 2011;19:3504 [Google Scholar]
- 139.Ouwerkerk R, Bottomley PA, Solaiyappan M, Spooner AE, Tomaselli GF, Wu KC, et al. Tissue sodium concentration in myocardial infarction in humans: a quantitative 23Na MR imaging study. Radiology 2008;248:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Furlan A, Moon C-H, Kim J-H, He X, Park B, Zaho T, et al. Sodium MR imaging of human kidney using a dual-tuned (23Na/1H) body RF coil at 3T: quantitative assessment of sodium concentration and corticomedullary gradient in healthy subjects. Proc Intl Soc Mag Reson Med 2011;19:2947 [Google Scholar]
- 141.Kalayciyan R, Wetterling F, Neudecker S, Schad LR. In vivo sodium imaging of kidney using 3D ultrashort echo time sequence. Proc Intl Soc Mag Reson Med 2011;19:1489 [Google Scholar]
- 142.Madelin G, Lee JS, Inati S, Jerschow A, Regatte RR. Sodium inversion recovery MRI of the knee joint in vivo at 7T. J Magn Reson 2010;207:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang L, Wu Y, Chang G, Oesingmann N, Schweitzer ME, Jerschow A, et al. Rapid isotropic 3D-sodium MRI of the knee joint in vivo at 7T. J Magn Reson Imaging 2009;30:606–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang G, Wang L, Schweitzer ME, Regatte RR. 3D 23Na MRI of human skeletal muscle at 7 Tesla: initial experience. Eur Radiol 2010;20:2039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Robson MD, Tyler DJ, Neubauer S. Ultrashort TE chemical shift imaging (UTE-CSI). Magn Reson Med 2005;53:267–74 [DOI] [PubMed] [Google Scholar]
- 146.Ackerman JL, Wu Y, Reese TG, Cao H, Hrovat MI, Toddes SP, et al. In vivo 31P solid state MRI of human wrists: short-T2 MRI using the scanner 1H channel. Proc Intl Soc Mag Reson Med 2011;19:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hitchens TK, Ye Q, Ho C. 3D ultra short TE MRI for whole subject imaging of perfluorocarbon-labeled cell biodistribution. Proc Intl Soc Mag Reson Med 2011;19:1706 [Google Scholar]
- 148.Qian Y, Williams AA, Chu CR, Boada FE. Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med 2010;64:1426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage 2010;18:539–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Williams A, Qian Y, Chu CR. UTE- T2* mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage 2011;19:84–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Filho GH, Du J, Pak BC, Statum S, Znamorowski R, Haghighi P, et al. Quantitative characterization of the Achilles tendon in cadaveric specimens: T1 and T2* measurements using ultrashort-TE MRI at 3 T. AJR Am J Roentgenol 2009;192:W117–24 [DOI] [PubMed] [Google Scholar]
- 152.Kirsch S, Schad LR. Single-slice mapping of ultrashort T(2). J Magn Reson 2011;210:133–6 [DOI] [PubMed] [Google Scholar]
- 153.Du J, Pak BC, Znamirowski R, Statum S, Tatkahashi A, Chung CB, et al. Magic angle effect in magnetic resonance imaging of the Achilles tendon and enthesis. Magn Reson Imaging 2009;27:557–64 [DOI] [PubMed] [Google Scholar]
- 154.Szeverenyi NM, Bydder GM. Dipolar anisotropy fibre imaging in a goat knee meniscus. Magn Reson Med 2011;65:463–70 [DOI] [PubMed] [Google Scholar]
- 155.Atkinson IC, Lu A, Thulborn KR. Characterization and correction of system delays and eddy currents for MR imaging with ultrashort echo-time and time-varying gradients. Magn Reson Med 2009;62:532–7 [DOI] [PubMed] [Google Scholar]
- 156.Josan S, Pauly JM, Daniel BL, Pauly KB. Double half RF pulses for reduced sensitivity to eddy currents in UTE imaging. Magn Reson Med 2009;61:1083–9 [DOI] [PubMed] [Google Scholar]
- 157.Josan S, Kaye E, Pauly JM, Daniel BL, Pauly KB. Improved half RF slice selectivity in the presence of eddy currents with out-of-slice saturation. Magn Reson Med 2009;61:1090–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Takizawa M, Hanada H, Oka K, Takahashi T. Correcting K-trajectory by using multiple function models of gradient waveform for ultrashort TE (UTE). Proc Intl Soc Mag Reson Med 2011;19:4385 [Google Scholar]
- 159.Madio DP, Lowe IJ. Ultra-fast imaging using low flip angles and FIDs. Magn Reson Med 1995;34:525–9 [DOI] [PubMed] [Google Scholar]
- 160.Hsu J-J, Lowe IJ. Signal recovery in free induction decay imaging using a stimulated spin echo. Magn Reson Med 2002;47:409–14 [DOI] [PubMed] [Google Scholar]
- 161.Paley MNJ, Kryukov E, Lamperth M, Young IR. An independent multichannel imaging research system for ultrashort echo time imaging on clinical MR systems. Concepts Mag Reson Part B: Mag Reson Eng 2009;35B:80–8 [Google Scholar]
- 162.Hafner S. Fast imaging in liquids and solids with the Back-projection Low Angle ShoT (BLAST) technique. Magn Reson Imaging 1994;12:1047–51 [DOI] [PubMed] [Google Scholar]
- 163.Grodzki DM, Jakob PM, Heismann B. Ultra short echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Proc Intl Soc Mag Reson Med 2011;19:2815. [DOI] [PubMed] [Google Scholar]
- 164.Weiger M, Stampanoni M, Pruessmann KP. Direct depiction of bone microstructure using ZTE imaging. Proc Intl Soc Mag Reson Med 2011;19:563 [Google Scholar]
- 165.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med 2006;55:575–82 [DOI] [PubMed] [Google Scholar]
- 166.Du J, Bydder M, Takahashi AM, Chung CB. Two-dimensional ultrashort echo time imaging using a spiral trajectory. Magn Reson Imaging 2008;26:304–12 [DOI] [PubMed] [Google Scholar]
- 167.Hetzer S, Mildner T, Möller HE. A modified EPI sequence for high-resolution imaging at ultra-short echo time. Magn Reson Med 2011;65:165–75 [DOI] [PubMed] [Google Scholar]
- 168.Krämer P, Konstandin S, Heilmann M, Schad LR. 3D radial twisted projection imaging for DCE-MRI with variable flip angles. Proc Intl Soc Mag Reson Med 2011;19:2055 [Google Scholar]
- 169.Martirosian P, Schraml C, Schwenzer NF, Springer F, Schick F, Deimling M. Magnetic resonance imaging of tendons, ligaments and menisci by subtraction of two steady free precession signals. Proc Intl Soc Mag Reson Med 2011;19:2724. [DOI] [PubMed] [Google Scholar]
- 170.Diwoky C, Stollberger R. 3D radial bUTE. Proc Intl Soc Mag Reson Med 2011;19:387 [Google Scholar]
- 171.Kijowski R, Klaers J, Lee K, Rosas H, Hernandez L, Block W. Rapid multi-planar assessment of the articular cartilage of the knee joint using isotropic resolution VIPR-ATR imaging. Proc Intl Soc Mag Reson Med 2011;19:503 [Google Scholar]
- 172.Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anat Embryol 1998;197:317–24 [DOI] [PubMed] [Google Scholar]
- 173.Bogduk N. The inter-body joint and the intervertebral discs. Clinical anatomy of the lumbar spine and sacrum. 3rd edn Churchill Livingstone: Edinburgh, UK: 1997;13 [Google Scholar]