Abstract
This commentary will discuss the use of the “stroke window” settings in the evaluation of CT head examinations and advocate their more widespread use in patients who present with neurological symptoms in addition to patients with suspected stroke. We present examples of the use of stroke windows, which revealed subtle abnormalities that were not readily apparent on default brain window settings and were subsequently confirmed on MRI or follow-up CT. As a result we suggest that stroke windows should be routine in the review of all CT head examinations.
The initial use of CT rather than MRI in outpatients with vague or non-specific neurological symptoms, including suspected non-sinus headache, is easily defensible because CT is relatively cheap, fast and widely available. CT is able to detect the presence of most significant intracranial pathologies, thereby facilitating a “filtered” fast-track for patients to MRI and justifying the higher radiation burden to the individual.
Unfortunately, soft tissue contrast resolution of CT is inferior to MRI [1] even with modern multidetector and dual source scanners, which means that some important intracranial lesions that would have been detected by MRI could be missed on CT.
However, the use of narrow window width (and therefore high-contrast) CT review settings (i.e. “stroke windows” [2] 40 WW 40 WL) as an integral part of the general evaluation of CT brain examination helps to increase detection of subtle, potentially significant lesions.
This article provides examples to support the continued use of “stroke” review parameters and advocates their more widespread routine use in the radiological evaluation of non-contrast CT brain examination.
Case examples
The importance of stroke windows to review CT brain examinations in either inpatient or emergency situations in individuals presenting with a sudden onset of focal neurological signs is self-evident.
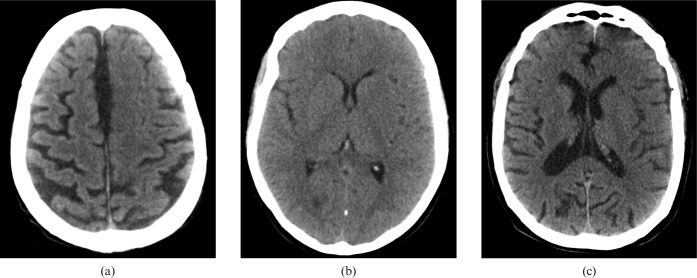
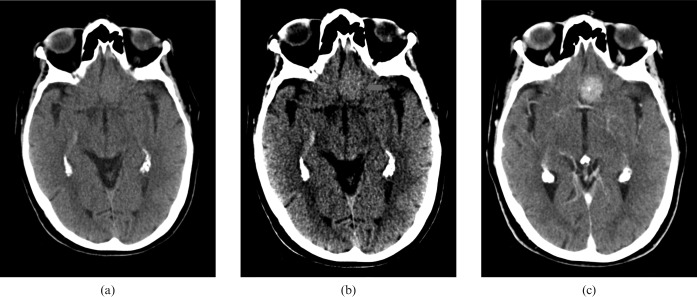
Figure 1 shows the CT images of three different patients, who each presented with sudden onset of focal neurology, displayed as they would have been on the local patient archive and communication system (PACS) with the default “brain” review settings (100 WW 40 WL). All were initially reported as normal.
Figure 1.
Default “brain” window settings on CT images of three different patients (a), (b) and (c), who each present with sudden onset of focal neurology.
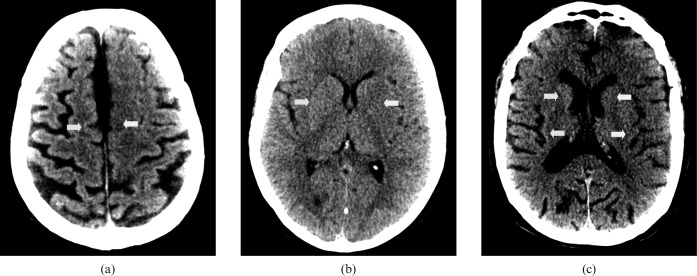
As a part of our department's ongoing quality assurance programme, all these cases were reviewed early the following day. Using the stroke review settings (40 WW 40 WL) shown in Figure 2, the conspicuity of the asymmetry resulting from reduced attenuation of the grey matter due to local cytotoxic oedema secondary to anoxic damage (white arrows) is clearly increased. The corresponding contralateral unaffected grey matter is indicated with yellow arrows.
Figure 2.
Harsh “stroke” window settings on review the following day of the same patients as Figure 1 (a), (b) and (c). The visualisation of local cytotoxic oedema secondary to anoxic damage (white arrows) is clearly increased. The corresponding contralateral unaffected grey matter is indicated with yellow arrows.
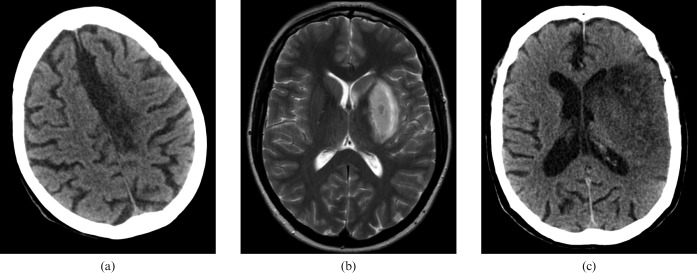
Follow-up imaging confirmed the suspected ischaemic lesions and is shown in Figure 3.
Figure 3.
Subsequent confirmatory imaging of the same three patients in Figures 1 and 2 (a), (b) and (c).
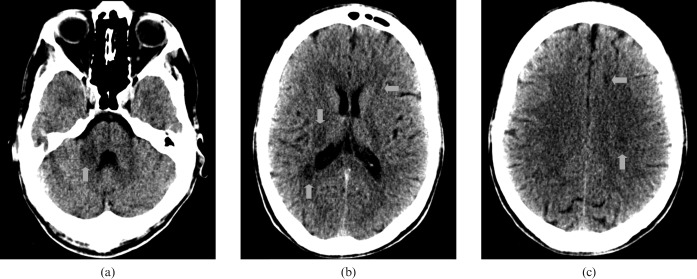
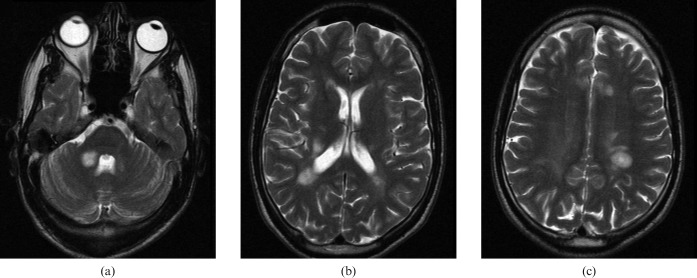
The benefits of these relatively harsh review parameters extend beyond their use in suspected cerebrovascular accident (CVA). Figure 4 shows the CT image of a 26-year-old male undergoing outpatient imaging because of an ataxic gait.
Figure 4.
(a), (b) and (c). Three CT images of a 26-year-old male outpatient with an ataxic gait. Subtle areas of reduced attenuation are more evident on these stroke windows (green arrows).
Subtle areas of reduced attenuation are more evident on stroke windows (green arrows) and suggest possible demyelination. MRI (Figure 5) confirms this diagnosis.
Figure 5.
(a), (b), (c). Confirmatory MRI of same patient as Figure 4 (comparable anatomical images shown).
A significant benefit of the routine use of these review parameters is appreciated with the increased identification of tumours, especially those that have subtly different tissue density to brain, but are not causing any significant mass effect or oedematous changes at the time of imaging.
The following individuals all attended as outpatients and had a non-contrast scan. Again, many were subsequently reported as normal, but were identified as being potentially abnormal during our daily quality assurance regime and were subsequently scheduled for either urgent MRI or, in one case because of the patient's pacemaker, contrast-enhanced CT.
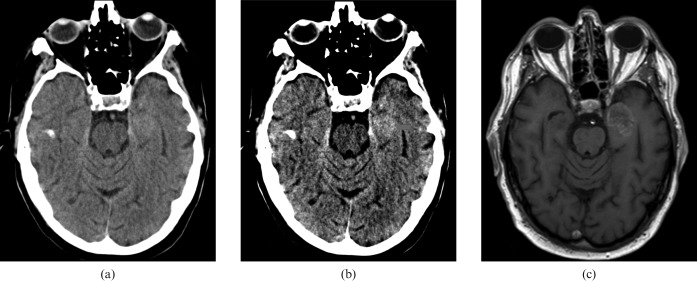
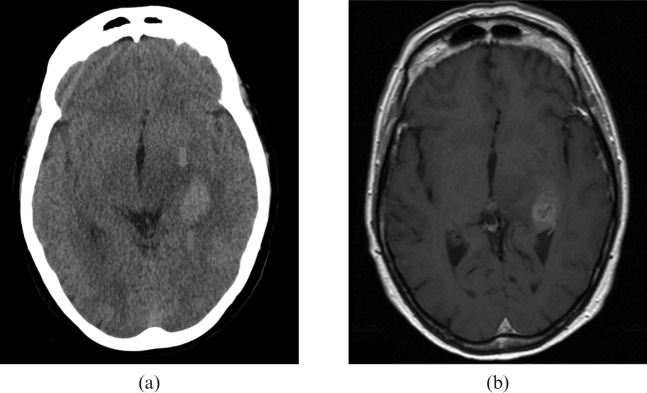
Figure 6 shows the imaging of a 77-year-old male, who presented with multiple episodes of confusion associated with dysphasia. The red arrow indicates a left anteromedial temporal lobe lesion that is not readily apparent on the standard window settings. MRI confirmed the diagnosis.
Figure 6.
Imaging of a 77-year-old male patient with confusion and dysphasia. (a) Standard and brain window settings, (b) harsh “stroks” window settings and (c) confirmatory MRI. The red arrow indicates a left anteromedial temporal lobe lesion that is not readily apparent on the standard window settings.
Imaging of a 69-year-old female is represented in Figure 7. This patient was reported as having a witnessed episode of unresponsiveness. On examination, her neurology was unremarkable, although she was complaining of a persistent left-sided headache. Of note, her medical history included a transient ischaemic attack affecting the left arm.
Figure 7.
69-year-old female patient with period of unresponsiveness and headache. (a) Standard and brain window settings, (b) harsh “stroke” window settings and (c) confirmatory MRI. The red arrow indicates a right medial temporal lobe lesion.
The red arrow indicates a right medial temporal lobe lesion. Unfortunately, due to failure to attend, this patient did not undergo MRI for approximately 6 weeks; it is suspected that during this time the lesion progressed relatively rapidly to frank glioblastoma with intratumoural necrosis.
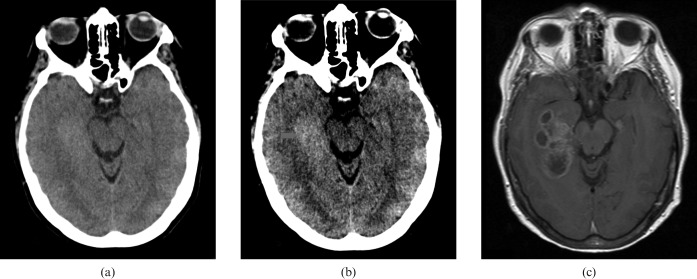
Figure 8 is the imaging results of a 74-year-old female, who was reported to have had an episode of loss of consciousness; during this time her partner reported some limb shaking.
Figure 8.
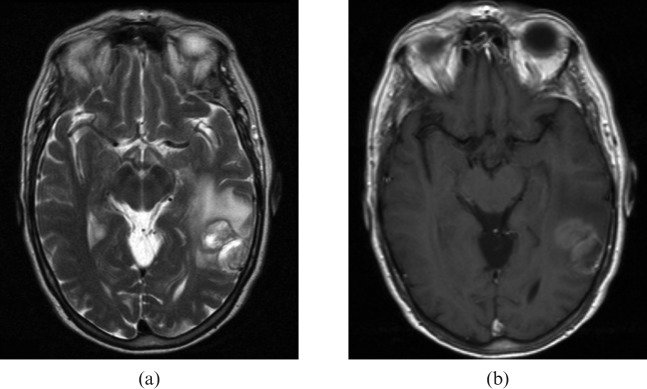
74-year-old female patient with loss of consciousness and potential seizure. (a) Standard brain window settings, (b) harsh “stroke” window settings and (c) initial MRI. CT scan showing evidence of an increased cellularity related to the lesion within the posterior left temporal lobe (red arrow).
This is an interesting case because, despite the CT scan showing evidence of an increased cellularity related to the lesion within the posterior left temporal lobe (red arrow), the initial MRI (Figure 8) was reported as likely to represent a dysembryoplastic neuroepithelial tumour (DNET). However, follow-up MRI 5 weeks later identified that the lesion had progressed to a probable glioblastoma with intratumoural necrosis with surrounding oedema (Figure 9).
Figure 9.
Follow-up MRI of same patient as Figure 8 showing tumour progression. (a) T2 weighted and (b) contrast-enhanced T1 weighted.
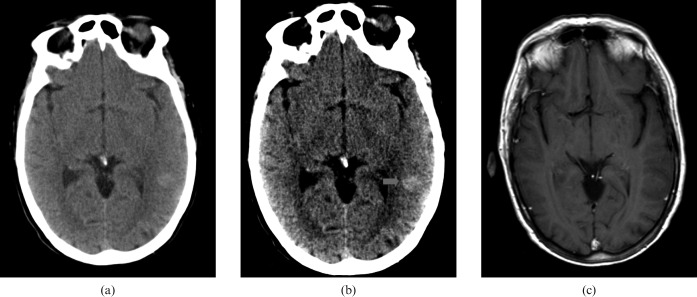
Finally, Figure 10 shows the imaging results of an 84-year-old male who presented via the ophthalmology clinic with a progression of visual field defects; the suspected cause was normal pressure glaucoma.
Figure 10.
84-year-old male who presented via the ophthalmology clinic with a progression of visual field defects. (a) Standard brain window settings, (b) harsh “stroke” window settings and (c) confirmatory contrast-enhanced CT.
The red arrow suggests a left frontobasal lesion and recall CT the following day identified a probable meningioma.
Discussion
It is common reporting practice to alter review windows when reporting CT examinations of the body; indeed, the routine use of narrow “liver windows” has been advocated for decades to improve soft tissue contrast within the liver and therefore increase the identification of subtle lesions [3]. It would also be ridiculous to suggest that thoracic CT reporting should be performed without reviewing both lung parenchyma and mediastinal window settings.
However, somewhat paradoxically, there seems to have been more of a resistance to review CT brain examinations on anything other than default brain settings (with additional bone windows in trauma). This may be explained by the pre-PACS era when CT head images were frequently reported from a single sheet of film without any soft copy review facilities.
Nevertheless, the capacity for lesion identification is markedly improved by using a narrow window width and adequate centre level settings [4]. Lev et al [5] showed that the detection of acute ischaemic lesions was increased by merely altering the window settings of the images being reviewed, such that narrower window width, higher-contrast images (stroke settings) were more sensitive in detecting both subtle grey/white matter changes and the “hyperdense middle cerebral artery” sign than standard brain review settings (sensitivities of 71% vs 57%, respectively).
In the same way that ischaemic hypodense cortical lesions are rendered more apparent, low-density lesions within the white matter are also more easily identified.
Demyelinating plaques in the brains of patients with multiple sclerosis (representative of myelin loss, destruction of oligodendrocytes and reactive astrogliosis) are often associated with oedema [6] and as such they can occasionally be detected on careful CT review.
As previously demonstrated, these review settings do not only highlight subtle low-density lesions. Of the four tumours presented, three were likely to be anaplastic astrocytoma (WHO grade III), two of which probably progressed to glioblastoma (one confirmed histologically).
As the name implies, anaplastic astrocytomas (WHO grade III) exhibit considerable variation in cellular morphology, with high cellularity and frequent mitosis regularly reported [7]. It is this property that we believe occasionally makes these tumours minimally hyperdense in comparison to the surrounding white matter. Unlike higher grade glioblastoma, necrosis is usually absent in anaplastic astrocytomas on either gross inspection or microscopic evaluation [8].
Figure 11 demonstrates the CT appearance of an anaplastic astrocytoma (red arrow) with related oedema (blue arrow), which at the time of the MRI was developing areas of intratumoural necrosis. The median survival of patients with anaplastic astrocytoma is 2−3 years with tumours progressing rapidly to frank glioblastoma with intratumoural necrosis [9], which was likely in several of our cases.
Figure 11.
Patient with an anaplastic (WHO grade III) astrocytoma. CT appearance (a) of an anaplastic astrocytoma (red arrow) with related oedema (blue arrow), and confirmatory MRI (b).
Similarly, meningiomas (and cerebral lymphomas) tend to have a denser cellular matrix than the surrounding brain [10], and appear subtly hyperdense in comparison with the adjacent white matter, potentially making them more apparent on CT at narrower window widths.
While we are not advocating the use of CT to replace MRI in the imaging work-up of patients with vague or non-specific neurological presentations, we are suggesting that by thoroughly interrogating the CT image information acquired it could be used more effectively to fast-track patients who potentially do have subtle imaging abnormalities that might benefit from more rapid MRI. It is clear that the routine use of CT window settings that increase the visibility of subtle abnormalities on a CT image of the brain will speed up the diagnostic journey for these patients.
Conclusion
The use of stroke windows, an undoubted essential soft-copy tool in the detection of hyperacute and acute ischaemic changes, should be used more universally in the reporting of CT brain images, and not just considered in cases of suspected CVA. The increased visualisation of subtle abnormalities when using these harsh review settings justifies their use.
The term stroke windows is an unfortunate misleading misnomer, which has probably contributed to their use being largely restricted to looking for suspected ischaemic lesions, potentially allowing other significant pathologies to go undetected.
References
- 1.Chawla S, Poptani H, Melham ER. Introduction to anatomic, physiologic and metabolic imaging in neuro-oncology. Cancer Treat Res 2008;143:3–42 [DOI] [PubMed] [Google Scholar]
- 2.Mullins ME. Modern emergent stroke imaging: pearls, protocols and pitfalls. Radiolo Clin North Am 2006;44:41–62 [DOI] [PubMed] [Google Scholar]
- 3.Webb WR, Brant WE, Helms CA. Introduction to CT of the abdomen and pelvis. Webb WR, Brant WE, Helms CA, ed. Fundamentals of body CT. London: Saunders, 1991:137 [Google Scholar]
- 4.Srinivasan A, Goyal M, Al Azri F, Lum C. State-of-the-art imaging of acute stroke. RadioGraphics 2006;26:S75–S95 [DOI] [PubMed] [Google Scholar]
- 5.Lev MH, Farkas J, Gemmete JJ, Hossain ST, Hunter GJ, Koroshetz WJ, et al. Acute stroke: improved nonenhanced CT detection—benefits of soft-copy interpretation by using variable window width and center level settings. Radiology 1999;213:150–5 [DOI] [PubMed] [Google Scholar]
- 6.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 2006;354:942–55 [DOI] [PubMed] [Google Scholar]
- 7.Smirniotopoulos JG. The new WHO classification of brain tumors. Neuroimaging Clin N Am 1999;9:595–613 [PubMed] [Google Scholar]
- 8.Ricci PE. Imaging of adult brain tumors. Neuroimaging Clin N Am 1999;9:651–69 [PubMed] [Google Scholar]
- 9.Kieffer SA, Chang JK. Intracranial Neoplasms. Haaga JR, Lanzieri CF, Gilkeson RC, eds. CT and MR imaging of the whole body (volume one) 4th edn Maryland Heights, MO: Mosby; 2002 [Google Scholar]
- 10.Osborn AG, Blaser SI, Salzman KL, Provenzale J, et al. Diagnostic imaging. 2005 Amirsys 2005: 4–56 [Google Scholar]