Abstract
Objective
The aim of the study is to compare CT enterography with polyethylene glycol solution (PEG-CT) with CT enteroclysis (CT-E) in patients with suspected small bowel disease.
Methods
145 patients underwent abdominal contrast-enhanced 16-row multidetector CT after administration of 2000 ml of PEG by mouth (n = 75) or after administration of 2000 ml of methylcellulose by nasojejunal tube (n = 70). Small bowel distension, luminal and extraluminal findings were evaluated and compared with small bowel follow-through examination in 60 patients, double contrast enema in 50, surgery in 25 and endoscopy in 35. Statistical evaluation was carried out by χ2 testing. For both techniques we have also calculated the effective dose and the equivalent dose in a standard patient.
Results
Crohn's disease was diagnosed in 64 patients, neoplasms in 16, adhesions in 6. Distension of the jejunum was better with CT-E than PEG-CT (p<0.05: statistically significant difference). No significant difference was present for others sites (p>0.05). Evaluation of pathological ileal loops was good with both techniques. The values of sensitivity, specificity and diagnostic accuracy were respectively 94%, 100% and 96% with CT-E, and 93%, 94% and 93% with PEG-CT. The effective dose for PEG-CT was less than the dose for the CT-E (34.7 mSv vs 39.91 mSv).
Conclusion
PEG-CT shows findings of Crohn's disease as well as CT-E does, although CT-E gives better bowel distension, especially in the jejunum, and has higher specificity than PEG-CT.
The radiological evaluation of the small bowel includes conventional modalities, such as barium studies, and imaging studies targeted to evaluate the intestinal wall and to identify extraluminal pathological patterns, such as sonography, CT and MRI.
An adequate visceral distension is the primary requirement of CT imaging of the small bowel. There are different modalities of administration and different types of contrast agents used to obtain the distension of the small bowel loops [1]. The small bowel is commonly opacified with positive contrast agents (1–2% barium sulphate suspension, 2–3% water-soluble iodinated solution) or with negative contrast agents (oral water, oral oil emulsions, polyethylene glycol solution (PEG), suspension of 0.1% barium sulphate (Volumen), Mucofalk or methylcellulose by nasojejunal tube) [1–4]. Positive contrast agents allow intestinal loops to be delimited and help verify whether an expansive process is intra- or extraluminal, but they do not allow, given their high density, accurate evaluation of wall characteristics and they interfere with possible angiography-like three-dimensional (3D) reconstructions [5]. Low-density agents are to be preferred because they allow better depiction of wall enhancement between the hypodensity of the intraluminal fluid and the hypodensity of the extraluminal fat and do not interfere with angiography-like 3D reconstructions [6].
Contrast agents can be administered by the oral route (CT enterography) or by nasojejunal tube (CT enteroclysis (CT-E)). In CT-E, contrast material is infused through a nasojejunal tube and contiguous axial images are obtained after total opacification of the small intestine. This technique has a unique theoretical advantage in its ability to simultaneously show intraluminal, mural and extra-intestinal complications of small bowel Crohn's disease, combining the advantages of CT and double contrast enema into one technique [2]. The most important limitations of CT-E are the increased radiation dose, patient discomfort with nasojejunal tube insertion and the increased time (almost 1 h from intubation to CT examination) [4].
The first and main aim of the present study is to compare technical quality and the patient's discomfort during CT enterography, performed after oral administration of polyethylene glycol solution (PEG-CT), with CT-E, performed after administration of methylcellulose by nasojejunal tube, in patients with suspected small bowel disease.
In addition, we evaluated the diagnostic quality of the CT.
Methods and materials
We have evaluated 145 patients (65 men and 80 women; age range 18–85 years, mean 48 years), who had undergone abdominal 16-row multidetector CT (Light Speed Pro 16, GE Medical Systems, Milwaukee, WI).
The selection criteria for inclusion in our study were diarrhoea (>3 bouts/day) (80 patients), abdominal pain pertinent to small bowel (62 patients), bleeding pertinent to small bowel (20 patients), histological data suspected or positive for Crohn's disease (15 patients), endoscopy (25 patients), radiological examinations positive for small bowel disease (35 patients performed double contrast enema, 40 patients performed small bowel follow-through). Double contrast enema was performed according to Herlinger's method [7]. Patients received 200–250 ml of a barium sulphate suspension (Prontobario 60%, Bracco, Milan, Italy) followed by an infusion of 1000–2000 ml of 0.5% methylcellulose by nasojejunal tube. Posteroanterior and anteroposterior films were obtained with and without loop compression. If the radiological studies performed before CT were positive for small bowel disease, CT examination was performed to evaluate the mural and extramural involvement. If radiological examination performed before CT was negative, but there were symptoms suspected for small bowel disease or endoscopy was positive for small bowel disease, CT examination was also performed.
Exclusion criteria included pregnancy, renal insufficiency, documented reaction to iodinated contrast material and previous intestinal resection.
Before examination, all patients underwent intestinal preparation according to the following plan: 2 days before, a light diet, free of fruit and vegetables; the day before, 150 mg of a mixture in equal parts of sennosides A and B with a cup of sugared tea at 8.00 a.m.; at 1.00 p.m., a semi-liquid diet; at 5.00 p.m., 15 g of magnesium sulphate in three-quarters of a glass of lukewarm water followed by consumption of 3 l of water during the following 4–5 h; at 9.00 p.m., a cup of hot soup; fasting from 9.00 p.m.
We explained to the patients the importance of obtaining good distension of the small bowel loops by administration of the liquid through a nasojejunal tube or by the ingestion of a large quantity of the liquid. CT-E was proposed to all patients as the first examination. Patients who refused the nasojejunal tube or patients in whom intubation failed underwent PEG-CT. All patients gave oral informed consent for the procedure.
After CT examination, radiological studies were performed only if the CT examination was negative or the CT findings were unclear in a patient with clear clinical suspicion of small bowel disease. If the CT was positive for small bowel disease, we did not perform further radiological studies.
Technique
70 patients (32 men and 38 women; age range 18–75 years, mean 45 years) underwent CT-E. Diarrhoea was present in 45 patients, abdominal pain pertinent to small bowel in 32, bleeding pertinent to small bowel in 12 patients, histological data suspected or positive for Crohn's disease in 7, endoscopy in 15, radiological examinations positive for small bowel disease in 43 (25 patients had a double contrast enema, and 18 patients had a small bowel follow-through). They underwent fluoroscopic placement of a 12–16-French nasojejunal tube, and then they were brought into the CT room. Contrast material (1500–2500 ml of 0.5% methylcellulose) was hand infused using 60 ml syringes. We aimed to have constant and continuous injection, approximately four syringes per minute. An anticholinergic compound (N-butyl hyoscine bromide) was administered intravenously to avoid spasms, to obtain homogeneous small bowel distension and to reduce patient abdominal discomfort. We administered 10 mg when the patient complained about abdominal discomfort and 10 mg just before a CT scan.
At the end of the infusion of the methylcellulose, the patients underwent 16-row multidetector CT. Unenhanced CT was performed using the following scanning parameters: collimation (mm), 5; table speed (mm/rot), 5; pitch, 1.375; 120 kVp; 300 mAs. Contrast-enhanced CT was performed using the following scanning parameters: collimation 1.25 mm; table speed 13.75 mm per rotation; pitch 1.375, 120 kVp; 300 mAs. Unenhanced and contrast-enhanced CT was performed with the patient in the supine position from the diaphragm to the perineum during a single breath-hold. Unenhanced scans were performed to evaluate the grade of the distension of the small bowel loops, so that in the case of inadequate small bowel distension a further 200–250 ml of methylcellulose was given after the unenhanced scans. Unenhanced examination was useful also to evaluate density values of the pathological wall before iv administration or the presence of other signs, e.g. calcifications in the case of carcinoid tumours. Contrast-enhanced CT images were acquired 40 s after iv injection of 130–150 ml contrast agent at a rate of 3 ml s−1 (Ultravist 370, Schering AG, Berlin, Germany). The iv administration of contrast agents was done to evaluate the pattern and extent of wall pathological enhancement. Two-dimensional (2D) image processing was performed with a computer workstation (Advantage Windows, GE Medical Systems, Milwaukee, WI). We also performed maximum intensity projection (MIP) reconstructions.
Nasojejunal intubation was refused by 60 patients and failed in 15 patients; therefore, 75 patients underwent PEG-CT (35 men and 40 women; age range 22–85 years, mean 50 years). In these patients diarrhoea was present in 35, abdominal pain pertinent to small bowel in 30, bleeding pertinent to small bowel in 8, histological data suspected or positive for Crohn's disease in 8, endoscopy in 10 patients, radiological examinations positive for small bowel disease in 32 (10 patients had a double contrast enema with barium and methylcellulose, 22 patients had a small bowel follow-through). They obtained small bowel distension with oral administration of 1.5–2.0 l of iso-osmotic PEG (Isocolan, Giuliani S.P.A, Milan, Italy) administered in equal doses of 100 ml starting 45 min before the CT examination. We administered 10 mg of the anticholinergic compound (N-butyl-hyoscine bromide) when the patient complained about abdominal discomfort and 10 mg just before the CT scan. A further 200–250 ml of PEG was given after the unenhanced scans in cases of inadequate small bowel distension. CT parameters were the same as for CT-E.
In patients studied by CT-E, we evaluated the discomfort of the patients from intubation, from methylcellulose infusion and during the CT examination. In patients studied by PEG-CT, we evaluated the degree of discomfort during PEG administration and during CT examination. The evaluation was performed by a patient questionnaire (presence and grade of abdominal pain, nausea and vomiting in a four-point scale from 0 = absent to 3 = high grade).
For both techniques, we have calculated the effective dose and the equivalent dose. The equivalent dose is a measure of the radiation dose to the tissue where an attempt has been made to allow for the different relative biological effects of different types of ionizing radiation. The effective dose equivalent (now replaced by effective dose) is used to compare radiation doses on different body parts on an equivalent basis, because radiation does not affect different parts in the same way. The effective dose to an individual is found by calculating a weighted average of the equivalent dose to different body tissues, with the weighting factors designed to reflect the different radiosensitivities of the tissues. Impact CT patient dosimetry calculator software from the Medicine and Healthcare Products Regulatory Agency (MHRA) Evaluation Centre was used to evaluate the dose in the CT examination. PCXMC software from the STUK (Radiation and Nuclear Safety Authority) was used to evaluate the dose during nasojejunal intubation. We considered a standard patient (adult, weight 70 kg).
Analysis of images
Two gastrointestinal radiologists, who were not involved in the CT examination and were blinded to the clinical and radiological data, consensually reviewed all CT images. We defined the anatomy of the small bowel loops in the coronal reformat images and we used the same criteria as in the radiological study (barium study and double contrast enema). The small bowel loop occupies the inframesocolic space of the peritoneal cavity. The jejunum generally occupies the left upper and mid-portions and the ileum the right mid- and lower portions of the abdominal cavity. Proximal jejunal loops are folded in the left upper abdomen, usually showing an almost vertical course. Distal jejunal loops cross the spine to the right side and continue as the ileum proximal. Most of the ileum occupies the area above the pelvic inlet. The distal ileum is usually directed cephalic and to right. Jejunal loops are generally more anteriorly located in the abdomen than are the loops of the ileum [8]. The CT results were classified as normal, Crohn's disease or other disease. Subsequently, the CT results were compared with those of the other examinations carried out (endoscopy, radiological examinations). Discrepancies were resolved with agreement by all parts.
In CT after iodinated contrast medium injection, the normal wall of the distended loop (normal parietal thickness <3 mm) shows a linear and homogeneous hyperdense appearance between the endoluminal low-density solution and extraparietal hypodensity of the peritoneal fat.
The CT criterion for the diagnosis of small bowel disease was parietal thickening. In particular, we analysed density (HU), grade (mm) and symmetry of parietal thickening, and the presence of associated extraluminal anomalies to perform a differential diagnosis among several small bowel diseases (tumours, inflammatory disease, others).
The CT criterion for the diagnosis of small bowel Crohn's disease was parietal thickening (>3 mm) in association with at least one extraparietal inflammatory involvement. In patients with Crohn's disease we also considered the diameter of the small bowel lumen (diameter of the loop excluding the thickness of the wall); diameter of the loop (diameter of the loop including the thickness of the wall); presence of stenosis; presence, site and number of abnormal altered bowel segments; degree of mural thickening; mural enhancement; presence of target sign (alternating rings of high and low density); presence of comb sign (hypervascularity of the involved mesentery); presence of perienteric stranding (loss of the normal sharp interface between the bowel wall and mesentery); presence of fibrofatty proliferation (excess of mesenteric fat); presence of fistulas; presence of sinus tract (linear extension from small bowel loop into an exoenteric inflammatory process); lymphadenopathy (diameter >1 cm); presence of abscesses; and other signs.
We have also looked for small bowel distension and evaluated the difference between the two CT techniques. The distension of each small bowel segment (proximal jejunum, distal jejunum, proximal ileum, distal ileum, last ileal loop) was classified in a four-point scale (0 = absent, 1 = incomplete, 2 = partial, 3 = complete). The complex anatomy of the small intestine and its extreme length make it difficult to objectively define the degree of distension of the bowel loop. As far as we know, there has been no objective definition of the degree of distension of the bowel loop in the literature. In our study we carried out a subjective evaluation. Having identified the different bowel segments without pathologies in the coronal reformat images, we defined grade 3 (complete distension) as when most of the loops in the examined section had a complete distension, that is well-distended lumen with thin wall; grade 0 (distension absent) as when most of the segment loops examined were collapsed, that is undistended lumen and walls not thin. Grades 1 and 2 were intermediate forms between grade 0 and grade 3.
Comparable statistical evaluations of small bowel loop distension and the patient's discomfort were carried out by χ2 testing (Yates corrected). A value of p<0.05 was considered statistically significant.
Results
We did not find any statistical difference between PEG-CT and CT-E concerning the age and the sex of the patients (p = 0.96).
With both techniques, all patients underwent successful CT without any complications even if at the end of the examination all patients needed to defecate because of the well-known laxative effects of methylcellulose and PEG.
When we performed statistical analysis of discomfort, we found a positive statistical difference between PEG-CT and CT-E concerning pain during infusion, nausea during infusion and nausea during CT examination (p<0.05) (Table 1). In other words, the patients who underwent CT-E complained of more discomfort than those who underwent PEG-CT because of the effects described above. A statistically significant difference was not present for other discomfort. Moreover, discomfort to nasojejunal tube insertion was present only in patients who underwent CT-E; thus, statistical comparison with PEG-CT cannot be applied.
Table 1. Degree of discomfort in CT enterography with polyethylene glycol (PEG-CT) (75 patients) and in CT enteroclysis (CT-E) (70 patients).
| Pain |
Nausea |
Vomiting |
||||
| CT-E | PEG-CT | CT-E | PEG-CT | CT-E | PEG-CT | |
| Intubation | ||||||
| Grade 0 | 50 | – | 10 | – | 58 | – |
| Grade 1 | 10 | – | 28 | – | 8 | – |
| Grade 2 | 8 | – | 20 | – | 3 | – |
| Grade 3 | 2 | – | 12 | – | 1 | – |
| p-value | Statistical test cannot be applied | |||||
| Infusion | ||||||
| Grade 0 | 30 | 55 | 25 | 50 | 60 | 60 |
| Grade 1 | 20 | 10 | 35 | 15 | 8 | 5 |
| Grade 2 | 15 | 8 | 10 | 8 | 0 | 10 |
| Grade 3 | 5 | 2 | 0 | 2 | 2 | 0 |
| p-value | 0.0004* | 0.0004* | 0.49 | |||
| During CT | ||||||
| Grade 0 | 65 | 67 | 55 | 70 | 65 | 71 |
| Grade 1 | 5 | 8 | 15 | 5 | 3 | 2 |
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Grade 3 | 0 | 0 | 0 | 0 | 2 | 0 |
| p-value | 0.65 | 0.019* | 0.91 | |||
Results are presented as number of patients.
*p<0.05: statistically significant difference.
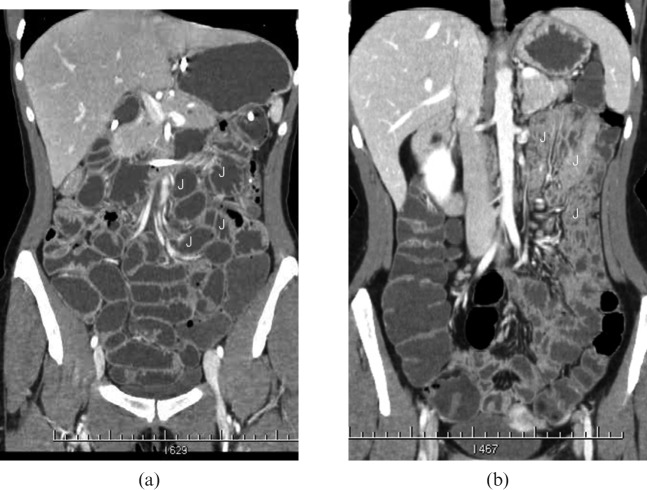
The different degrees of distension of the loops (classified with a four-point scale) are summarised in Table 2. In patients examined using PEG-CT, we found complete distension of the proximal jejunal loops in 30/75 (40%) patients, distal jejunal loops in 45/75 (60%) patients, proximal ileum in 58/75 (77.5%) patients, distal ileum in 64/75 (85.5%) patients and the last ileal loop in 60/75 (80%) patients. In patients examined using CT-E, we found complete distension of proximal jejunal loops in 54/70 (77%) patients, distal jejunal loops in 59/70 (84.5%) patients, proximal ileum in 62/70 (88.5%) patients, distal ileum in 64/70 (91%) patients and the last ileal loop in 63/70 (90%) patients. Distension of the proximal and distal jejunum was found to be significantly better in patients examined using CT-E than those examined using PEG-CT (Figure 1), as confirmed by the χ2 test with Yates correction (p<0.05: statistically significant difference). No significant difference (Table 2) was present for others sites (p>0.05).
Table 2. Degree of small bowel loop's distension in CT enterography with polyethylene glycol (PEG-CT) and in CT enteroclysis (CT-E).
| 0 Absent |
1 Incomplete |
2 Partial |
3 Complete |
||||||
| PEG-CT | CT-E | PEG-CT | CT-E | PEG-CT | CT-E | PEG-CT | CT-E | p-value | |
| PJ | 10 (13.5) | 3 (4) | 15 (20) | 4 (6) | 20 (26.5) | 9 (13) | 30 (40) | 54 (77) | 0.0000* |
| DJ | 5 (6.5) | 3 (4) | 10 (13.5) | 3 (4) | 15 (20) | 5 (7.5) | 45 (60) | 59 (84.5) | 0.0022* |
| PI | 2 (2.5) | 1 (1.5) | 5 (6.5) | 3 (4) | 10 (13.5) | 4 (6) | 58 (77.5) | 62 (88.5) | 0.1164 |
| DI | 2 (2.5) | 0 (0) | 4 (5.5) | 2 (3) | 5 (6.5) | 4 (6) | 64 (85.5) | 64 (91) | 0.3779 |
| LIL | 1 (1.5) | 2 (3) | 5 (6.5) | 2 (3) | 9 (12) | 3 (4) | 60 (80) | 63 (90) | 0.1483 |
Results are presented as number of patients with corresponding percentage of total patient number in parentheses. PJ, proximal jejunum; DJ, distal jejunum; PI, proximal ileum; DI, distal ileum; LIL, last ileal loop.
*p<0.05: statistically significant difference.
Figure 1.
CT enteroclysis coronal image (a) shows better distension of the jejunal loops (J) than CT enterography with polyethylene glycol (PEG-CT) coronal image (b).
A further 200–250 ml of methylcellulose or PEG was given after the unenhanced scans for the inadequate small bowel distension in 20/145 (14%) patients (5 studied by CT-E and 15 studied by PEG-CT).
Concerning the use of N-butyl-hyoscine bromide, the first dose was usually administered intravenously before 1–1.5 l of methylcellulose or PEG; no patients had any contraindications to the drug (glaucoma, prostatic hypertrophy, tachyarrhythmia) and no side-effects were observed.
In total, Crohn's disease was diagnosed in 64 patients (34 using CT-E and 30 using PEG-CT). In these patients the pathological loops showed wall thickening of between 4 and 12 mm (mean 7 mm), loop diameter of between 12 and 31 mm (mean 23 mm), luminal diameter between 2 and 19 mm (mean 9 mm), longitudinal extent between 10 and 32 cm (mean 15 cm). Unenhanced CT depicted density values of involved segments of between 20 and 57 HU and the degree of their contrast enhancement as between 75 and 208 HU. The distal ileum and last ileal loop were the most frequently involved sites; we found only one jejunal localisation. A target sign was observed in 57 patients, perienteric stranding in 18 patients and comb sign in 24 patients (Figure 2). Other signs were fibrofatty proliferation in 14 patients, stenosis in 26 patients and fistulas in 12). In four patients abscesses were present.
Figure 2.
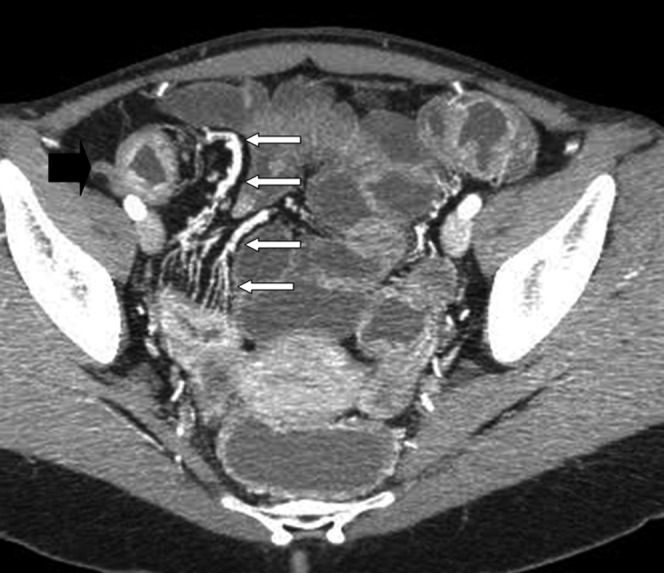
CT enteroclysis (MIP reconstruction) shows clearly the comb sign (white arrows) associated to the thickening of the last ileal loop (black arrow).
We found neoplasms in 16 patients, non-Hodgkin's lymphoma in 6 (4 studied with CT- E and 2 with PEG-CT), carcinoid tumours in 3 (2 studied with CT-E, 1 with PEG-CT), Peutz–Jeghers syndrome in 2 (studied with PEG-CT), adenocarcinomas in 2 (studied with CT-E), lipomas in 2 (studied with PEG-CT), metastasis from melanoma in 1 (studied with CT-E) (Figures 3 and 4). In all 16 patients with neoplasms the diagnosis was confirmed by surgery; the mean time between CT and surgery was 25 days. Finally, adhesions were present in six patients, four examined with CT-E and two with PEG-CT.
Figure 3.
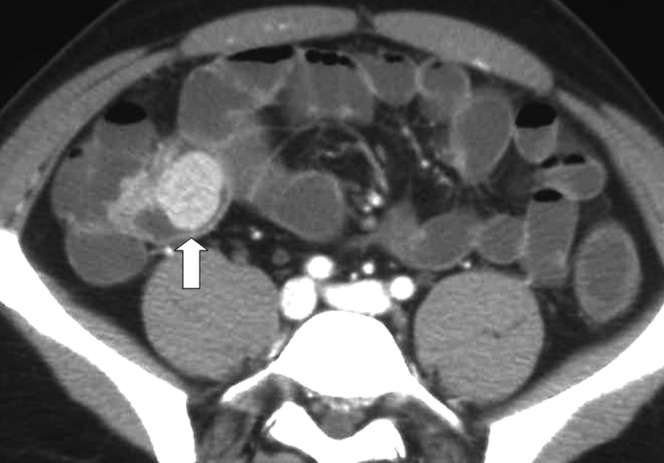
CT enterography with polyethylene glycol shows a polyp (arrow) in the last ileal loop of a patient with Peutz–Jeghers syndrome.
Figure 4.
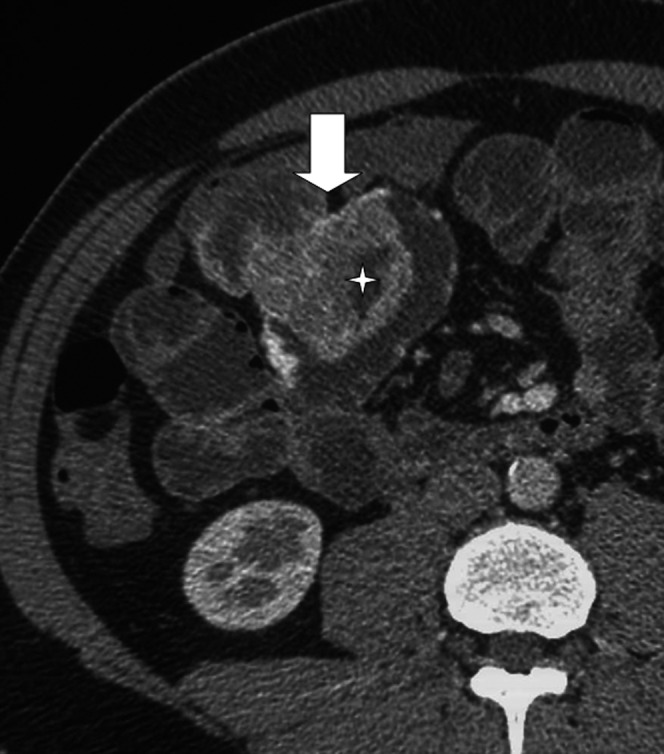
CT enteroclysis shows a large parietal mass (arrow) with ulceration. Surgical report: adenocarcinoma.
Ileoscopy was performed in 35 patients (before CT examination in 25, after CT examination in 10); the mean time between multidetector CT and ileoscopy was 10 days. Double contrast enema was performed in 50 patients (before CT examination in 35, after CT examination in 15) and small bowel follow-through was performed in 60 patients (before CT examination in 40, after CT examination in 20 patients); the mean time between CT and barium studies was 7 days. In total, we found six false-negative CT cases (three with PEG-CT and three with CT-E) due to early Crohn's disease (erosions and/or ulcers) and two false-positive CT cases (only of the PEG-CT) due to suboptimal distension of the loops. Barium study and endoscopy confirmed the absence or the presence of disease respectively in false-positive and false-negative cases (Figures 5 and 6).
Figure 5.
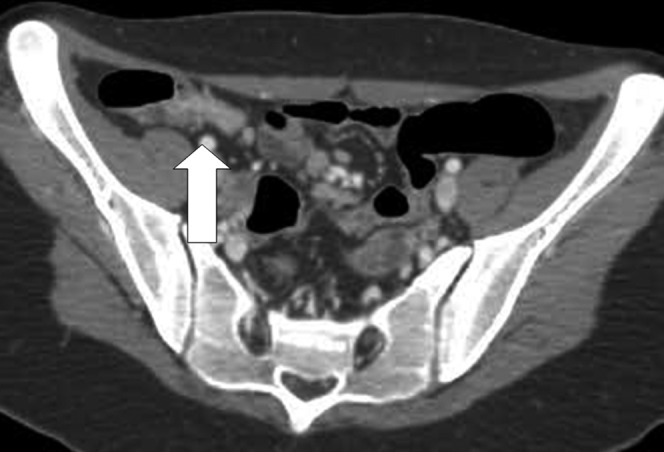
An example of false-positive case. CT enterography with polyethylene glycol shows a small bowel loop with narrowing (arrow) but the endoscopy did not show any alterations.
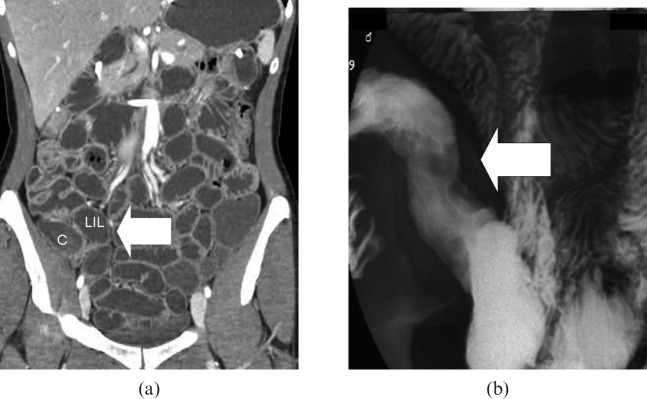
Figure 6.
An example of a false-negative CT case. (a) No alterations of the last ileal loop were present in CT enteroclysis (arrow); C, colon; LIL, last ileal loop. (b) Barium study performed after CT examination showed some erosions and ulcers in the last ileal loop (arrow).
The values of sensitivity, specificity and diagnostic accuracy were respectively 94%, 100% and 96% with CT-E, and 93%, 94% and 93% with PEG-CT (Table 3).
Table 3. Sensitivity, specificity and diagnostic accuracy of CT enterography with polyethylene glycol (PEG-CT) and in CT enteroclysis (CT-E).
| CT-E (70 patients) | PEG-CT (75 patients) | |
| False-positive cases (PF) | 0 | 2 |
| False-negative cases (NF) | 3 | 3 |
| True-negative cases (NT) | 20 | 31 |
| True-positive cases (PT) | 34 Crohn's disease, 9 neoplasms, 4 adhesions | 30 Crohn's disease 7 neoplasms, 2 adhesions |
| Sensitivity (PT/PT+NF) | 94% | 93% |
| Specificity (NT/NT+PF) | 100% | 94% |
| Accuracy (PT+NT/total) | 96% | 93% |
Results are presented as number of patients.
Concerning the radiation dose, the effective dose for PEG-CT examination is 34.7 mSv.
The effective dose applied to the standard patient during the nasojejunal intubation is 1.7 mSv min−1 of fluoroscopy. In our patients the time to place the nasojejunal tube was 10–14 min and the maximum fluoroscopic time was 3 min, so the average effective dose applied to the patients was 5.21 mSv. The total dose applied during the CT-E was 39.91 mSv. The values of the equivalent dose and the effective dose are given in Table 4.
Table 4. Doses in CT enterography with polyethylene glycol (PEG-CT) and in CT enteroclysis (CT-E).
| Organs | Equivalent dose (mGy) |
|
| PEG-CT | CT-E | |
| Ovary | 12.1 | 13.0 |
| Gonad | 13.35 | 13.4 |
| Uterus | 81.4 | 81.9 |
| Stomach | 77.9 | 81.1 |
| Haematopoietic tissue | 31.0 | 31.3 |
| Colon | 70.0 | 72.5 |
| Spleen | 85.5 | 88.2 |
| Liver | 68.1 | 70.3 |
| Total effective dose (mSv) | 34.7 | 39.91 |
The dose for scout view is included in the table. In CT-E we have also included the dose for nasojejunal intubation (3 min).
Discussion
The recent development of faster machines, the rapid increase in the spatial resolution with the introduction of multidetector CT and better multiplanar reconstructions have modified the approach to small bowel imaging [9, 10]; thus, CT has become one of the most important techniques to evaluate patients with suspected or known small bowel disease.
The use of CT in small bowel disease is aimed at visualisation of the entire organ and adequate distension, the elimination of respiratory and peristaltic motion artefacts, and the iv administration of contrast agents to evaluate the pattern and extent of wall enhancement.
Accurate intestinal cleansing is required as in conventional radiology [1]. We are aware that there is no agreement in the literature about the usefulness of performing intestinal preparation before CT examination. Many authors [1, 6, 11] advise a light diet free of fruit and vegetables for 2 days before CT and the administration of laxatives the day before. A few authors [12, 13] advise fasting for 8–12 h prior to CT. We have been recommending our intestinal preparation for radiological study since 1980 and since 2000 for CT examination, and have found that the intestinal loops are usually clean so we plan to continue recommending this preparation.
Independent of the CT technique used, it is essential to have a fluid-distended loop because mural wall thickening is the hallmark of intestinal disease.
In the literature, we found oral use of oil emulsions, water, air, Mucofalk and PEG [1, 3, 6, 13–16,]. Oil emulsions provide lower density values than do water, but because of their taste, patients do not appreciate them, and their high cost limits their routine clinical use. Water is cheap, well tolerated by patients and allows accurate evaluation of the intestinal wall and its enhancement; however, it is absorbed rapidly and tends to stimulate peristalsis, failing to ensure adequate distension of the distal jejunum and the ileum. Air, administered either orally through delayed-release effervescent substances or rectally, for the study of the terminal ileum, has produced satisfactory results in the demonstration of intraluminal and mural diseases, but the excessive difference between its density (−800 HU) and that of the enhanced intestinal wall (100–135 HU) often gives rise to artefacts, which have limited its use. Recently, some authors proposed the oral use of Mucofalk, which contains fibre from the outer shell seeds of podorozhnika, Plantago ovata; these seeds retain water in quantities much greater than their weight [17]. In contrast, Maglinte et al [4] proposed a suspension of 0.1% barium sulphate (Volumen) for oral use.
In accordance with other authors [6], we decided to use a solution of PEG as an oral hypodense contrast medium. The choice of PEG is based on the fact that it is a well-known solution in widespread use for the preparation of endoscopic studies. Its main characteristics are an agreeable flavour and lack of toxicity; moreover, it has the same density as water but is not adsorbed in the intestine.
The technique of administration of oral contrast agents also influences the grade of distension of the loops. The oral dose of contrast agent can be single, double or triple in variable amounts between 600 and 1500 ml, fractionated over 1–2 h prior to the examination. In our study, we administered 2 l of PEG in equal doses of 100 ml starting 45 min before the CT examination. The last two doses were administered just before the CT examination to obtain distension of the proximal jejunum. Because PEG is an intestinal transit accelerator, we did not administer any transit accelerator agent (e.g. metoclopramide), but we did administer an anticholinergic agent (N-butyl-hyoscine bromide). In contrast to other authors [18, 19], we preferred to administer the hypotonic agent intravenously in 2 doses: 10 mg was administered when the patient began feeling abdominal pain (generally after receiving of 1.3–1.5 l of the PEG), and another 10 mg was given immediately before starting the CT examination. Following these procedures, the patient was able to drink the total amount of the contrast agent and we obtained good distension of the small bowel loops, as shown in Table 2.
A common drawback of oral contrast agents is that they fail to ensure suitable and uniform distension of all small bowel loops, giving rise to significant problems in differentiating between real wall thickening and inadequate distension or spasm. The problem can be overcome, although with greater invasiveness, time and costs, by using CT-E, a method developed in the early 1990s in which variable amounts (2000–2750 ml) of low-density (methylcellulose or water) or high-density (4–5% sodium diatrizoate, 1% barium sulphate) contrast material are infused by hand or with a peristaltic pump through a nasojejunal tube before the CT scan with and without iv contrast medium [2–4, 7, 19, 20, 22–28]. Manual infusion is limiting because the distension is better when the peristaltic pump is used, even if its use does not always allow optimal distension of all the loops. Turetschek et al [19], for example, did not recognise a jejunal stenosis owing to unsatisfactory distension and misinterpreted it as a spasm. Wold et al [16] did not use a peristaltic pump to perform CT-E and did not find a significant difference in the adequacy of luminal distension between peroral water CT enterography and CT-E. In this study CT-E showed a distension score of 2.75, whereas peroral water CT showed a score distension of 2; the sensitivity of CT-E is similar to peroral CT (78% vs 75%), while specificity and accuracy of CT-E is superior to peroral CT (100% vs 83% and 88% vs 80%, respectively). We administered methylcellulose by hand, trying to obtain a constant and continuous injection. CT was performed at the end of the infusion; if the unenhanced scans showed less than optimal distension, we administered an additional dose of methylcellulose (about 200–250 ml). We also used a hypotonic agent to obtain homogeneous bowel distension and to reduce abdominal discomfort. The first dose was administered when the patients felt abdominal pain (usually after the infusion of about 1.5 l of methylcellulose) and permitted them to tolerate the infusion of the total amount of methylcellulose. Following these procedures, we obtained good distension of the small bowel loops in CT-E as shown in Table 2.
When we applied out the statistical test, distension of the proximal and distal jejunum was found to differ significantly in patients studied with CT-E and those studied with PEG-CT. In other words, distension of the proximal and distal jejunum was significantly better in patients studied with CT-E than those studied with PEG-CT. In fact, CT-E had a higher specificity than PEG-CT because of the absence of false-positive cases owing to better distension.
Moreover, one of the most important limitations of CT-E is patient discomfort and the time needed for all manoeuvres (almost 1 h from intubation to CT examination) [4]. We have confirmed this in our study. In fact, the patients who underwent CT-E complained of more discomfort than those who underwent PEG-CT (p<0.05: statistically significant difference).
Finally, there is evidence in the literature that another limitation of CT-E is the increased radiation dose [4].
Our study has some methodological limitations. First is the non-randomisation of the patients. In fact, CT-E was proposed to all patients as first examination. Patients who refused the nasojejunal tube or patients in whom intubation failed underwent PEG-CT. Another limitation could be that we used two different techniques to study the patients: CT-E and PEG-CT, with two different contrast agents, respectively methylcellulose and PEG solution. The last limitation is the absence of a complete reference standard (i.e. some cases were reported as normal and had no further investigations).
Conclusion
Even if the quality of images is better with CT-E than with PEG-CT, PEG-CT shows terminal ileum involvement, extraluminal findings and complications of Crohn's disease as well as CT-E does. In fact, even if it was not possible to obtain an adequate study of the jejunum with PEG-CT, the jejunum is rarely affected by Crohn's disease.
Patients accepted the oral solution after attempting intubation with a nasojejunal tube; the radiation dose of PEG-CT is lower than CT-E.
In conclusion, it is our opinion that PEG CT can be a valid alternative examination to CT-E.
Acknowledgment
We thank Dr Fabrizio Cichocki, medical physics expert, for his help in the evaluation of the radiation doses in CT enteroclysis.
References
- 1.Birnbaum BA. Computed tomography of the small bowel. Technique and principles of interpretation. Herlinger H, Maglinte DDT, Birnbaum BA, editors. Clinical imaging of the small intestine. Berlin, Germany: Springer, 1999: 153–66 [Google Scholar]
- 2.Bender GN, Timmons JH, Williard WC, Carter J. Computed tomographic enteroclysis. One methodology. Invest Radiol 1996;31:43–9 [DOI] [PubMed] [Google Scholar]
- 3.Engin G. Computed tomography enteroclysis in the diagnosis of intestinal diseases. J Comput Assist 2008;32:9–16 [DOI] [PubMed] [Google Scholar]
- 4.Maglinte DDT, Sandrasegaran K, Lappas JC, Chiorean M. CT enteroclysis. Radiology 2007;245:661–71 [DOI] [PubMed] [Google Scholar]
- 5.Makó EK, Mester AR, Tarján Z, Karlinger K, Tóth G. Enteroclysis and spiral CT examination in diagnosis and evaluation of small bowel Crohn's disease. Eur J Radiol 2000;35:168–75 [PubMed] [Google Scholar]
- 6.Mazzeo S, Caramella D, Belcari A, Melai L, Cappelli C, Fontana F, et al. Multidetector CT of the small bowel: evaluation after oral hyperhydration with isotonic solution. Radiol Med 2005;109:516–26 [PubMed] [Google Scholar]
- 7.Herlinger H. A modified technique for the double contrast small bowel enema. Gastrointestin Radiol 1978;3:201–7 [DOI] [PubMed] [Google Scholar]
- 8.Herlinger H. Anatomy of the small intestine. Herlinger H, Maglinte DDT, Birnbaum BA, editors. Clinical imaging of the small intestine. Berlin, Germany: Springer, 1999: 3–12 [Google Scholar]
- 9.Macari M, Megibow AJ, Balthazar EJ. A pattern approach to the abnormal small bowel: observations at MDCT and CT enterography. AJR Am J Roentgenol 2007;188:1344–55 [DOI] [PubMed] [Google Scholar]
- 10.Minordi LM, Vecchioli A, Poloni G, Bonomo L. CT enteroclysis: Multidetector technique (MDCT) versus single-detector technique (SDCT) in patients with suspected small-bowel Crohn's disease. Radiol Med 2007;112:1188–200 [DOI] [PubMed] [Google Scholar]
- 11.Schmidt S, Felley C, Meuwly JY, Schnyder P, Denys A. CT enteroclysis: technique and clinical applications. Eur Radiol 2006;16:648–60 [DOI] [PubMed] [Google Scholar]
- 12.Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn's disease. Am J Gastroenterol 2007;102:2541–50 [DOI] [PubMed] [Google Scholar]
- 13.Paulsen SR, Huprich JE, Fletcher JG, Booya F, Young BM, Fidler JL, et al. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics 2006;26:641–57 [DOI] [PubMed] [Google Scholar]
- 14.Megibow AJ, Babb JS, Hecht EM, Cho JJ, Houston C, Boruch MM, et al. Evaluation of bowel distention and bowel wall appearance by using neutral oral contrast agent for multi-detector row CT. Radiology 2006;238:87–95 [DOI] [PubMed] [Google Scholar]
- 15.Minordi LM, Vecchioli A, Mirk P, Filigrana E, Poloni G, Bonomo L. Multidetector CT in small-bowel neoplasms. Radiol Med 2007;112:1013–25 [DOI] [PubMed] [Google Scholar]
- 16.Wold PB, Fletcher JG, Johnson CD, Sandborn WJ. Assessment of small bowel Crohn's disease: non invasive peroral CT enterography compared with other imaging methods and endoscopy-feasibility study. Radiology 2003;229:275–81 [DOI] [PubMed] [Google Scholar]
- 17.Doerfler OC, Ruppert-Kohlmayr AJ, Reittner P, Hinterleitner T, Petritsch W, Szolar DH. Helical CT of the small bowel with an alternative oral contrast material in patients with Crohn's disease. Abdom Imaging 2003;28:313–18 [DOI] [PubMed] [Google Scholar]
- 18.Balthazar EJ. CT of the gastrointestinal tract: principles and interpretation. AJR Am J Roentgenol 1991;156:23–32 [DOI] [PubMed] [Google Scholar]
- 19.Turetschek K, Schober E, Wunderbaldinger P, Bernhard C, Schima W, Puespoek A, et al. Findings at helical CT-enteroclysis in symptomatic patients with Crohn's disease: correlation with endoscopic and surgical findings. J Comput Assist Tomogr 2002;26:488–92 [DOI] [PubMed] [Google Scholar]
- 20.Maglinte DD, Sandrasegaran K, Lappas JC. CT enteroclysis: techniques and applications. Radiol Clin North Am 2007;45:289–301 [DOI] [PubMed] [Google Scholar]
- 21.Di Mizio R, Rollandi GA, Bellomi M, Meloni GB, Cappabianca S, Grassi R. Multidetector-row helical CT enteroclysis. Radiol Med 2006;111:1–10 [DOI] [PubMed] [Google Scholar]
- 22.La Seta F, Buccellato A, Tesè L, Biscaldi E, Rollandi GA, Barbiera F, et al. Multidetector-row CT enteroclysis: indications and clinical applications. Radiol Med 2006;111:141–58 [DOI] [PubMed] [Google Scholar]
- 23.Minordi LM, Vecchioli A, Guidi L, Mirk P, Fiorentini L, Bonomo L. Multidetector CT enteroclysis versus barium enteroclysis with methylcellulose in patients with suspected small bowel disease. Eur Radiol 2006;16:1527–36 [DOI] [PubMed] [Google Scholar]
- 24.Minordi LM, Vecchioli A, Poloni G, Guidi L, De Vitis I, Bonomo L. Enteroclysis CT and PEG-CT in patients with previous small-bowel surgical resection for Crohn's disease: CT findings and correlation with endoscopy. Eur Radiol 2009;19:2432–40 [DOI] [PubMed] [Google Scholar]
- 25.Minordi LM, Vecchioli A, Guidi L, Poloni G, Fedeli G, Bonomo L. CT findings and clinical activity in Crohn's disease. Clin Imaging 2009;33:123–9 [DOI] [PubMed] [Google Scholar]
- 26.Pilleul F, Penigaud M, Milot L, Saurin JC, Chayvialle JA, Valette PJ. Possible small-bowel neoplasms: contrast-enhanced and water-enhanced multidetector CT enteroclysis. Radiology 2006;241:796–801 [DOI] [PubMed] [Google Scholar]
- 27.Rajesh A, Maglinte DD. Multislice CT enteroclysis: technique and clinical applications. Clin Radiol 2006;61:31–9 [DOI] [PubMed] [Google Scholar]
- 28.Rollandi GA, Curone PF, Biscaldi E, Nardi F, Bonifacino E, Conzi R, et al. Spiral CT of the abdomen after distension of small bowel loops with transparent enema in patients with Crohn's disease. Abdom Imaging 1999;24:544–9 [DOI] [PubMed] [Google Scholar]