Abstract
Objectives
The aim of this study was to evaluate the diagnostic accuracy of fused fluoro-deoxy-D-glucose positron emission tomography/magnetic resonance mammography (FDG-PET/MRM) in breast cancer patients and to compare FDG-PET/MRM with MRM.
Methods
27 breast cancer patients (mean age 58.9±9.9 years) underwent MRM and prone FDG-PET. Images were fused software-based to FDG-PET/MRM images. Histopathology served as the reference standard to define the following parameters for both MRM and FDG-PET/MRM: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy for the detection of breast cancer lesions. Furthermore, the number of patients with correctly determined lesion focality was assessed. Differences between both modalities were assessed by McNemaŕs test (p<0.05). The number of patients in whom FDG-PET/MRM would have changed the surgical approach was determined.
Results
58 breast lesions were evaluated. The sensitivity, specificity, PPV, NPV and accuracy were 93%, 60%, 87%, 75% and 85% for MRM, respectively. For FDG-PET/MRM they were 88%, 73%, 90%, 69% and 92%, respectively. FDG-PET/MRM was as accurate for lesion detection (p = 1) and determination of the lesions' focality (p = 0.7722) as MRM. In only 1 patient FDG-PET/MRM would have changed the surgical treatment.
Conclusion
FDG-PET/MRM is as accurate as MRM for the evaluation of local breast cancer. FDG-PET/MRM defines the tumours' focality as accurately as MRM and may have an impact on the surgical treatment in only a small portion of patients. Based on these results, FDG-PET/MRM cannot be recommended as an adjunct or alternative to MRM.
Introduction
Magnetic resonance mammography (MRM) is a valuable adjunct to conventional imaging methods, such as X-ray mammography and ultrasound, to determine the local operative treatment strategy for a primary breast cancer lesion [1, 2]. It is well established that MRM is superior to X-ray mammography with and without concomitant ultrasound to define the local extent of breast cancer [3]; it is used both for the detection of breast cancer lesions that are occult on other imaging modalities [4, 5] and for differential diagnosis of breast findings [6]. Hence, MRM has increasingly been used in clinical routine [7]. Notably, in contrast to its high sensitivity for the detection of breast cancer lesions and its high negative predictive value (NPV), MRM has been found to have a substantial rate of false-positive findings in breast cancer patients resulting in an only moderate specificity of approximately 65% [2, 8]. These false-positive findings, however, may lead to overtreatment in patients falsely characterised as multicentric or multifocal by MRI [3, 9]. As a consequence, pre-operative biopsies are required in most cases to minimise the risk of overtreatment. Improving the specificity of MRM scans in the detection of breast cancer lesions would spare the patient the additional trauma of pre-operative invasive procedures [2, 10].
It has been shown that (18F)-2-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET)/CT combining anatomical and functional information in a single examination session leads to more accurate oncological staging than functional or anatomical information alone for several tumour entities [11–13]. To test the hypothesis that additional (functional) FDG-PET information may improve the specificity of (anatomical) MRM scans in the detection of breast cancer lesions, Moy and colleagues [14, 15] conducted an initial study and fused MRM data sets and prone FDG-PET scans of 23 patients in whom breast cancer was suspected. The authors found an increase in the specificity from 52% to 95% and an increase in the positive predictive value (PPV) from 69% to 94% with fused FDG-PET/MR images compared with MRM alone. However, these results were bought at the (not tolerable) expense of a declining sensitivity and NPV (92% to 63% and 85% to 69%, respectively).
To verify these initial results, data sets of prone FDG-PET/CT scans of the breast and MRM were retrospectively fused depending on software. The aim was to assess whether there were statistically significant differences between the accuracy of MRM and fused PET/MRM. Both imaging modalities were compared with regard to their accuracy when characterising (a) the lesion's character (benign vs malignant) and (b) the lesion's focality (unifocal vs multifocal vs multicentric). Furthermore, a potential influence of fused PET/MRM on patient management was assessed compared with MRM alone.
Methods and materials
Patients
27 women (mean age, 58.9 years; range, 42.9–78.4 years; standard deviation (SD), 9.9 years) with suspected malignancy on conventional X-ray mammograms (either performed for reason of screening or for reason of a clinically suspicious, palpable breast mass) and/or with suspected malignancy on breast ultrasound were referred for whole-body FDG-PET/CT. Malignant breast tumour was histopathologically verified in all patients following the imaging procedures. The whole-body scan included an FDG-PET/CT mammography scan in the prone position. Furthermore, all patients underwent MRM (mean interval between MRM and FDG-PET/CT mammography 0.76 days (0–6 days; SD 1.8 days)). All patients signed an informed consent that detailed the use of iv FDG, CT contrast material and MR contrast material and rare potential side effects. No patient underwent anticancer treatment at the time of imaging. This study was performed after full approval of the local ethics committee.
Reference standard
Histopathology of resected breast tumours served as the standard of reference in every patient. Histopathological specimens were evaluated by two pathologists.
PET mammography
FDG-PET mammography data were extracted from a whole-body FDG-PET/CT investigation data set that was performed for initial breast cancer staging. FDG-PET/CT data sets were acquired on a Biograph™ PET/CT system (Siemens Molecular Imaging, Hoffman Estates, IL). Patients fasted for at least 6 h before receiving an iv application of a mean of 270 MBq (range 210–350 MBq; SD 37 MBq) of 18F-FDG. Before the injection of the FDG, a blood sample was taken to ensure that blood glucose levels were below 150 mg dl−1. First, a supine whole-body FDG-PET/CT scan was performed 60 min after iv application of 18F-FDG covering a field of view from the head to the upper thighs. CT image acquisition was performed in a caudocranial direction with 100 mAs at 130 kV. 140 ml of an iodinated contrast agent (Ultravist 300™, Schering AG, Berlin, Germany) containing 300 mg ml−1 of iodine was administered with an automated injector (XD 5500™, Ulrich Medical Systems, Ulm, Germany) using a biphasic injection technique with a flow rate of 3 ml s−1 for the first 90 ml and 1.5 ml s−1 for the following 50 ml. The start delay was set to 50 s. All images were reconstructed with a 5 mm slice thickness and a 2.4 mm increment. A limited breath-hold technique was used to avoid motion-induced artefacts in the area of the diaphragm [16]. Following the acquisition of the CT data, PET images were obtained in three-dimensional (3D) mode. The PET emission time for the whole-body scan was adapted to the patients' body weight: <65 kg, 4 min per bed position; 65–85 kg, 5 min; >85 kg, 6 min. Iterative algorithms (FORE and AWOSEM, non-linear) with two iterations and eight subsets were used for image reconstruction. Data were filtered (full width at half maximum (FWHM) 5.0 mm) and scatter corrected. Subsequently, patients were positioned prone for FDG-PET/CT mammography, using the same positioning device as on MRM. Based on the findings of Kumar et al [17], who reported increased FDG uptake of malignant breast cancer lesions over time, the prone FDG-PET scan was started 110 min after the FDG injection. A topogram in the lateral view was performed to define the scan range from the axillary fossa to the lower end of the breasts. CT was performed first, followed by PET. No additional contrast material was applied for the PET/CT scan in the prone position. Image acquisition was performed in the caudocranial direction. The CT parameters were the same as in supine imaging. The number of PET bed positions depended on the size of the field of view from the axilla to the lower end of the breasts. The PET emission time was set to times between 6 and 10 min, depending on the volume of the breast (cup A, 6 min; cup B, 7 min, cup C, 8 min; cup D, 9 min; bigger than cup D, 10 min). PET image reconstruction was performed according to the supine protocol. The attenuation-corrected PET data acquired in the prone position were used for subsequent MR fusion.
MR mammography
All patients underwent MRM in the prone position. Dynamic contrast-enhanced breast MRI was performed on a 1.5 T MR scanner with multichannel capability (Siemens Magnetom Espree™, Siemens Medical Solutions, Erlangen, Germany). A standard eight-channel phased-array breast coil (Siemens Phased-array Breast Coil™, Siemens Medical Solutions) was used. After obtaining a localiser sequence an axial T2 weighted turbo spin-echo (TSE) sequence (6.18 min; repetition time/echo time (TR/TE), 7000/95; flip angle, 180°; field of view, 37 cm; slice thickness, 2 mm, no gap; number of excitations (nex), 3; matrix, 384 × 384) was acquired. Subsequently, a dynamic axial T1 3D fast low angle shot (FLASH) sequence (11.59 min; TR/TE, 11/4.76; flip angle, 15°; field of view, 37 cm; thickness, 2 mm, 0.4 mm gap; nex, 1; matrix, 365 × 384) was acquired, followed by an iv injection (power injector: Spectris Solaris™, Indianola, PA) of gadopentetate dimeglumine (Magnevist™, Schering, Berlin, Germany) at a concentration of 0.1 mmol kg−1 body weight, followed by a 20 ml saline flush. The flow rate was set to 2 ml s−1. After four subsequent dynamic series, image subtraction of the contrast-enhanced images from the pre-contrast images was performed.
PET/MR mammography
The FDG-PET/CT data sets and the MRM data sets were transferred to a picture archiving and communication system (PACS, General Electrics Healthcare, General Electrics, Munich, Germany) separately. From each patient, the attenuation-corrected FDG-PET mammography images acquired in the prone position as well as the first dynamic MRM series were transferred to a TrueD™ fusion workstation (Siemens, Malvern, PA) via the hospital network. The MRM data set was treated as the reference volume, and the FDG-PET data set was treated as the volume to be registered, although the software supports either choice. Both data sets were fused semi-automatically by using the “landmark matching” tool of the TrueD™ workstation. Corresponding points in both data sets were defined using a ninefold screen partition with the following images displayed at the same time: MRM, PET and PET/MRM data in the axial, coronal and sagittal views each. We defined absolute and relative landmarks. Absolute landmarks were represented by reproducible anatomic structures, for example the nipple of the breast or the apex of the heart, relative landmarks were represented by anatomic structures relative to an absolute landmark, for example the lung margins or the breast skin boundaries. Once a landmark was chosen, the pixel was marked in the MRM and PET data set and a sequential number was assigned. The landmark matching tool fuses both data sets semi-automatically in all three planes considering all determined landmarks. Each fused PET/MRM series was saved as a separate DICOM file.
Image analysis
In clinical routine, breast lesions on MRM were rated according to the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) lexicon [18]. Abnormal enhancement was characterised as a mass or non-mass lesion. Both the morphological appearance (size, shape and pattern of enhancement) and the temporal enhancement pattern were evaluated. Time–signal intensity curves (progressive, plateau or washout) were generated for all enhancing lesions. Additionally, a three time-point (3TP) analysis was performed using the 3TP software package (CAD sciences, White Plains, NY) as described in the literature [19–22]. To test whether fused FDG-PET data sets can improve the characterisation of breast lesions suspicious for malignancy on MRI, only lesions classified as “suspicious abnormality” (BI-RADS 4) or higher were included in the analysis.
On PET/MRM, findings were categorised as positive or negative for malignancy. According to a previously published study, the following functional criteria had to be fulfilled in addition to MR criteria, to rate a lesion as malignant [15]:
A lesion embedded in tissue showing mild metabolic activity was classified positive for malignancy if the FDG uptake was greater than blood pool activity.
A lesion within tissue with moderate or high physiological activity was rated positive for malignancy if the activity was focally greater than the adjacent physiologic activity.
According to the ACRBI-RADS™ MRI lexicon, lesions classified as “foci” (<5 mm, no morphological descriptors and dynamic data applicable) were excluded, whereas “focal areas” and “masses” (>5 mm) were included in the analysis. Findings rated as malignant with MRM or PET/MRM were classified as unifocal lesions (solitary lesion), multifocal lesions (more than one lesion in the same quadrant) or multicentric lesions (more than one quadrant affected by breast cancer lesions, or distance between breast cancer lesions more than 4 cm within the same quadrant) according to a previous study [23].
Analysis of the MRM data and the PET/MRM data was performed by two radiologists (one consultant radiologist specialised in breast imaging and one fourth-year resident) and a nuclear medicine consultant. Diagnoses were made in consensus. All MRM images were evaluated on a PACS workstation (General Electrics Healthcare, General Electrics), PET/MRM data were evaluated on the TrueD™ workstation (Siemens). PET/MRM scans were reviewed in three orthogonal planes (axial, coronal, sagittal). MRM images were evaluated first, followed by PET/MRM. The time interval between the evaluation of the MRM images and the PET/MRM images was 1 month to avoid a bias resulting from the knowledge of the MRM findings.
Statistical analysis
Using histopathology as the reference standard, the number of true-positive (TP), true-negative (TN), false-negative (FN) and false-positive (FP) results was assessed for MRM and PET/MRM on a lesion-by-lesion analysis. The following parameters for the detection of malignant breast lesions were calculated for both modalities:
sensitivity;
specificity;
PPV;
NPV; and
accuracy.
Furthermore, the number of patients with correct classification of the lesion's focality (solitary, multifocal, multicentric) was assessed for MRM and PET/MRM. McNemar's test was used to test whether there was a statistically significant difference concerning (a) lesion detection accuracy and (b) the correct characterisation of the breast cancer lesion's focality on a patient-based analysis. A p-value of less than 0.5 indicated a significant difference. The number of patients in which PET/MRM would have changed patient management was determined compared with MRM alone.
Results
Reference standard
According to the reference standard, 28 breasts were affected by breast cancer in 27 patients. For an overview of the pT stage, the histopathological type, the tumour grading and the breast cancer lesion's focality see Table 1. The reference standard identified 58 breast lesions in these 28 breasts. 43 lesions represented malignant lesions; 15 lesions represented benign lesions.
Table 1. pT stage, grading, histopathological type and the focality of all tumours according to the reference standard. The type of tissue sampling (ablation, excision or biopsy) is reported as are all evaluation results by MR mammography and positron emission tomography (PET)/MR mammography.
| Patient number | Lesion number | pT stage | Grading | Histopathology | Tumours' focality (according to the gold standard) | Gold standard (histopathology) sampled by | Lesion evaluation by MR | Lesion evaluation by PET/MR |
| 1 | 1 | 2 | 2 | IDC | Unifocal | E | tp | tp |
| 2 | 1 | 1c | 3 | ILC | Unifocal | A | tp | tp |
| 2 | 2 | Fibrocystic changes | A | fp | tn | |||
| 2 | 3 | Sclerosing adenosis | A | tn | tn | |||
| 3 | 1 | 1c | 2 | Mixed IDC/ILC | Multicentric | A | fn | fn |
| 3 | 2 | 1c | 2 | Mixed IDC/ILC | A | tp | tp | |
| 4 | 1 | 2 | 2 | IDC | Multicentric | A | tp | tp |
| 4 | 2 | 2 | 2 | IDC | A | tp | tp | |
| 4 | 3 | 2 | 2 | IDC | A | tp | tp | |
| 4 | 4 | Sclerotic changes | A | tn | tn | |||
| 5 | 1 | 2 | 3 | IDC | Unifocal | A | tp | tp |
| 5 | 2 | Scar | A | fp | fp | |||
| 5 | 3 | Fibrocystic changes | A | tn | tn | |||
| 5 | 4 | Fibrotic changes | A | tn | tn | |||
| 6 | 1 | 2 | 2 | IDC | Unifocal | E | tp | tp |
| 7 | 1 | 1c | 2 | Mixed IDC/ILC | Multicentric | E | tp | tp |
| 7 | 2 | 1c | 2 | Mixed IDC/ILC | E | tp | fn | |
| 8 | 1 | 2 | 2 | Mixed IDC/ILC | Unifocal | E | tp | tp |
| 9 | 1 | 1b | 2 | Mixed IDC/ILC | Unifocal | E | tp | tp |
| 10 | 1 | Fibroadenoma | E | fp | fp | |||
| 10 | 2 | 1c | 2 | IDC | Unifocal | E | tp | tp |
| 11 | 1 | 2 | 2 | ILC | Unifocal | A | tp | tp |
| 12 | 1 | mic | na | IDC | Unifocal | E | tp | tp |
| 13 | 1 | 1c | 3 | IDC | Unifocal | E | tp | tp |
| 14 | 1 | 1c | 1 | Tubular carcinoma | Unifocal | E | tp | tp |
| 14 | 2 | Fibrocystic changes | B | fp | tn | |||
| 15 | 1 | 1b | 2 | ILC | Unifocal | E | tp | tp |
| 16 | 1 | 1c | 1 | IDC | Unifocal | E | tp | tp |
| 16 | 2 | Fibrocystic changes | E | fp | fp | |||
| 17 | 1 | 2c | 2 | IDC | Multicentric | A | tp | tp |
| 17 | 2 | 2c | 2 | IDC | A | tp | tp | |
| 17 | 3 | 2c | 2 | IDC | A | tp | tp | |
| 17 | 4 | Lymph node | A | tn | tn | |||
| 18 | 1 | 2c | 2 | ILC | Unifocal | E | tp | tp |
| 18 | 2 | Sclerotic changes | E | tn | fp | |||
| 19 | 1 | 1c | 3 | Micropapillar | Multicentric | A | tp | tp |
| 19 | 2 | 1c | 3 | Invasive micropapillar carcinoma | A | tp | tp | |
| 20 | 1 | 3 | 2 | ILC | Multicentric | A | tp | tp |
| 20 | 2 | 3 | 2 | ILC | A | tp | tp | |
| 20 | 3 | 3 | 2 | ILC | A | tp | tp | |
| 20 | 4 | 3 | 2 | ILC | A | tp | tp | |
| 21 | 2 | 2 | 3 | IDC | Unifocal | A | tp | tp |
| 22 | 1 | 1c | 3 | IDC | Unifocal | E | tp | tp |
| 23 | 1 | 1c | 1 | IDC | Multifocal | E | tp | tp |
| 23 | 2 | 1c | 1 | IDC | E | tp | tp | |
| 23 | 3 | Scar | E | tn | tn | |||
| 23 | 4 | Sclerosing adenosis | E | tn | tn | |||
| 24 | 1 | 2 | 2 | IDC | Unifocal | E | tp | tp |
| 24 | 2 | Fibrocystic changes | E | fp | tn | |||
| 25 | 1 (left) | 3 | 2 | Mixed IDC/ILC | Multifocal | A | tp | tp |
| 25 | 2 (left) | 3 | 2 | Mixed IDC/ILC | A | tp | tp | |
| 25 | 3 (left) | 3 | 2 | Mixed IDC/ILC | A | fn | fn | |
| 25 | 1 (right) | 1b | 2 | ILC | Unifocal | E | tp | tp |
| 26 | 1 | 3 | 3 | IDC | Unifocal | A | tp | tp |
| 26 | 2 | Fibrocystic changes | A | tn | tn | |||
| 27 | 1 | 1c | 2 | IDC | Multicentric | E | tp | tp |
| 27 | 2 | 1c | 2 | IDC | E | tp | fn | |
| 27 | 3 | 1c | 2 | IDC | E | fn | fn |
IDC, invasive ductal carcinoma; LIC, invasive lobular carcinoma; E, excision; A, ablation; B, biopsy; tp, true-positive; tn, true-negative; fp, false-positive; fn, false-negative; mic, microinvasive carcinoma; na, not available.
Lesion-based analysis
When defining malignancy, MRM was TP in 40 lesions (69%) (Figure 1), TN in 9 lesions (16%), FN in 3 lesions (5%) and FP in 6 lesions (10%). PET/MRM was TP in 38 lesions (65%), TN in 11 lesions (19%), FN in 5 lesions (9%) and FP in 4 (7%) lesions. The sensitivity, specificity, PPV, NPV and accuracy for MRM detecting malignant lesions were 93%, 60%, 87%, 75%, and 85%, respectively. For PET/MRM they were 88%, 73%, 90%, 69% and 92%, respectively. There was no statistically significant difference when comparing the diagnostic accuracy of MRM and PET/MRM for detection of breast cancer lesions (p = 1).
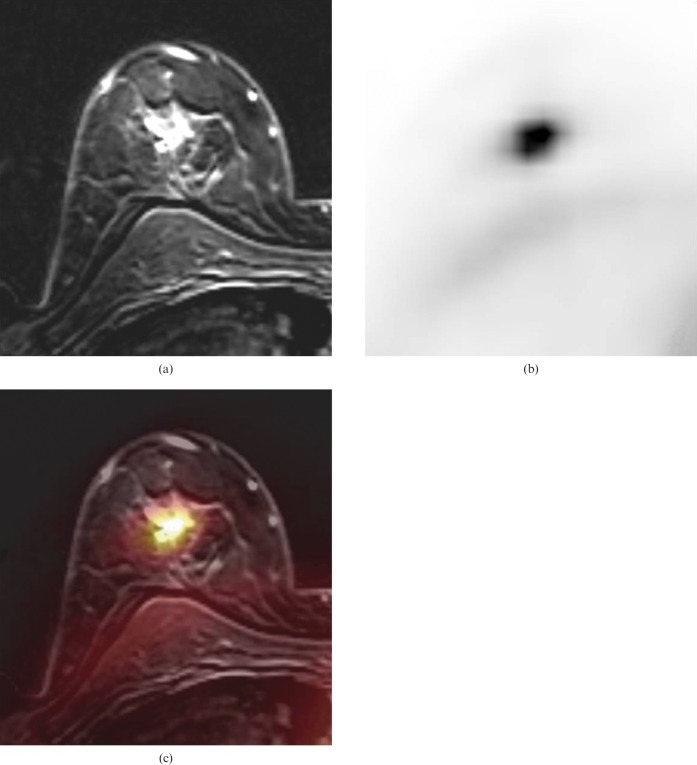
Figure 1.
(a) Magnetic resonance mammography (MRM) of a 47-year-old woman suffering from a solitary invasive ductal pT1c Grade 3 carcinoma (14 × 13 × 12 mm) of the right breast. (b) (18F)-2-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) scan showed intense FDG uptake (maximum standard uptake value 5.9) of the tumour. (c) FDG-PET/MRM with fused anatomical and functional information.
Definition of lesion's focality/Influence on patient's management
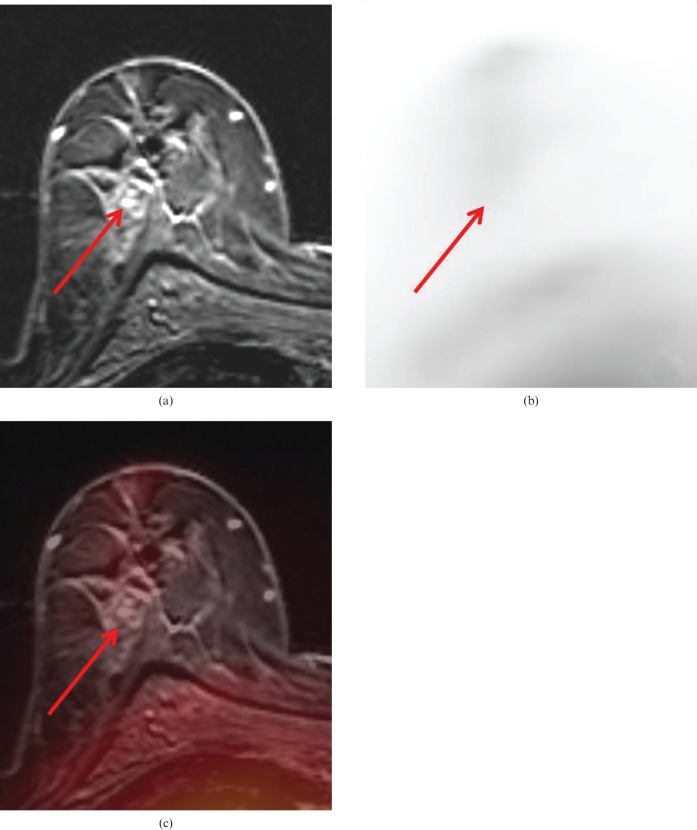
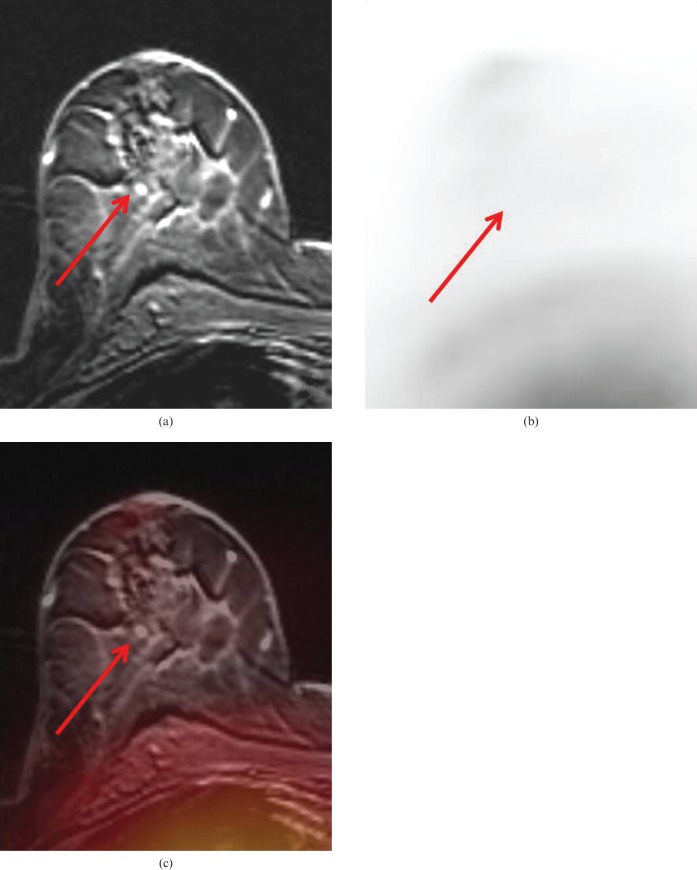
In 19 breasts (68%) MRM determined the lesion's focality correctly, whereas PET/MRM was able to determine the lesion's focality correctly in 20 breasts (71%). MRM failed to determine the lesion's focality in nine breasts (32%), PET/MRM failed in eight breasts (29%) (p = 0.7722). Thus, PET/MRM would have changed the surgical therapy decision in one patient (4%) compared with MRM alone (Figures 2 and 3). Based on PET/MRM the patient would have undergone breast-conserving therapy rather than a breast ablation.
(a) Magnetic resonance mammography (MRM) of the same patient as shown in Figure 1. MRM falsely rated the enhancing lesions (arrows) in this focal area as additional tumour nodules, rating this lesion as multicentric. Histopathology verified fibrocystic changes in this lesion rather than malignancy. (b) (18F)-2-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) showed no evidence of tumour (arrows), maximum standard uptake values were 0.4 and 0.7, respectively. (c) FDG-PET/MRM correctly rated these additional lesions as benign fibrocystic changes.
(a) Magnetic resonance mammography (MRM) of the same patient as shown in Figure 1. MRM falsely rated the enhancing lesions (arrows) in this focal area as additional tumour nodules, rating this lesion as multicentric. Histopathology verified fibrocystic changes in this lesion rather than malignancy. (b) (18F)-2-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) showed no evidence of tumour (arrows), maximum standard uptake values were 0.4 and 0.7, respectively. (c) FDG-PET/MR mammography correctly rated these additional lesions as benign fibrocystic changes.
Discussion
MRM has been shown to be very sensitive for the detection of breast cancer lesions [24]. Even lesions that are occult on clinical breast examination, X-ray mammography and sonography may be detected by MR imaging [25, 26]. However, this high sensitivity for lesion detection has been coupled with a limited specificity [8, 27, 28]. This study demonstrates that software-based fused PET/MRM is only able to marginally increase the specificity over MRM alone by providing additional functional FDG-PET information. PET/MRM was not able to improve the accuracy of breast cancer detection compared with MRM alone. These results are in contrast to the report of Moy and colleagues [15], who have found an improvement of specificity when including PET in MR data sets.
Although the T stage of enclosed patients in both studies seems comparable, (i.e. what influences the tumour detectability on the PET images [29]), several reasons may serve as an explanation for the different findings in the current study and the reported data of Moy et al. In the current analysis all non-mass lesions were excluded from analysis (with the exception of “focal areas” as they may contain tumour cells, e.g. ductal carcinoma in situ [30]). If these lesions had been included in the analysis with MRM and PET/MRM (as was the case in the study of Moy et al), a considerable increase in the specificity may have been expected when including the PET data over MRM alone. These non-mass lesions are mostly benign and small and will therefore not show increased FDG-PET uptake. This difference concerning the inclusion criteria has to be considered the most important topic for achieving different results in both studies.
It has to be taken into consideration that because of the limited spatial resolution of PET scanners, the detection rate will decline with decreasing tumour size [29]. Avril et al [29] described a rapid increase in tumour detection with FDG-PET from 25% detection rate of T1b cancers to 84.4% detection rate of T1c cancers. In our study, 85% of all investigated tumours were at least T1c tumours. When including a higher number of small primary breast cancer lesions, the detection rate of the primary tumour will be less than our results. Further technical developments like PET time-of-flight imaging capability may qualify even smaller lesions for detection by FDG-PET in the future [31]. Alternative PET tracers such as 18F-16-alpha-17-beta-fluoroestradiol, as an oestrogen receptor marker [32, 33] used for PET-based therapy control [34], will have to be evaluated in conjunction with PET/MRM in the future. These specific oestrogen receptor tracers may outperform the rather unspecific FDG when evaluating patients with breast cancer.
Other factors influencing data evaluation are histopathology and tumour grading: different histological breast cancer types typically go along with differences in FDG avidity. Infiltrating ductal carcinomas usually show a higher maximum standardised uptake value (SUV) level than other histopathological subtypes [35] and may, therefore, particularly qualify for FDG-PET/MRM imaging. In our study more than 50% of tumours were infiltrating ductal carcinomas. Although this contribution of the histopathological breast cancer type resembles the normal contribution in Western countries [36], studies including a higher amount of other breast cancer subtypes may report different accuracies of PET/MRM, according to different PET information. In Moy's study, more than 75% of enclosed cancer patients suffered from intraductal carcinoma [15]. Thus, tumours with a typically strongly increased FDG uptake were included. The difference between the study populations in Moy's study compared with the current analysis must, therefore, be taken into consideration. Another issue has to be taken into consideration when evaluating breast cancer lesions with FDG-PET: tumour grading is an important factor influencing FDG avidity and may, therefore, be responsible for the detectability of breast cancer lesions with FDG-PET. Grade 3 carcinomas are known to take up significantly more FDG than grade 1–2 tumours [35]. The reason that the relatively high number of lower-grade tumours (grade 2 or less: more than 70% in this study) seems to have a negligible effect in our study may be explained by the absolute SUVmax of grade 1 and grade 2 tumours. Although grade 1 or grade 2 tumours accumulate less FDG than higher-grade lesions, it has been known that the absolute median SUV of these lesions is 4.9 [35], implicating a clear detectability even of these lower-grade lesions embedded in physiological breast tissue with a median SUV of 0.4 [37].
A limitation of our study compared with the study of Moy and colleagues [15] is the use of a static FDG-PET and MR fusion device that is not able to distort the images. We have tried to address this limitation by using the same breast positioning device for MRM and PET mammography. However, even then it cannot be avoided that breast structures may be in a slightly different position during both scans. A manually adjusted alignment was used to minimise possible mismatches caused by the static fusion approach. It can be expected that combined dual-modality PET/MRI scanners will reduce those potential mismatches in the future, as has been shown for hardware-aligned PET/CT scanners for other indications [11]. First results on prototype PET/MR scanners show promising results, combining high-resolution morphology with functional information isochronously [38–42]. Future studies on combined PET/MR scanners may answer the question of whether PET/MR may find its place in local breast cancer staging.
Conclusion
FDG-PET/MRM is as accurate as MRM concerning the evaluation of local breast cancer. FDG-PET/MRM defines the tumours' focality as accurately as MRM and may have an impact on the surgical treatment in only a small portion of patients. Based on these results FDG-PET/MRM cannot be recommended as an adjunct or alternative to MRM alone.
References
- 1.Van Goethem M, Schelfout K, Kersschot E, Colpaert C, Verslegers I, Biltjes I, et al. MR mammography is useful in the preoperative locoregional staging of breast carcinomas with extensive intraductal component. Eur J Radiol 2007;62:273–82 [DOI] [PubMed] [Google Scholar]
- 2.Rim A, Chellman-Jeffers M. Trends in breast cancer screening and diagnosis. Cleve Clin J Med 2008;75:S2–9 [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK, Braun M. [Magnetic resonance imaging in preoperative staging for breast cancer: pros and cons]. Radiologe 2008;48:358–66 [DOI] [PubMed] [Google Scholar]
- 4.Warner E, Plewes DB, Shumak RS, Catzavelos GC, Di Prospero LS, Yaffe MJ, et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol 2001;19:3524–31 [DOI] [PubMed] [Google Scholar]
- 5.Wright H, Listinsky J, Rim A, Chellman-Jeffers M, Patrick R, Rybicki L, et al. Magnetic resonance imaging as a diagnostic tool for breast cancer in premenopausal women. Am J Surg 2005;190:572–5 [DOI] [PubMed] [Google Scholar]
- 6.Rausch DR, Hendrick RE. How to optimize clinical breast MR imaging practices and techniques on your 1.5-T system. Radiographics 2006;26:1469–84 [DOI] [PubMed] [Google Scholar]
- 7.Van Goethem M, Tjalma W, Schelfout K, Verslegers I, Biltjes I, Parizel P. Magnetic resonance imaging in breast cancer. Eur J Surg Oncol 2006;32:901–10 [DOI] [PubMed] [Google Scholar]
- 8.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999;213:881–8 [DOI] [PubMed] [Google Scholar]
- 9.Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast 2007;16:S34–44 [DOI] [PubMed] [Google Scholar]
- 10.Simonelli LE, Fowler J, Maxwell GL, Andersen BL. Physical sequelae and depressive symptoms in gynecologic cancer survivors: meaning in life as a mediator. Ann Behav Med 2008;35:275–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, et al. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol 2004;22:4357–68 [DOI] [PubMed] [Google Scholar]
- 12.Antoch G, Stattaus J, Nemat AT, Marnitz S, Beyer T, Kuehl H, et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology 2003;229:526–33 [DOI] [PubMed] [Google Scholar]
- 13.Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med 2004;45:357–65 [PubMed] [Google Scholar]
- 14.Moy L, Noz ME, Maguire GQ, Jr, Ponzo F, Deans AE, Murphy-Walcott AD, et al. Prone mammoPET acquisition improves the ability to fuse MRI and PET breast scans. Clin Nucl Med 2007;32:194–8 [DOI] [PubMed] [Google Scholar]
- 15.Moy L, Ponzo F, Noz ME, Maguire GQ, Jr, Murphy-Walcott AD, Deans AE, et al. Improving specificity of breast MRI using prone PET and fused MRI and PET 3D volume datasets. J Nucl Med 2007;48:528–37 [DOI] [PubMed] [Google Scholar]
- 16.Beyer T, Antoch G, Blodgett T, Freudenberg LF, Akhurst T, Mueller S. Dual-modality PET/CT imaging: the effect of respiratory motion on combined image quality in clinical oncology. Eur J Nucl Med Mol Imaging 2003;30:588–96 [DOI] [PubMed] [Google Scholar]
- 17.Kumar R, Loving VA, Chauhan A, Zhuang H, Mitchell S, Alavi A. Potential of dual-time-point imaging to improve breast cancer diagnosis with (18)F-FDG PET. J Nucl Med 2005;46:1819–24 [PubMed] [Google Scholar]
- 18.Tardivon AA, Athanasiou A, Thibault F, El Khoury C. Breast imaging and reporting data system (BIRADS): magnetic resonance imaging. Eur J Radiol 2007;61:212–15 [DOI] [PubMed] [Google Scholar]
- 19.Degani H, Gusis V, Weinstein D, Fields S, Strano S. Mapping pathophysiological features of breast tumors by MRI at high spatial resolution. Nat Med 1997;3:780–2 [DOI] [PubMed] [Google Scholar]
- 20.Furman-Haran E, Grobgeld D, Margalit R, Degani H. Response of MCF7 human breast cancer to tamoxifen: evaluation by the three-time-point, contrast-enhanced magnetic resonance imaging method. Clin Cancer Res 1998;4:2299–304 [PubMed] [Google Scholar]
- 21.Hauth EA, Jaeger H, Maderwald S, Stockamp C, Muhler A, Kimmig R, et al. Evaluation of quantitative parametric analysis for characterization of breast lesions in contrast-enhanced MR mammography. Eur Radiol 2006;16:2834–41 [DOI] [PubMed] [Google Scholar]
- 22.Hauth EA, Stockamp C, Maderwald S, Muhler A, Kimmig R, Jaeger H, et al. Evaluation of the three-time-point method for diagnosis of breast lesions in contrast-enhanced MR mammography. Clin Imaging 2006;30:160–5 [DOI] [PubMed] [Google Scholar]
- 23.Heusner TA, Kuemmel S, Umutlu L, Koeninger A, Freudenberg LS, Hauth EA, et al. Breast cancer staging in a single session: whole-body PET/CT mammography. J Nucl Med 2008;49:1215–22 [DOI] [PubMed] [Google Scholar]
- 24.Zakhireh J, Gomez R, Esserman L. Converting evidence to practice: A guide for the clinical application of MRI for the screening and management of breast cancer. Eur J Cancer 2008;44:2742–52 [DOI] [PubMed] [Google Scholar]
- 25.Stoutjesdijk MJ, Boetes C, Jager GJ, Beex L, Bult P, Hendriks JH, et al. Magnetic resonance imaging and mammography in women with a hereditary risk of breast cancer. J Natl Cancer Inst 2001;93:1095–102 [DOI] [PubMed] [Google Scholar]
- 26.Swayampakula AK, Dillis C, Abraham J. Role of MRI in screening, diagnosis and management of breast cancer. Expert Rev Anticancer Ther 2008;8:811–17 [DOI] [PubMed] [Google Scholar]
- 27.Winnekendonk G, Krug B, Warm M, Gohring UJ, Mallmann P, Lackner K. Diagnostic value of preoperative contrast-enhanced MR imaging of the breast. Rofo 2004;176:688–93 (In German) [DOI] [PubMed] [Google Scholar]
- 28.Taieb S, Ceugnart L. [Breast MR imaging: validated indications and unsolved problems]. Bull Cancer 2008;95:147–52 [DOI] [PubMed] [Google Scholar]
- 29.Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol 2000;18:3495–502 [DOI] [PubMed] [Google Scholar]
- 30.Van Goethem M, Schelfout K, Kersschot E, Colpaert C, Weyler J, Verslegers I, et al. Comparison of MRI features of different grades of DCIS and invasive carcinoma of the breast. JBR-BTR 2005;88:225–32 [DOI] [PubMed] [Google Scholar]
- 31.Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med 2007;48:471–80 [PubMed] [Google Scholar]
- 32.Seimbille Y, Rousseau J, Benard F, Morin C, Ali H, Avvakumov G, et al. 18F-labeled difluoroestradiols: preparation and preclinical evaluation as estrogen receptor-binding radiopharmaceuticals. Steroids 2002;67:765–75 [DOI] [PubMed] [Google Scholar]
- 33.Jonson SD, Welch MJ. PET imaging of breast cancer with fluorine-18 radiolabeled estrogens and progestins. Q J Nucl Med 1998;42:8–17 [PubMed] [Google Scholar]
- 34.Sundararajan L, Linden HM, Link JM, Krohn KA, Mankoff DA. 18F-Fluoroestradiol. Semin Nucl Med 2007;37:470–6 [DOI] [PubMed] [Google Scholar]
- 35.Crippa F, Seregni E, Agresti R, Chiesa C, Pascali C, Bogni A, et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med 1998;25:1429–34 [DOI] [PubMed] [Google Scholar]
- 36.Hammer C, Fanning A, Crowe J. Overview of breast cancer staging and surgical treatment options. Cleve Clin J Med 2008;75:S10–16 [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Chiu E, Rosenberg J, Gambhir SS. Standardized uptake value atlas: characterization of physiological 2-deoxy-2-[18F]fluoro-D-glucose uptake in normal tissues. Mol Imaging Biol 2007;9:83–90 [DOI] [PubMed] [Google Scholar]
- 38.Pichler BJ, Judenhofer MS, Catana C, Walton JH, Kneilling M, Nutt RE, et al. Performance test of an LSO-APD detector in a 7-T MRI scanner for simultaneous PET/MRI. J Nucl Med 2006;47:639–47 [PubMed] [Google Scholar]
- 39.Pichler BJ, Judenhofer MS, Pfannenberg C. Multimodal imaging approaches: PET/CT and PET/MRI. Handb Exp Pharmacol 2008;109:32. [DOI] [PubMed] [Google Scholar]
- 40.Pichler BJ, Judenhofer MS, Wehrl HF. PET/MRI hybrid imaging: devices and initial results. Eur Radiol 2008;18:1077–86 [DOI] [PubMed] [Google Scholar]
- 41.Pichler BJ, Wehrl HF, Kolb A, Judenhofer MS. Positron emission tomography/magnetic resonance imaging: the next generation of multimodality imaging? Semin Nucl Med 2008;38:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beyer T, Weigert M, Quick HH, Pietrzyk U, Vogt F, Palm C, et al. MR-based attenuation correction for torso-PET/MR imaging: pitfalls in mapping MR to CT data. Eur J Nucl Med Mol Imaging 2008;35:1142–6 [DOI] [PubMed] [Google Scholar]