Abstract
Calcifying fibrous tumour (CFT) is a recently recognised rare benign lesion characterised by dense hyalinised collagenous tissue, psammomatous or dystrophic calcifications and a lymphoplasmacytic infiltrate. The usual locations of the lesion are the soft tissues of the extremities, but rarely it occurs in the abdomen. Recently, we experienced a case of CFT found in the liver of a 29-year-old woman. Here, we describe the characteristic radiological and histopathological findings, along with a review of the relevant literature.
Calcifying fibrous tumour (CFT) is a recently recognised rare benign lesion characterised by dense hyalinised collagenous tissue interspersed with benign spindle cells, psammomatous or dystrophic calcifications and a lymphoplasmacytic infiltrate. To date, fewer than 80 cases have been reported in the literature. Usually, these tumours occur in the soft tissues of the extremities and trunk, followed by the neck, axilla and pleura. Rare sporadic cases have been reported, with the tumour occurring in various anatomical sites such as the groin, scrotum, adrenal gland, breast, peritoneum and mesentery [1–9].
We present a case of a CFT found in the liver and describe the characteristic radiological and histopathological findings, along with a review of the relevant literature.
Case report
A 29-year-old woman was admitted to our hospital because of a palpable mass on the right upper quadrant of the abdomen that had been present for 3 weeks. She had given birth approximately 3 months previously and her medical history was unremarkable. On physical examination, a hard mass was palpable on the right upper quadrant of the abdomen and there was minimal tenderness. Viral markers for hepatitis and the level of tumour markers such as serum α-fetoprotein and carcinoembryonic antigen were within normal limits. Other laboratory findings were also normal.
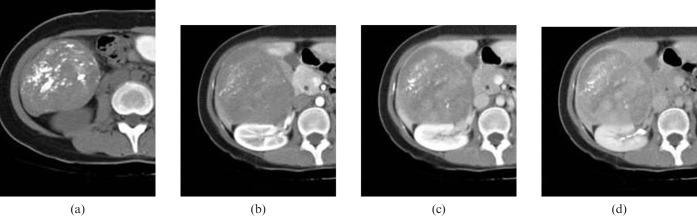
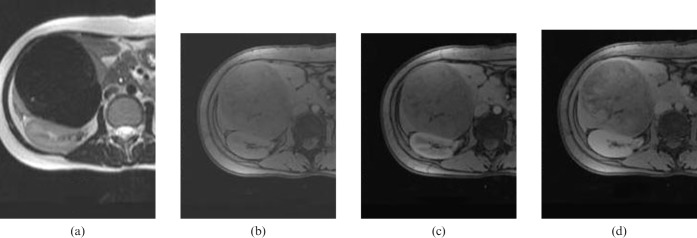
The patient underwent a helical CT scan (Somatom Plus 32, Siemens Medical Systems, Erlangen, Germany) of the liver. Contrast-enhanced dynamic CT revealed a 10 × 10 cm solid mass in segments V and VI of the right lobe of the liver. The mass was well demarcated and showed an exophytic growth pattern. On pre-contrast images, multiple laminated and amorphous calcifications were detected within the mass. After iv injection of contrast material, the mass was slowly enhanced. On 4 min delayed images, a large portion of the mass was enhanced (Figure 1). Liver MRI (1.5 T Magnetom Vision, Siemens Medical Systems) was performed for further evaluation of the mass 4 days after the CT scan. The mass showed as dark signal intensity on T2 weighted images and slightly low signal intensity on T1 weighted images. The contrast enhancement pattern on MRI was the same as with the CT scan (Figure 2). Ultrasound study of the abdomen showed severe posterior acoustic shadowing from the anterior portion of the mass, owing to dense calcifications. The mass could not be penetrated by an 18-gauge biopsy needle because of hardness from calcification. Consequently, we could not obtain sufficient tissue to conduct a diagnosis from the mass. The mass was hypovascular on hepatic artery angiography.
Figure 1.
CT findings of a calcifying fibrous tumour of the liver. (a) Pre-contrast CT scan showing a large solid mass in segments V and VI of the liver with exophytic growth and multifocal laminated and amorphous calcifications. (b) Arterial-phased contrast-enhanced CT scan showing subtle internal enhancement. (c) Equilibrium-phased contrast-enhanced CT scan showing a progressive heterogeneous enhancement pattern of the mass with capsule-like peripheral rim enhancement.
Figure 2.
MRI findings of a calcifying fibrous tumour of the liver. (a) T2 weighted image (T2WI) showing a dark signal-intensity mass in the right lobe of the liver. (b) Pre-contrast fat-suppressed T1 weighted image (T1WI) showing a lower signal-intensity mass corresponding to the T2WI. (c) Arterial and (d) 5 min-delayed contrast-enhanced T1WI showing a progressive delayed contrast-enhancement pattern of the mass similar to that of CT findings.
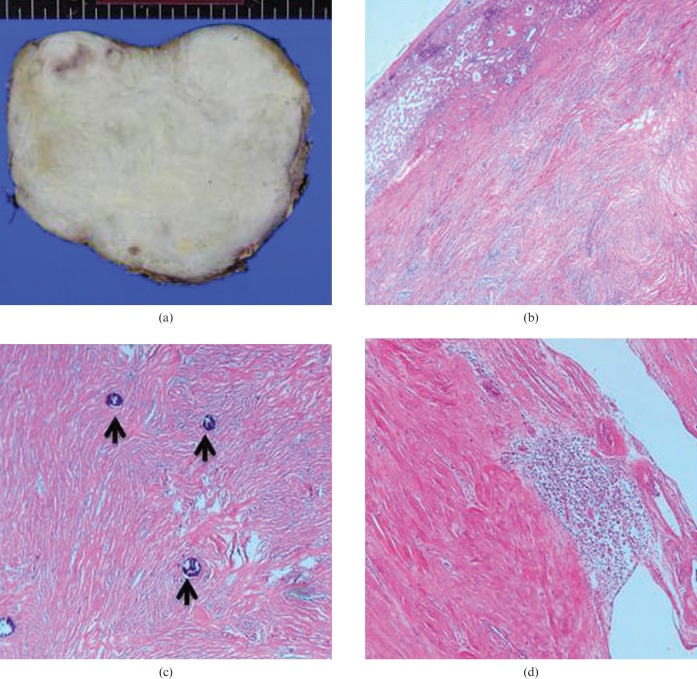
The patient underwent resection of segments V and VI of the right lobe of the liver with mass excision. The hepatic origin was then confirmed and involvement of adjacent structures was ruled out. The resected specimen showed a well-circumscribed grey-white firm to hard mass, measuring 15 × 10 × 10 cm (Figure 3a).
Figure 3.
Surgical and pathological findings of a calcifying fibrous tumour of the liver. (a) Surgical resection specimen of the mass showing a well-circumscribed grey-white firm to hard mass, measuring 15 × 10 × 10 cm. (b) Microscopic finding of the junction between normal liver and the mass. The normal liver parenchyma is shown in the left upper corner. The mass was composed of short fascicles of spindle cells in a collagenous stroma (haematoxylin and eosin (H&E), 40×). (c) The mass was generally hypocellular and consisted of bland spindle cells embedded within the vascularised collagenous stroma. Deposits of psammomatous calcifications (arrows) are characteristic (H&E, 100×). (d) Multifocal aggregations of inflammatory cells composed of lymphocytes and plasma cells are noted (H&E, 100×).
On histology, the mass was generally hypocellular and consisted of spindle cells embedded in a vascularised collagenous stroma. Deposits of psammomatous calcifications were also noted. Multiple aggregations of inflammatory cells composed of lymphocytes and plasma cells were noted. The immunohistochemical staining for CD34 showed equivocal positivity. The above findings suggested the diagnosis of calcifying fibrous tumour of the liver (Figure 3b–d). The patient had an uneventful post-operative course and has been followed-up for recurrence. There was no evidence of tumour recurrence on the 1 year follow-up CT scan.
Discussion
CFT is a rare benign tumour with a predilection for children and young adults [1]. It is characterised by dense hyalinised collagenous tissue interspersed with benign spindle cells, psammomatous or dystrophic calcifications and a lymphoplasmacytic infiltrate [2]. The extremities, followed by the trunk, neck and head, are mainly affected. Rare cases have been described of the tumour occurring in the mediastinum, pleura, lung, adrenal gland, epididymis and lymph node [1–9].
This disease entity was originally reported as a childhood fibrous tumour with psammoma bodies by Rosenthal et al [1]. Fetsch et al [2] reported 10 similar cases and first used the term calcifying fibrous pseudotumour. The cause and pathogenesis of calcifying fibrous pseudotumour were unclear. Van Dorpe et al [10] reported an unusual case with multiple peritoneal inflammatory pseudotumours and calcifying fibrous pseudotumours simultaneously and a transitional stage of the lesions, suggesting that calcifying pseudotumour is a late sclerosing stage of inflammatory myofibroblastic tumour (IMT), at least in some cases. However, Nascimento et al [11] analysed 15 cases of calcifying fibrous pseudotumours and proposed that calcifying fibrous pseudotumour is a distinctive benign mesenchymal neoplasm with a low risk for recurrence and, therefore, best labelled as calcifying fibrous tumour as there is no convincing evidence to support an association between calcifying fibrous pseudotumour and IMT. Hill et al [12] also suggested that the CFPs have distinct histological and immunohistochemical features from IMTs. Currently, it has been renamed as calcifying fibrous tumour owing to its tendency to recur locally [13].
CFT is a painless benign soft-tissue or subserosal lesion that occurs in various anatomical sites, mainly in children, adolescents and young adults, without obvious gender predilection [11]. Most lesions are well-circumscribed, solitary or multiple lesions with thick band-like or punctuate calcifications. These lesions are easily shelled out during operation, but are not encapsulated pathologically [14].
To date, about 30 cases of abdominal CFTs have been reported. Most cases have occurred in the mesentery or omentum, or on the serosal surface of the stomach or intestines [12].
The mean age of patients with CFTs occurring in the superficial soft tissue is 16.2 years compared with 34 years in abdominal CFTs. There is a female predominance in abdominal CFTs [2, 12].
To our knowledge, the case presented here is the first of CFT occurring within the liver that has been clinically, radiologically and pathologically documented. Our patient was a 29-year-old woman without any significant medical history who presented with a large hepatic mass with multiple laminated and amorphous calcifications. The mass was well demarcated and showed a progressive delayed contrast-enhancement pattern. There could be many differential diagnoses for calcified hepatic tumours. The radiological differential diagnosis of hepatic CFT includes hepatic localised fibrous tumour, fibrous tissue containing sarcomas such as fibrosarcoma and malignant fibrous histiocytoma (MFH), chondrosarcoma and rarely carcinosarcoma. Hepatic localised fibrous tumour is a rare disease entity and shows an early contrast-enhancement pattern that is correlated with the prominent vascular structure. Although sporadic cases with delayed contrast-enhancing hepatic localised fibrous tumour have been reported, large quantities of calcifications within the mass and a delayed enhancing pattern of CFT was somewhat different from the usual hepatic localised fibrous tumour [15]. Other malignant mesenchymal sarcomas such as MFH, chondrosarcoma and carcinosarcoma can sometimes show tumoural calcifications, but these lesions usually show more indistinct margins and larger cystic or necrotic changes, suggesting their aggressiveness [16, 17]. Most reported hepatic inflammatory pseudotumours (IPTs) have been hypovascular, with scarce enhancement at the arterial phase, and this could present a dilemma in the differential diagnosis between CFT and IPT. However, infrequency of calcifications in IPT could be a differential point [18]. Although rare, calcifying nested stromal–epithelial tumour of the liver shows similar radiological and clinical features to CFT in that it usually occurs in young females with a well-demarcated mass with psammoma-like calcifications. This low-grade malignancy has a characteristic histological appearance of irregular, sharply circumscribed nests and islands of bland-appearing spindled to focally epithelioid cells, surrounded by a cellular desmoplastic stroma [19]. Lastly, hepatic metastases with calcifications, such as mucin-producing adenocarcinoma, calcified gastrointestinal stromal tumour or calcified hepatocellular/cholangiocellular carcinoma, can be easily differentiated from CFT owing to their invasiveness and aggressiveness and relevant clinical history.
Although local recurrence has been reported in a few cases of CFT, these lesions are usually treated by local excision with clear margins. CFT does not have a propensity to metastasise and malignant transformation has not yet been reported [7].
In conclusion, we suggest that if the solid mass in the liver shows a well-circumscribed and calcified mass with a delayed contrast-enhancement pattern on CT, CFT should be considered as a differential diagnosis.
Acknowledgments
The authors are grateful for the critical review of the radiological images by Professor Jeong-Sik Yu from Yonsei University Medical College. We thank Suk Hee Kim and Seon Ha Yun for their help in the preparation of this manuscript.
References
- 1.Rosenthal NS, Abdul-Karim FW. Childhood fibrous tumor with psammoma bodies. Clinicopathologic features in two cases. Arch Pathol Lab Med 1988;112:798–800 [PubMed] [Google Scholar]
- 2.Fetsch JF, Montgomery EA, Meis JM. Calcifying fibrous pseudotumor. Am J Surg Pathol 1993;17:502–8 [DOI] [PubMed] [Google Scholar]
- 3.Erasmus JJ, McAdams HP, Patz EF, Jr , Murray JG, Pinkard NB. Calcifying fibrous pseudotumor of pleura: radiologic features in three cases. J Comput Assist Tomogr 1996;20:763–5 [DOI] [PubMed] [Google Scholar]
- 4.Suh JH, Shin OR, Kim YH. Multiple calcifying fibrous pseudotumor of the pleura. J Thorac Oncol 2008;3:1356–8 [DOI] [PubMed] [Google Scholar]
- 5.Mangat A, Schiller C, Mengoni P, Reynolds C, Jeruss JS. Calcifying fibrous pseudotumor of the breast. Breast J 2009;15:299–301 [DOI] [PubMed] [Google Scholar]
- 6.Jeong HS, Lee GK, Sung R, Ahn JH, Song HG. Calcifying fibrous pseudotumor of mediastinum: a case report. J Korean Med Sci 1997;12:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein EB, Savel RH, Sen F, Shamamian P. Calcifying fibrous pseudotumor of the neck: diagnostic challenges of a rare benign lesion. Am Surg 2005;71:1051–4 [PubMed] [Google Scholar]
- 8.Sudhakar S, Mistry Y, Dastidar A, Sen S, Gibikote S. Calcifying fibrous tumour: an unusual omental lesion. Pediatr Radiol 2008;38:1246–8 [DOI] [PubMed] [Google Scholar]
- 9.Eftekhari F, Ater JL, Ayala AG, Czerniak BA. Case report: Calcifying fibrous pseudotumour of the adrenal gland. Br J Radiol 2001;74:452–4 [DOI] [PubMed] [Google Scholar]
- 10.Van Dorpe J, Ectors N, Geboes K, D'Hoore A, Sciot R. Is calcifying fibrous pseudotumor a late sclerosing stage of inflammatory myofibroblastic tumor? Am J Surg Pathol 1999;23:329–35 [DOI] [PubMed] [Google Scholar]
- 11.Nascimento AF, Ruiz R, Hornick JL, Fletcher CDM. Calcifying fibrous ‘pseudotumor’: clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol 2002;10:189–96 [DOI] [PubMed] [Google Scholar]
- 12.Hill KA, Gonzalez-Crussi F, Chou PM. Calcifying fibrous pseudotumor versus inflammatory myofibroblastic tumor: a histological and immunohistochemical comparison. Mod Pathol 2001;14:784–90 [DOI] [PubMed] [Google Scholar]
- 13.Montgomery E. Calcifying fibrous tumor. In: Fletcher CD, Unni KK, Mertens F, editor. World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2002: 77–8 [Google Scholar]
- 14.Sudhakar S, Mistry Y, Dastidar A, Sen S, Gibikote S. Calcifying fibrous tumour: an unusual omental lesion. Pediatr Radiol 2008;38:1246–8 [DOI] [PubMed] [Google Scholar]
- 15.Moser T, Nogueira TS, Riehm S, Averous G, Weber JC. Delayed enhancement pattern in a localized fibrous tumor of the liver. AJR Am J Roentgenol 2005;184:1578–80 [DOI] [PubMed] [Google Scholar]
- 16.Yu RS, Chen Y, Jiang B, Wang LH, Xu XF. Primary hepatic sarcomas: CT findings. Eur Radiol 2008;18:2196–205 [DOI] [PubMed] [Google Scholar]
- 17.Kwon JH, Kang YN, Kang KJ. Carcinosarcoma of the liver: a case report. Korean J Radiol 2007;8:343–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesan K, Viamonte B, Peterson M, Kono Y, Santillan C, Middleton M, et al. Capsular retraction: an uncommon imaging finding in hepatic inflammatory pseudotumor. Br J Radiol 2009;82:e256–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makhlouf H, Abdul-Al HM, Wang G, Goodman ZD. Calcifying nested stromal-epithelial tumors of the liver. A clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up. Am Surg Pathol 2009;33:976–83 [DOI] [PubMed] [Google Scholar]