Abstract
Objectives
We describe the spectrum of findings and the diagnostic value of MR defecography in patients referred with suspicion of dyssynergic defecation.
Methods
48 patients (34 females, 14 males; mean age 48 years) with constipation and clinically suspected dyssynergic defecation underwent MR defecography. Patients were divided into patients with dyssynergic defecation (n = 18) and constipated patients without dyssynergic defecation (control group, n = 30). MRIs were analysed for evacuation ability, time to initiate evacuation, time of evacuation, changes in the anorectal angle (ARA-change), presence of paradoxical sphincter contraction and presence of additional pelvic floor abnormalities. Sensitivity, specificity, positive and negative predictive values and accuracy for the diagnosis of dyssynergic defecation were calculated.
Results
The most frequent finding was impaired evacuation, which was seen in 100% of patients with dyssynergic defecation and in 83% of the control group, yielding a sensitivity for MR defecography for the diagnosis of dyssynergic defecation of 100% (95% confidence interval (CI) 97–100%), but a specificity of only 23% (95% CI 7–40%). A lower sensitivity (50%; 95% CI 24–76%) and a high specificity (97%; 95% CI 89–100%) were seen with abnormal ARA-change. The sensitivity of paradoxical sphincter contraction was relatively high (83%; 95% CI 63–100%). A combined analysis of abnormal ARA-change and paradoxical sphincter contraction allowed for the detection of 94% (95% CI 81–100%) of the patients with dyssynergic defecation.
Conclusion
MR defecography detects functional and structural abnormal findings in patients with clinically suspected dyssynergic defecation. Impaired evacuation is seen in patients with functional constipation owing to other pelvic floor abnormalities than dyssynergic defecation.
Dyssynergic defecation, which produces functional outlet obstruction during defecation, is one of the causes of chronic constipation. Dyssynergic defecation is a functional disorder characterised by either paradoxical contraction, an inability to relax the anal sphincter and/or puborectalis muscle, or impaired abdominal and rectal pushing forces. In the literature, many other terms such as anismus [1], dyskinetic puborectalis muscle [2], non-relaxing puborectalis syndrome [3], spastic pelvic floor syndrome [4, 5] and pelvic floor dyssynergia [6] have been used. An expert group (Rome III) [7] recently proposed the term “dyssynergic defecation” to appropriately describe the failure of co-ordination or dyssynergia of the abdominal and pelvic floor muscles involved in defecation.
Different physiological tests can be used to investigate this functional disorder, including the balloon expulsion test, electromyography (EMG) of the puborectalis muscle and anorectal manometry. Defecography can be performed to rule out structural rectal abnormalities and provide an estimate of the degree of rectal emptying. As false-positive and false-negative results are common with these different tests, none can be used by itself as a gold standard for identifying patients with dyssynergic defecation.
Most authorities recommend using a combination of diagnostic tests and clinical history. The Rome III expert group defined the criteria for the diagnosis of dyssynergic defecation based on clinical history, anorectal manometry, balloon expulsion test, EMG and conventional defecography (evacuation proctography) [7]. Functional imaging with conventional defecography is considered to be a useful adjunct in establishing the diagnosis of dyssynergic defecation. Delayed initiation of evacuation and impaired evacuation in particular, as seen on conventional defecography, are highly predicitive for the presence of dyssynergic defecation [8, 9]. Different structural imaging findings in conventional defecography have been described in patients with dyssynergic defecation; however, the usefulness of these findings is discussed controversially [8, 10, 11].
The experience with MR defecography, which has shown to be a valuable alternative to evacuation proctography [12–15], is limited in dyssynergic defecation patients. There is only one study which has focused on the MR defecography findings in a study setting in patients with dyssynergic defecation [16]. Hence, the purpose of this study was to describe the spectrum of findings in MR defecography in patients referred with the suspicion of dyssynergic defecation and to assess the value of MR defecography in establishing this diagnosis. For the latter, the patients with dyssynergic defecation were compared with a group of constipated patients without dyssynergic defecation.
Methods and materials
The study consisted of a retrospective analysis of data collected as part of routine clinical work-up. The review and retrospective analysis of the clinical data were approved by the institutional review board, and informed consent was waived.
All patients who were referred for MR defecography because of clinical suspicion of dyssynergic defecation from the gastroenterology department in our hospital between March 2000 and January 2005 were identified from our MRI database (n = 79).
The gastroenterology department in our hospital is a tertiary centre for the physiological assessment and treatment of patients with anorectal disorders. The clinical suspicion of dyssynergic defecation was based on a history of functional constipation with obstructive defecation patterns [17]. Patients who did not undergo additional anorectal manometry testing (n = 19) and patients who underwent prior anal or pelvic floor surgery (n = 12) were excluded from the study. Thus, our study population comprised 48 patients.
The final diagnosis of dyssynergic defecation was established by an interdisciplinary panel including two gastroenterologists with special interest in anorectal diseases, a surgical proctologist and a radiologist after reviewing the clinical history, anorectal manometry and MR defecography. Clinical history, anorectal manometry and MR defecography were available in all 48 patients. The diagnosis made by the interdisciplinary panel was used as the gold standard for further analysis. According to the final diagnosis, the 48 patients were divided into 2 groups, the dyssynergic defecation group and the control group. Patients were included in the dyssynergic defecation group if they fulfilled the Rome III criteria for dyssynergic defecation [7]. To fulfil this, patients had to have symptoms of functional constipation [17] and at least two of the following features during repeated attempts to defecate: (1) evidence of impaired evacuation based on imaging; (2) inappropriate contraction of the pelvic floor muscles (i.e. anal sphincter or puborectalis) or inadequate relaxation of sphincter pressure by manometry or imaging; (3) adequate propulsive forces assessed by manometry or imaging. The control group had only symptoms of functional constipation without evidence of dyssynergic defecation in the diagnostic tests according to Rome III criteria.
MR defecography
All patients, including those of the dyssynergic defecation and those of the control group, underwent MR defecography according to our standard protocol for patients with pelvic floor disorders. MR defecography was performed using a superconducting open-configuration 0.5 T MRI system (Signa SP; GE Healthcare, Waukesha, WI) in the sitting position for the first 33 patients (69%). Afterwards, because of technical reasons, the open-configuration system was no longer available, and the following 15 patients (31%) were examined using a closed-configuration 1.5 T MRI system (Signa EchoSpeed EXCITE® HD or HDx; GE Healthcare) in the supine position. There was no significant difference with regard to the distribution of patients in the dyssynergic defecation group and in the control group who underwent MR defecography in the sitting or supine body position.
Prior to MRI, the patient’s rectum was filled with a synthetic stool. A convenience food product of potato starch was used, and 125 g of the potato starch powder was mixed with 200 ml of water and 1.5 ml of gadopentate dimeglumine (377 mg ml–1) (Magnevist®; Bayer Schering AG, Berlin, Germany), producing a gadolinium concentration of 2.5 mmol l–1. 300 ml of the enema was administrated via a rectal tube with the patient on the MR table in the lateral position. No other particular preparation was performed on the patients (e.g. voiding or bowel preparation).
When the MR defecography was performed in the open-configuration system, the patient was placed upright on a wooden chair between the magnet rings. A flexible transmit–receive radiofrequency coil was wrapped around the pelvis. A phased array coil was used if the examination was performed in the closed-configuration system with the patient in supine position. On the basis of localising images in the axial, coronal and sagittal planes, imaging was performed on a single slice in the midsagittal plane of the rectal anal canal in both scanners. In order to keep the imaging plane, patients were instructed not to move on the chair or table.
In the 1.5 T scanner a steady-state free-precession (SSFP) sequence (repetition time (TR)/echo time (TE), 3.5/1.5 ms; flip angle, 45°; section thickness, 10 mm with no interslice gap; bandwidth, 125 kHz; rectangular field of view (FOV), 31 cm; matrix, 224 × 160; two signals acquired) was first obtained at rest, at maximal voluntary sphincter and pelvic floor muscle contraction (squeezing) and at straining. Subsequently, the manoeuvre of evacuation was imaged with a T1 weighted multiphase fast spoiled gradient-recalled (FSPGR) echo sequence (TR/TE, 7.4/1.7 ms; flip angle, 80°; section thickness, 10 mm with no interslice gap; bandwidth, 15.6 kHz; rectangular FOV, 31 cm; matrix, 256 × 160; two signals acquired) with an image update every 2 s. In the 0.5 T scanner, all imaging (at rest, squeezing, straining and during evacuation) was performed with a T1 weighted multiphase FSPGR sequence (TR/TE, 22.4/10.7 ms; flip angle, 90°; section thickness, 15 mm with no interslice gap; bandwidth, 12.5 kHz; rectangular FOV, 29–32 cm; matrix, 256 × 160; one signal acquired). Image updates were provided every 2 s. The acquisition time for the FSPGR sequence varied from 2.5 min to 5 min depending on the evacuation ability and collaboration of the patient. The overall MRI time, including patient preparation, was 25 min at the longest. In order to obtain images at the different pelvic positions, the patients were coached by the technician performing the examination using a microphone and a headset. All images acquired at the different positions were formatted into a cine loop presentation to allow assessment of the dynamics of rectal emptying and of pelvic floor movement.
Imaging analysis
All MR images were retrospectively and independently analysed by two radiologists with similar levels of experience in reading MR defecography (AS and CSR, with 3 and 2 years of experience, respectively) in order to assess interobserver agreement on the presence or absence of an abnormal finding and interobserver reliability for quantitative measurements. For cases with disagreement on the presence or absence of an abnormal finding between the two readers, consensus was achieved in a separate reading by a third independent reader (blinded for review process) with more than 8 years of experience in MR defecography who served as a tiebreaker. The third reader was blinded to the results of analysis of the two first readers and analysed the whole study with discordant results. Although the readers knew the reason of referral for MR defecography (i.e. clinical suspicion of dyssynergic defecation), they were blinded to all of the results of the other diagnostic tests. All three radiologists who performed this retrospective image analysis were not involved in the prospective analysis of the interdisciplinary panel described above and thus were not aware of the final diagnosis of the patients. The imaging analysis was performed on a separate workstation (Advantage Windowing Workstation; GE Healthcare Europe, Buc, France). The MR defecography image interpretations were based on all source images and cine loops obtained at different positions (at rest, squeezing, straining and during evacuation). The measurements described below were performed with electronic callipers, which were included in the standard software delivered with the workstation. All readers analysed the images with regard to the evacuation ability (inability to evacuate or impaired evacuation ability), time to initiate evacuation (TIE), time of evacuation (TOE), changes in the anorectal angle (ARA) and the presence of paradoxical sphincter contraction.
The presence of impaired evacuation ability was defined as the inability to expel any contrast enema within the whole examination or two-thirds of the contrast enema within 60 s. The 60 s cut-off value for a normal evacuation time at MR defecography with the technique used in this study is based on published data in the literature where conventional defecography was performed in patients with dyssynergic defecation [18]. The percentage of contrast enema expelled was assessed subjectively on midsagittal multiphase images.
In patients who were able to evacuate some of the contrast enema, the time to initiate evacuation and the time of evacuation were measured. The time to initiate evacuation was defined as the interval between the initial pelvic floor descent and the opening of the anal canal. The time of evacuation was measured between the opening of the anal canal and the completion of the rectal emptying in seconds.
The ARA (the angle between the central axis of the anal canal and the posterior wall of the distal part of the rectum) [19] was measured at rest and during straining. ARA changes between rest and straining were calculated as [(ARA at straining) – (ARA at rest)]. Normally, the ARA increases between rest and straining owing to the normal pelvic floor descent at straining. Similar to the previous studies, abnormal changes of ARA were defined if the ARA decreased from rest to straining [8, 16].
A presence of paradoxical sphincter contraction was diagnosed when there was a marked impression of the puborectalis muscle or anal sphincter in the posterior anorectal wall and a poorly relaxing puborectalis muscle or anal sphincter with a lack of lowering the pelvic floor during straining and defecation.
In addition, each reader was asked to analyse all MR images with regard to pelvic floor structure abnormalities, including pelvic floor relaxation, pelvic organ prolapse, anterior rectoceles and intussusceptions (internal rectal prolapse). Pelvic floor relaxation and pelvic organ prolapse were measured on midsagittal images during maximal straining according to the HMO system (H-line, levator hiatus width; M-line, muscular pelvic floor relaxation; organ prolapse (HMO) classification) introduced by Comiter et al [20] and described in detail by Boyadzhyan et al [21]. The rectal descent was measured as the shortest distance between the pubococcygeal (PCL) and the anorectal junction [13].
An anterior rectocele was defined as a protrusion of the rectal wall anterior to a line extending upward through the anal canal. The size of an anterior rectocele was identified as the depth of wall protrusion beyond the expected margin of the normal rectal wall [13, 22].
The extent of any cystocele, vaginal vault descent and enterocele was graded according to Comiter et al [20] and Boyadzhyan et al [21], and rectal descent and anterior rectoceles were graded according to Roos et al [13], each with a three-grade scoring system, as small, moderate or large.
Finally, the length of an intussusception was measured on the midsagittal image during maximal straining or evacuation (depending on the position where the intussusception was obvious).
Statistical analysis
The statistical analysis of the collected data was performed using commercially available SPSS software (SPSS 15.0, Chicago, IL).
The interobserver agreement between the independent reader’s interpretations (Readers 1 and 2) was determined by calculating κ values and 95% of the confidence intervals (CIs). The κ values were tested for a significant difference from zero. A κ value of 0.00 indicated no agreement; 0.01–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, excellent agreement [23]. Bland–Altman analysis for interobserver agreement was used to compare the differences in measurements with the mean of measurements performed by the two readers [24].
For the comparison of the quantitative measurements (TIE, TOE and ARA) between the dyssynergic defecation group and the control group, mean values of measurements performed by Readers 1 and 2 were calculated and the Mann–Whitney U-test was performed. The proportions of abnormal findings (as obtained in consensus) in the dyssynergic defecation group and in the control group were compared with the χ2 test. If small numbers invalidated the χ2 approximation, Fisher’s exact test was used [25].
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of each pathological MR finding for the final diagnosis of dyssynergic defecation were calculated. For these calculations, the results of the “consensus” reading were used. For all tests a p-value of 0.05 or less was considered a statistically significant difference.
Results
18 patients (9 men (50%), 9 women (50%); mean age, 46 years; range, 20–76 years) constituted the dyssynergic defecation group. 30 patients (5 men (17%), (83%) 25 women; mean age, 56 years; range, 31–78 years) with functional constipation, but without evidence of dyssynergic defecation according to Rome III criteria, were included in the control group. The diagnosis in the control group was outlet obstruction owing to abnormal pelvic floor relaxation, pelvic organ prolapse, enterocele or intussusception, or a combination of these findings. The frequency of the MR findings, including impaired evacuation, abnormal ARA-change and paradoxical sphincter contraction in patients with dyssynergic defecation and constipated patients without dyssynergic defecation, are presented in Table 1.
Table 1. Frequency of abnormal findings in MR defecography.
| Dyssynergic defecation group (n = 18)a | Control group (n = 30)a | p-valueb | |
| Impaired evacuation | 18 (100) | 22 (73) | 0.04 |
| Abnormal ARA-change | 9 (50) | 1 (3) | <0.0001 |
| Paradoxical sphincter contraction | 16 (89) | 4 (13) | <0.0001 |
Numbers in parentheses are percentages.
ARA, anorectal angle. ARA-change calculated as [(ARA at straining)– (ARA at rest)].
aNumber of patients as assessed in consensus.
bCalculated with Fisher’s exact test.
Impaired evacuation
The most frequent finding was impaired evacuation, which was observed in the dyssynergic defecation group in all 18 patients (100%) and in the control group in 23 of 30 patients (77%) (Table 1, Figure 1). Among the 18 patients with dyssynergic defecation, 7 individuals (39%) were not able to evacuate any contrast enema during the whole examination. A statistically significant difference between the two groups was found for the presence of impaired evacuation (Table 1). In the dyssynergic defecation group, the time of evacuation (which was measured whenever any evacuation was possible) was prolonged (>60 s) in all 11 (100%) patients with the ability to evacuate, and in 22 of 30 (73%) patients in the control group. The mean time of evacuation was prolonged in the dyssynergic defecation group and in the control group, and was significantly longer in the dyssynergic defecation group than in the control group (Table 2). There was no significant difference in the mean time to initiate evacuation between the two groups (Table 2).
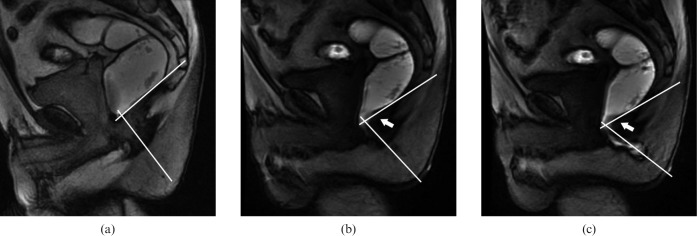
Figure 1.
Midsagittal MR images obtained with the closed-configuration system in a 65-year-old man with a final diagnosis of dyssynergic defecation (a) On steady-state free-precision images (5.7/2.6) obtained at (a) rest, the anorectal angle measures 94°. (b, c) T1 weighted multiphase fast-spoiled gradient-recalled echo images (8.4/2.1) show a decrease in the anorectal angle during straining (77°) (b) and during evacuation (69°) (c). In addition, paradoxical sphincter contraction is noted with impression of the dorsal anorectal wall during straining and evacuation (white arrow in b and c). White lines in a, b and c show anorectal angle measurements. The patient was able to evacuate only less than two-thirds of the contrast agent.
Table 2. Overview of the quantitative measurements on MR defecography images.
| Dyssynergic defecation group (n = 18) |
Control group (n = 30) |
p-valueb | |
| Meana | Meana | ||
| TIE (s) | 40.9 (4–143) | 20.8 (3–70) | 0.263 |
| TOE (s) | 143.5 (95–215) | 86.5 (24.234) | <0.0001 |
| ARA (degrees) | |||
| At rest | 105.6 (81–138) | 110.9 (77–141) | 0.133 |
| At straining | 105.7 (77–139) | 128.0 (87–155) | <0.0001 |
| ARA-change (degrees) | 0.1 (−33.5–23.5) | 17.1 (−2–42) | <0.0001 |
ARA, anorectal angle; ARA-change, calculated as [(ARA at straining)–(ARA at rest); TIE, time to initiate evacuation (measured only if evacuation was possible; dyssynergic defecation group, n = 11; control, n = 30); TOE, time of evacuation (measured only if evacuation was possible; dyssynergic defecation group, n = 11; control, n = 30).
aMean values calculated from measurements performed by Readers 1 and 2; numbers are mean values, numbers in parentheses are ranges.
bMann–Whitney U-test.
Anorectal angle
Abnormal changes, defined as a decrease between ARAs measured at rest and straining, were identified significantly more often in the dyssynergic defecation group (Table 1). In 50% (9/18) of patients with dyssynergic defecation (Figures 1 and 2) and in 3% (1/30) of patients in the control group, abnormal changes of ARA were noted (Table 1). No significant differences were observed between the two groups regarding the ARA measurements at rest. The ARA measurements at straining and the ARA-change, on the other hand, were significantly lower in the dyssynergic defecation group than in the control group (Table 2).
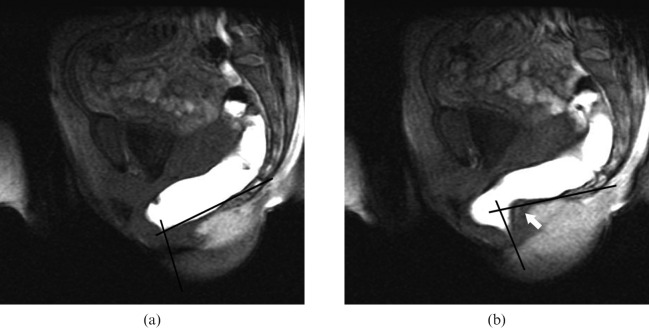
Figure 2.
Midsagittal MR images obtained with the open-configuration system in a 22-year-old woman with the final diagnosis of dyssynergic defecation. (a, b) T1 weighted multiphase fast-spoiled gradient-recalled echo images (22.4/10.7) were obtained at (a) rest and (b) straining. (b) During straining a paradoxical puborectalis contraction with impression of the posterior anorectal wall (white arrow) is seen. A normal anorectal angle was measured (a) at rest (110°). During straining (b), a lack of increase in the anorectal angle (83°) was present (black lines in (a) and (b)). The patient showed impaired evacuation with an increased time of evacuation.
Sphincter contraction
Paradoxical sphincter contraction was observed in 15/18 (83%) patients of the dyssynergic defecation group (Figures 1 and 2), and significantly less often in the control group (4/30 (13%) patients) (Table 1).
Diagnostic performance
The sensitivity, specificity, PPV, NPV and accuracy for the diagnosis of dyssynergic defecation of the main MR findings are shown in Table 3. Impaired evacuation showed the highest sensitivity for the detection of dyssynergic defecation. This finding, however, had a low specificity because impaired evacuation occurred with a high frequency in the control group as well. A lower sensitivity, but high specificity, was seen with abnormal ARA-change (see false-negative result in Figure 3). The sensitivity of paradoxical sphincter contraction was relatively high. When these findings were interpreted in combination, sensitivity improved. The highest sensitivity, NPV and accuracy were obtained if abnormal ARA-change and paradoxical sphincter contraction were interpreted in combination (Table 3).
Table 3. Diagnostic performance of MR defecography findings in the diagnosis of dyssynergic defecation.
| Measurements | TPRa | TNRa | FPRa | FNRa | Sensitivityb | Specificityb | PPVb | NPVb | Accuracyb |
| Impaired evacuation | 18 | 7 | 23 | 0 | 100 (97–100) | 23 (7–40) | 44 (27–60) | 100 (93–100) | 52 (37–67) |
| Abnormal ARA-change | 9 | 29 | 1 | 9 | 50 (24–76) | 97 (89–100) | 90 (66–100) | 76 (61–91) | 79 (67–92) |
| Paradoxical sphincter contraction | 15 | 26 | 4 | 3 | 83 (63–100) | 87 (73–100) | 79 (58–100) | 90 (77–100) | 85 (74–96) |
| ARA+paradoxical sphincter contraction | 17 | 26 | 4 | 1 | 94 (81–100) | 87 (73–100) | 81 (62–100) | 96 (87–100) | 90 (80–99) |
Data are results from the consensus reading. Numbers in parentheses are 95% confidence intervals.
ARA, anorectal angle; ARA-change, calculated as [(ARA at straining)–(ARA at rest)]; FNR, false-negative results; FPR, false-positive results; NPV, negative predictive value; PPV, positive predictive value; TNR, true-negative results; TPR, true-positive results.
aNumbers are absolute numbers of patients.
bNumbers are percentages.
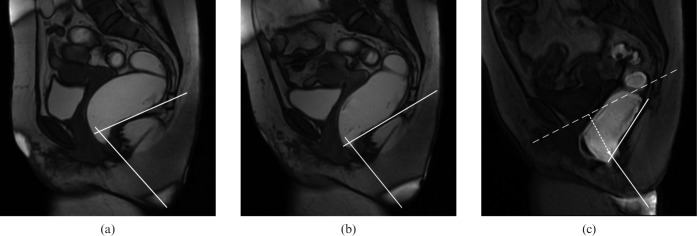
Figure 3.
Midsagittal MR images obtained with the closed-configuration system in a 57-year-old woman of the dyssynergic defecation group with positive manometry findings and clinically suspected dyssynergic defecation. The final diagnosis as established by the expert panel was dyssynergic defecation. (a, b) Steady-state free-precision images (3.6/1.4) obtained (a) at rest show an anorectal angle of 72°, and (b) at straining show a normal increase in the anorectal angle (84°) (white lines). (c) T1 weighted multiphase fast-spoiled gradient-recalled echo image (7.4/1.7) during evacuation shows a further increase in the anorectal angle to 117° (white lines). No evidence of paradoxical sphincter contraction (b) during straining or (c) evacuation is seen. A moderate posterior compartment descent of 38 mm (dotted arrow in c) below pubococcygeal line (dotted line in c) was detected. The patient experienced impaired evacuation with an increased time of evacuation of 90 s.
Interobserver agreement
The interobserver agreement for impaired evacuation, abnormal ARA-change and paradoxical sphincter contraction was good, revealing κ-values of 0.79 (95% CI 0.55–1.02), 0.65 (95% CI 0.43–0.87) and 0.67 (95% CI 0.41–0.93), respectively.
Bland–Altman analysis revealed a mean difference between the measurements of the ARA at rest and ARA at straining of both readers of 1.5° (standard deviation (SD) ±14.2) and −3.4° (SD±15.2) with limits of agreement of −26.4° to 29.4° and −33.1° to 26.3°. The mean interobserver difference of TIE and TOE were 0.2 s (SD±7.6) and −2.2 s (SD±14.2) with limits of agreement of −14.7 to 15.2 s and −30.2 to 25.7 s.
Additional pelvic floor abnormalities
The frequency of additional pelvic floor abnormalities, including abnormal pelvic floor relaxation, pelvic organ prolapse, rectal descent, anterior rectocele and intussusception in the two groups is shown in Table 4. No significant difference between the groups was found and, thus, no conclusions can be drawn for the differentiation between patients with and without dyssynergic defecation. The most frequent MR finding was rectal descent, which was diagnosed in 78% of patients with dyssynergic defecation (in 9/9 female and 5/9 male patients) (Figure 3) and in 100% of patients in the control group (Figure 4).
Table 4. Spectrum of additional MR defecography findings in patients with dyssynergic defecation and the control group.
| Dyssynergic defecation group (n = 18) |
Control group (n = 30) |
|||
| No. of patientsa | % | No. of patientsa | % | |
| Pelvic floor relaxation | ||||
| H-lineb | ||||
| Normal | 12 (4) | 67 | 5 (3) | 16 |
| Mild | 6 (5) | 33 | 20 (18) | 67 |
| Moderate | 0 | – | 5 (4) | 16 |
| M-lineb | ||||
| Normal | 6 (3) | 33 | 2 (0) | 7 |
| Mild | 6 (1) | 33 | 6 (5) | 20 |
| Moderate | 6 (5) | 33 | 15 (14) | 50 |
| Severe | 0 | – | 7 (6) | 23 |
| Pelvic organ prolapse and other findings | ||||
| Cystoceleb | ||||
| No prolapse | 16 (8) | 89 | 22 (17) | 73 |
| Small | 2 (1) | 7 | 6 (6) | 20 |
| Moderate | 0 | – | 2 (2) | 7 |
| Vaginal vault descentb,c | ||||
| No prolapse | 9 | 100 | 16 | 64 |
| Small | 0 | – | 9 | 36 |
| Enteroceleb | ||||
| No prolapse | 18 (9) | 100 | 25 (20) | 83 |
| Small | 0 | – | 4 (4) | 13 |
| Moderate | 0 | – | 1 (1) | 3 |
| Rectal descentd | ||||
| Small | 6 (4) | 33 | 3 (1) | 10 |
| Moderate | 7 (4) | 39 | 16 (13) | 53 |
| Large | 1 (1) | 6 | 11 (11) | 37 |
| Anterior rectoceled | ||||
| Small | 5 (4) | 28 | 11 (11) | 37 |
| Moderate | 2 (2) | 6 | 7 (7) | 23 |
| Large | 0 | – | 2 (2) | 7 |
| Intussusception | 0 | – | 7 (6) | 23 |
Figure 4.
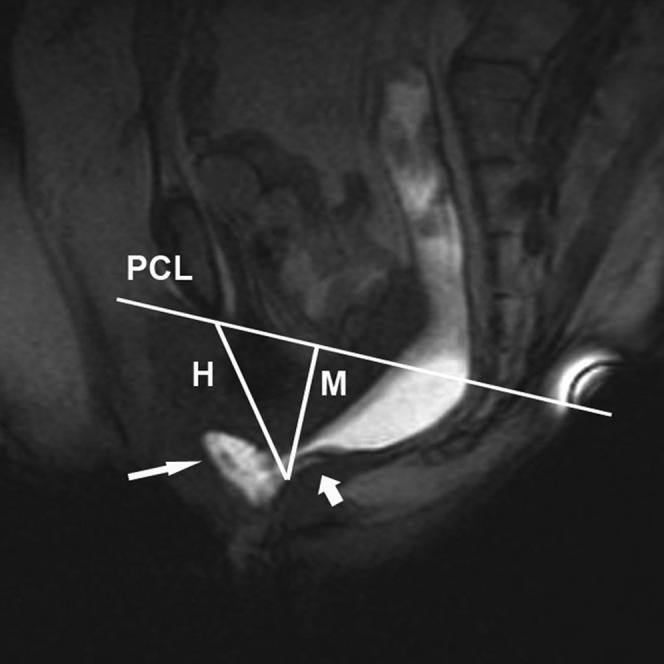
Midsagittal MR image obtained with the open-configuration system in a 58-year-old woman with constipation, but absence of dyssynergic defecation (control group). The T1 weighted multiphase fast-spoiled gradient-recalled echo image (22.4/10.7) obtained at the end of evacuation demonstrates an intussusception (small white arrow) and a moderate anterior rectocele of 24 mm (large white arrow). A mild hiatal widening (H line, 7 cm) and a mild hiatal descent (M line, 3.4 cm) are seen. The patient showed impaired evacuation with a slightly increased time of evacuation of 80 s. No abnormal anaorectal angle-change or paradoxical sphincter contraction was seen. PCL, pubococcygeal line; H, H-line; M, M-line.
Discussion
Compared with asymptomatic volunteers, patients with dyssynergic defecation demonstrate delayed initiation of evacuation and impaired evacuation [9]. Impaired evacuation is highly suggestive for the presence of dyssynergic defecation. In accordance with the results of two studies by Halligan et al [8, 9], the findings of the present study have also shown that impaired evacuation is the most frequent finding in patients with dyssynergic defecation. However, impaired evacuation yielded a low specificity and low PPV for the presence of dyssynergic defecation because it was also seen in patients of the control group. This fact may be explained by the consistency of our control group, which included patients with functional constipation without dyssynergic defecation, who by definition show signs of impaired evacuation as straining, sensation of incomplete evacuation and blockage. Our results suggest that impaired evacuation is not only seen in patients with dyssynergic defecation, but also in other patients presenting with functional constipation owing to pelvic floor abnormalities other than dyssynergic defecation.
Therefore, in the particular clinical setting among patients with functional constipation and clinically suspected dyssynergic defecation, other findings may be useful for identifying patients with dyssynergic defecation. Dyssynergic defecation includes paradoxical contraction or the inability to relax the pelvic floor musculature resulting in a lack of descent of the pelvic floor, a failure to increase the ARA and potentially a prominent impression of the puborectal sling [3, 5, 11]. Although the decrease in the ARA during straining is considered a typical sign of dyssynergic defecation, other studies with conventional defecography could not confirm this finding [8], and other authors also found this sign in asymptomatic volunteers [26, 27]. A recent study [16] on dynamic MR in paediatric patients with dyssynergic defecation described significant differences with regard to the ARA during straining and with regard to the ARA-change on straining between patients with dyssynergic defecation and healthy controls. However, we found abnormal ARA-changes not helpful for identifying patients with dyssynergic defecation (when interpreted independently), because only 50% of patients with dyssynergic defecation showed abnormal ARA-changes. Conversely, if an abnormal ARA-change was present, 90% of patients had dyssynergic defecation.
Concerning the presence of paradoxical sphincter contraction in patients with dyssynergic defecation, discordant results were noted in various studies. Karlbom et al [11] found a significant correlation between paradoxical sphincter contraction during straining and decreased rectal emptying. Another study found paradoxical sphincter contraction in healthy controls as well as in patients with chronic constipation, suggesting that paradoxical sphincter contraction is a non-specific finding [28]. In contrast to the studies performed with conventional defecography, our results have shown that paradoxical sphincter contraction is a useful finding for identifying patients with dyssynergic defecation. Similar results were seen in the above-mentioned study by Chu et al [16] on dynamic MR in paediatric patients with dyssynergic defecation, in whom a different puborectalis configuration concerning length and thickness was found compared with controls. We interpret this result based on the fact that MR defecography allows better visualisation of the pelvic floor muscles than conventional defecography because of the direct visualisation of the pelvic floor structures [14].
According to our results, the mean evacuation time was considerably longer in our dyssynergic defecation patients (mean evacuation time 143.5 s) compared with the results reported by Halligan et al [8] in a comparable set of patients (mean evacuation time 50 s). This difference in evacuation time can be explained by the larger amount of contrast used for the rectal enema (300 ml instead of 120 ml used by Halligan et al [8, 9]). Differences in enema viscosity (potato starch vs barium) may be another cause for longer evacuation times measured in the present study [10, 26, 29].
The inadequate relaxation of the pelvic floor during straining and evacuation, which is considered an important factor in the pathogenesis of dyssynergic defecation [1, 5], results in a stronger straining effort needed to initiate evacuation. Similar to the data from Halligan et al [8], the time to initiate evacuation (interval between initial pelvic floor descent and opening of the anal canal) was prolonged in patients with dyssynergic defecation in our study.
The analysis of the diagnostic performance of each of the individual parameters, which have been evaluated in the present study, has shown that the assessment of impaired evacuation alone is not useful in a clinical setting because it has almost no discriminatory power in patients presenting with functional constipation alone and in patients with dyssynergic defecation. We found the presence of abnormal ARA-change highly predictive for dyssynergic defecation, but interpretation of abnormal ARA-change revealed a low sensitivity. Interpreting paradoxical sphincter contraction alone showed a sensitivity of 83%. When interpreting abnormal ARA-changes and paradoxical sphincter contraction together, 94% of the patients with dyssynergic defecation could be detected.
The distinction between patients with functional constipation with or without dyssynergic defecation is important, as in our experience patients with MR defecography-documented dyssynergic defecation even in the absence of manometry-documented paradoxical sphincter contraction would profit more from pelvic floor rehabilitation than their counterparts with functional constipation and no other signs of dyssynergic defecation in MR defecography and/or anorectal manometry.
The main limitation of this study is that to date no single method has been universally adopted for the diagnosis of dyssynergic defecation. Thus, currently no true gold standard exists. A separation between patients with dyssynergic defecation and functional constipation might have been achieved by following up on the clinical response to pelvic floor rehabilitation using biofeedback therapy. This would also not have been perfect since some patients with dyssynergic defecation do not respond to physical therapy and, conversely, patients with functional constipation might report symptom improvement following physical therapy [30]. Under these circumstances, we used the most recent Rome III criteria [7] for the diagnosis of dyssynergic defecation, and the diagnosis was established by an independent interdisciplinary panel. Another major limitation of the study is that the MR defecography was also part of the consensus panel diagnosis, thus incorporation bias could have occurred. Because imaging is part of the Rome III criteria and no additional imaging studies (i.e. conventional defecography) were available, we had to provide MR defecography to the interdisciplinary panel. To minimise incorporation bias, the interdisciplinary panel did not perform any quantitative measurements on MR defecography images.
The relatively small number of our study group may also be considered as a limitation. Furthermore, we did not compare MR findings in patients with dyssynergic defecation with those in healthy subjects. We chose a control group of constipated patients with clinical suspicion of dyssynergic defecation but where the final diagnosis was different from dyssynergic defecation, because this reflects a realistic diagnostic challenge in clinical practice.
Finally, a potential limitation is the fact that MR defecography was not performed in the same body positions in all patients.
Conclusion
This study has shown that MR defecography detects functional and structural abnormal findings in patients with clinically suspected dyssynergic defecation, and some of these findings are helpful for establishing the diagnosis of dyssynergic defecation. Furthermore, the study has shown that impaired evacuation, a main finding in dyssynergic defecation, is also seen in patients with functional constipation owing to pelvic floor abnormalities other than dyssynergic defecation. Further studies including a larger number of patients are warranted to reproduce our findings.
In addition, studies incorporating interventional arms such as biofeedback therapy with follow-up on symptom, manometric and MR defecography changes would be of interest to evaluate the prognostic value of the parameters suggested.
References
- 1.Preston DM, Lennard-Jones JE. Anismus in chronic constipation. Dig Dis Sci 1985;30:413–18 [DOI] [PubMed] [Google Scholar]
- 2.Karasick S, Karasick D, Karasick SR. Functional disorders of the anus and rectum: findings on defecography. AJR Am J Roentgenol 1993;160:777–82 [DOI] [PubMed] [Google Scholar]
- 3.Jorge JM, Wexner SD, Ger GC, Salanga VD, Nogueras JJ, Jagelman DG. Cinedefecography and electromyography in the diagnosis of nonrelaxing puborectalis syndrome. Dis Colon Rectum 1993;36:668–76 [DOI] [PubMed] [Google Scholar]
- 4.Kelvin FM, Maglinte DD, Benson JT. Evacuation proctography (defecography): an aid to the investigation of pelvic floor disorders. Obstet Gynecol 1994;83:307–14 [PubMed] [Google Scholar]
- 5.Kuijpers HC, Bleijenberg G. The spastic pelvic floor syndrome. A cause of constipation. Dis Colon Rectum 1985;28:669–72 [DOI] [PubMed] [Google Scholar]
- 6.Whitehead WE, Wald A, Diamant NE, Enck P, Pemberton JH, Rao SS. Functional disorders of the anus and rectum. Gut 1999;45 (Suppl 2):II55–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology 2006;130:1510–18 [DOI] [PubMed] [Google Scholar]
- 8.Halligan S, Bartram CI, Park HJ, Kamm MA. Proctographic features of anismus. Radiology 1995;197:679–82 [DOI] [PubMed] [Google Scholar]
- 9.Halligan S, Malouf A, Bartram CI, Marshall M, Hollings N, Kamm MA. Predictive value of impaired evacuation at proctography in diagnosing anismus. AJR Am J Roentgenol 2001;177:633–6 [DOI] [PubMed] [Google Scholar]
- 10.Karlbom U, Nilsson S, Pahlman L, Graf W. Defecographic study of rectal evacuation in constipated patients and control subjects. Radiology 1999;210:103–8 [DOI] [PubMed] [Google Scholar]
- 11.Karlbom U, Pahlman L, Nilsson S, Graf W. Relationships between defecographic findings, rectal emptying, and colonic transit time in constipated patients. Gut 1995;36:907–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenenberger AW, Debatin JF, Guldenschuh I, Hany TF, Steiner P, Krestin GP. Dynamic MR defecography with a superconducting, open-configuration MR system. Radiology 1998;206:641–6 [DOI] [PubMed] [Google Scholar]
- 13.Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics 2002;22:817–32 [DOI] [PubMed] [Google Scholar]
- 14.Healy JC, Halligan S, Reznek RH, Watson S, Bartram CI, Phillips R, et al. Dynamic MR imaging compared with evacuation proctography when evaluating anorectal configuration and pelvic floor movement. AJR Am J Roentgenol 1997;169:775–9 [DOI] [PubMed] [Google Scholar]
- 15.Fletcher JG, Busse RF, Riederer SJ, Hough D, Gluecker T, Harper CM, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol 2003;98:399–411 [DOI] [PubMed] [Google Scholar]
- 16.Chu WC, Tam YH, Lam WW, Ng AW, Sit F, Yeung CK. Dynamic MR assessment of the anorectal angle and puborectalis muscle in pediatric patients with anismus: technique and feasibility. J Magn Reson Imaging 2007;25:1067–72 [DOI] [PubMed] [Google Scholar]
- 17.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–91 [DOI] [PubMed] [Google Scholar]
- 18.Minguez M, Herreros B, Sanchiz V, Hernandez V, Almela P, Anon R, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology 2004;126:57–62 [DOI] [PubMed] [Google Scholar]
- 19.Kruyt RH, Delemarre JB, Doornbos J, Vogel HJ. Normal anorectum: dynamic MR imaging anatomy. Radiology 1991;179:159–63 [DOI] [PubMed] [Google Scholar]
- 20.Comiter CV, Vasavada SP, Barbaric ZL, Gousse AE, Raz S. Grading pelvic prolapse and pelvic floor relaxation using dynamic magnetic resonance imaging. Urology 1999;54:454–7 [DOI] [PubMed] [Google Scholar]
- 21.Boyadzhyan L, Raman SS, Raz S. Role of static and dynamic MR imaging in surgical pelvic floor dysfunction. Radiographics 2008;28:949–67 [DOI] [PubMed] [Google Scholar]
- 22.Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology 2002;223:501–8 [DOI] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363–74 [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 25.Altmann DG. Practical statistics for medical research. London, UK: Chapman Hall, 1991 [Google Scholar]
- 26.Bartram CI, Turnbull GK, Lennard-Jones JE. Evacuation proctography: an investigation of rectal expulsion in 20 subjects without defecatory disturbance. Gastrointest Radiol 1988;13:72–80 [DOI] [PubMed] [Google Scholar]
- 27.Goei R, van Engelshoven J, Schouten H, Baeten C, Stassen C. Anorectal function: defecographic measurement in asymptomatic subjects. Radiology 1989;173:137–41 [DOI] [PubMed] [Google Scholar]
- 28.Voderholzer WA, Neuhaus DA, Klauser AG, Tzavella K, Muller-Lissner SA, Schindlbeck NE. Paradoxical sphincter contraction is rarely indicative of anismus. Gut 1997;41:258–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solopova AE, Hetzer FH, Marincek B, Weishaupt D. MR defecography: prospective comparison of two rectal enema compositions. AJR Am J Roentgenol 2008;190:W118–24 [DOI] [PubMed] [Google Scholar]
- 30.Battaglia E, Serra AM, Buonafede G, Dughera L, Chistolini F, Morelli A, et al. Long-term study on the effects of visual biofeedback and muscle training as a therapeutic modality in pelvic floor dyssynergia and slow-transit constipation. Dis Colon Rectum 2004;47:90–5 [DOI] [PubMed] [Google Scholar]