Abstract
Objective
The aim of this study was to find out on an unselected patient group whether crossing vessels have an influence on the width of the renal pelvis and what independent predictors of these target variables exist.
Methods
In this cross-sectional study, 1072 patients with arterially contrasted CT scans were included. The 2132 kidneys were supplied by 2736 arteries.
Results
On the right side, there were 293 additional and accessory arteries in 286 patients, and on the left side there were 304 in 271 patients. 154 renal pelves were more than 15 mm wide. The greatest independent factor for hydronephrosis on one side was hydronephrosis on the contralateral side (p<0.0001 each). Independent predictors for the width of the renal pelvis on the right side were the width of the renal pelvis on the left, female gender, increasing age and height; for the left side, predictors were the width of the renal pelvis on the right, concrements, parapelvic cysts and great rotation of the upper pole of the kidney to dorsal. Crossing vessels had no influence on the development of hydronephrosis. Only anterior crossing vessels on the right side are associated with widening of the renal pelvis by 1 mm, without making it possible to identify the vessel as an independent factor in multivariate regression models.
Conclusion
The width of the renal pelvis on the contralateral side is the strongest independent predictor for hydronephrosis and the width of the renal pelvis. There is no link between crossing vessels and the width of the renal pelvis.
Obstructions of the ureteropelvic junction (UPJ) can be caused by intrinsic or extrinsic factors [1]. Although there are no studies of this to date, crossing the UPJ by an aberrant crossing vessel is considered the most important [2] of the extrinsic factors [3]. Crossing vessels, which are thought to cause from 40% to over 50% of the extrinsic UPJ obstructions in adults [4, 5], are located ventral more often than dorsal to the UPJ. These are usually normal vessels of the lower pole segment [4, 6–9], which can be divided into additional renal arteries arising from the aorta, and accessoric renal arteries arising from branches of the aorta [10, 11]. The primary surgical therapy of choice is endoscopic endopyelotomy [12]. The success rate of 89–90% [12, 13] is thought to be noticeably poorer in patients with crossing vessels [12, 13]; however, this is not undisputed [14, 15]. Be that as it may, to prevent bleeding complications it is necessary to be familiar with the vascular situation around the UPJ prior to the procedure [3, 16–18]. CT angiography is used for this purpose, as it is highly accurate, quick to perform and shows all relevant anatomical structures in relation to one another [3, 19, 20]. The objective of this study was to determine whether or not there are vascular morphological patterns or other factors that influence the width of the renal collecting system, regardless of the definitions of hydronephrosis.
Methods and materials
Patients
Between February 2007 and March 2008, 1072 consecutive patients were included in the observation study. Of these, 473 were female (44.1%) and 599 were male (55.9%) with an average age of 59.5±17.6 years (range 1.7–99.4 years). Of these patients, 3.1% (n = 32) were children younger than 18 years. The indications for the CT scans were tumours for 288 (26.8%) of the patients, vascular pathologies for 221 (20.6%), pathologies of the liver for 213 (19.9%), haemorrhaging for 33 (3.1%) and various other causes for 317 (29.6%). The study was conducted subject to the guidelines of the Declaration of Helsinki. The study had no influence on the indication and execution of the CT scans or on the treatment of patients. Institutional ethical approval was waived as the study had no influence on treatment, and our institutional ethical review board did not require its approval for this retrospective cross-sectional study. All patients signed a written informed consent form before the examination.
Imaging
All examinations were conducted on the same 64-slice multidetector CT scanner (Lightspeed VCT XT, General Electric, Milwaukee, WI) and included at least an arterial-phase scan. Table 1 summarises the frequency and the details of the protocols that were used. The 0.625-mm collimated source images were saved temporarily on a General Electric workstation (AW 4.4, General Electric) in order to be able to perform reformations retrospectively from the pictures if this turns out to be clinically necessary.
Table 1. Scan protocols, scan parameters and frequency.
| Protocol | Liver | CT angiography | Pancreas | Protocol 4 | Protocol 5 |
| ST of native phase | 5 mm | – | 5 mm | – | 5 mm |
| ST of arterial phase | 0.625 mm | 0.625 mm | 0.625 mma | 0.625 mm | 0.625 mm |
| ST of portal-venous phase | 5 mm | 5 mm | 5 mm | 5 mm | 5 mm |
| ST of venous phase | 2.5 mm | – | – | – | – |
| Contrast media | Iodixanol 320 | Iodixanol 320 | Iodixanol 320 | Iodixanol 320 | Iodixanol 320 |
| Noise index | 20/16/18 | 40/18 | 20/16/18 | 16/18 | 20/16/18 |
| Number of patients | 444 | 371 | 134 | 35 | 88 |
| Percentage of patients | 41.4 | 34.6 | 12.5 | 3.3 | 8.2 |
aModified to show the pancreas with an extra delay of 3 s before the arterial phase.
ST, slice thickness.
Execution and design of the study
The study was designed as a retrospective cross-sectional study. If an examination fulfilled the inclusion criterion of a density of >200 HU in the aorta at the level of the renal arteries, the 0.625 mm collimated, temporarily saved source images were sent to the Picture Acquisition and Communication System (PACS). This was done once a week, retrospectively. Image analysis and all preliminary examinations were carried out on a diagnostic monitor (Lenovo 6659 HG2, IBM, Raleigh, Morrisville, NC) by a consensus of two radiologists with a wide experience of abdominal CT imaging stemming from several thousand examinations. For 1026/1072 patients (95.7%), there was more than one examination in the PACS. These examinations added up to a total of 4658 abdominal CT scans (4.3±4.2 examinations/patient; range 0–30). 3D-PACS software (Tiani 3D PACS software, version 3.3.16, Agfa-Gevaert N.V., Mortsel, Belgium) was used for the assessment of the examinations.
Inclusion and exclusion criteria
Inclusion criteria were a contrast of >200 HU in the aorta in the arterial phase and the existence of a venous contrast medium phase also with collimation of 0.625 mm. The 64-slice multidetector CT scanner was chosen to generate an unselected patient group consisting of 1600 patients because, owing to its location, this device is not used for urology or nephrology patients. Exclusion criteria were not reaching the threshold (468/1600 patients; 29.25%), incomplete visualisation of the urogenital system, movement artefacts, poor delimitation of a ureter or renal pelvis and technical defects (18/1600 patients; 1.125%). If there were any known diseases of the kidneys or the urinary tract prior to the examination, or if the indication for the examination was related to them, this also led to exclusion (31/1600 patients; 1.94%). None of these 31 patients suffered from hydronephrosis or obstruction of the UPJ. 11 patients with diseases of the renal parenchyma were excluded. No patient with dilatation of the renal pelvis was excluded. Consequently, the CT scans of 1072 patients without any symptoms with respect to the kidneys or urinary tract were assessed. Patients with a creatinine level >2 mg dl–1 were not examined.
Vessel contrast
In the 1072 CT scans, contrast of the vena cava was 110.2±58.4 HU; contrast of the aorta at the level of the renal arteries was 301.5±77.3 HU.
Parameters analysed and definitions
The primary target values were the width of the renal pelvis in the axial plane and the width of the ureters immediately caudal to the infundibulum; secondary target parameters were hydronephrosis and UPJ obstruction. The width of the renal pelvis (i.e. the anterior-posterior (AP) diameter) was measured in the axial plane, in the area where the pelvis leaves the kidney (as shown in Figure 1). Possible influencing factors that were compiled were age, gender, number of additional or accessory renal arteries from the aorta or its branches on each side and their relation to the renal pelvis or ureter, renal veins, rotation status of the renal pelvis in the axial scans in relation to a sagittal medial plane, rotation status of the kidney in relation to a coronal plane, diameter of the vena cava, length of the kidneys, width of the parenchyma, position related to the spine, infarctions, parapelvic cysts and concrements. Additional and accessory renal arteries were counted based on Satyapal's definition [10, 11]. The definition of hydronephrosis was a width of the renal pelvis ≥15 mm [13], which was met by 154/2132 pelves (7.2%). Pelves with diameters <15 mm but with clearly obstructed calyces did not occur. Pelves with diameters >15 mm combined with dilated ureters did not occur owing to the design of the study. A second definition of hydronephrosis was the occurrence of calyceal splaying, because criteria of obstruction are difficult to define with precision [13]. Measurements of ureteric and pelvic width are not always reliable, as these are dynamic structures and are influenced by factors such as peristaltic movement and hydration. However, the degree of reproducibility of the data taken from patients with more than one CT scan was good, as indicated by a correlation coefficient of p = 0.74 between the width of the pelves of the same patients obtained from examinations with an interval of 6.3±3.7 months. Owing to the pronounced variability of the arborisation of the renal arteries in patients without additional or accessory renal arteries, we abstained from the classification of branches of the renal arteries into kinds with and without a principle inferior branch [21].
Figure 1.
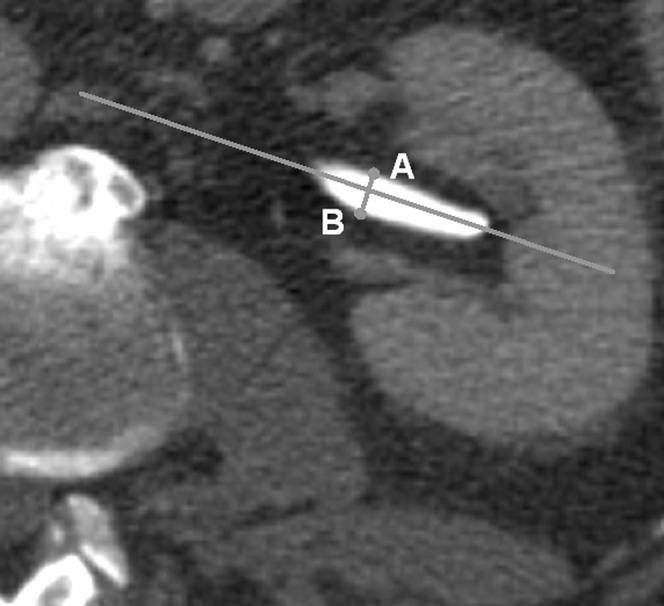
The width of the renal pelvis was measured in the axial plane in the area where the pelvis leaves the kidney. Perpendicular to the “longitudinal axis” drawn in the image, the largest distance between a point on the anterior wall of the pelvis (“A”) and another point on the posterior wall (“B”) was taken as a measure for the AP (anterior-posterior) diameter of the renal pelvis. In each case, the CT slice showing the largest distance between the anterior and the posterior wall of the pelvis was chosen for the measurement.
Statistics
Descriptive statistics were calculated using the Excel program (Microsoft, Seattle, WA). The Kolmogoroff–Smirnov test was used for the distribution analyses of the data. Group comparisons were made using t-tests where appropriate and indicated, comparisons of several groups were made using the one-way analysis of variance (one-way ANOVA) with post hoc Dunn test (GraphPad Prism version 4.00, GraphPad Software, San Diego, CA). Different multivariate logistic regression models with the target variable “hydronephrosis” were adapted, taking all existing combinations of crossing arteries and veins into consideration as covariates. Then, the variables with probability values of more than 0.10 were excluded in a forward stepwise variable selection procedure (SPSS 15, SPSS Inc., Chicago, IL). Finally, to identify influencing factors on the target variables width of the renal pelvis (WRP) and ureter width, multivariate regression models were adapted using a forward stepwise selection procedure. All values were given as mean value ± standard deviation; p<0.05 was considered statistically significant.
Results
Kidneys, renal pelves and ureters
In the patients studied, 2132 kidneys were found (1065 on the right and 1067 on the left side), as 13 patients (1.2%) possessed only one kidney. The renal pelves were 8.81±4.64 mm wide on the right side and 8.48±5.46 mm on the left side (p<0.0001); the right ureters were 3.15±1.18 mm wide and the left ureters were 3.07±1.15 mm wide (p&equals 0.0128). The renal pelves on both sides were wider in women than in men (right 9.63±4.77 vs 8.18±4.44 mm, p<0.0001; left 8.96±5.96 vs 8.11±5.02 mm, p<0.0001), but the ureters were not (right 3.13±1.36 vs 3.17±1.03, p > 0.05; left 3.02±1.06 vs 3.11±1.22, p>0.05). 154/2132 renal pelves (7.2%) were wider than 15 mm (78 on the right and 76 on the left side) and therefore considered to be “hydronephrotic” by definition. There was no hydronephrotic kidney that showed calyceal splaying without widening of the pelvis.
Renal vessels
There were 1065 main renal arteries on the right side and 1067 on the left side. In 262 patients (24.4%) there were 287 additional (Figure 2) and 6 accessory renal arteries on the right side, whereas on the left side there were 298 additional and 6 accessory renal arteries in 271 patients (25.3%). Two arteries on the right side and five arteries on the left side showed no relation to the ureters or the renal pelvis. Table 2 lists the vessels and their relation to the lower urinary tract.
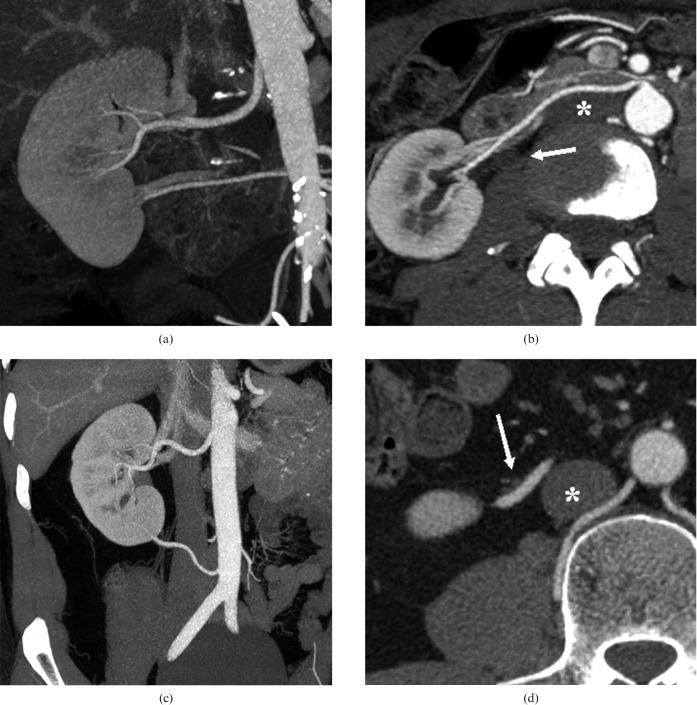
Figure 2.
Coronal maximum-intensity projection reconstruction of the right kidney in an arterial phase showing an additional renal artery (a), which passes by the caval vein (*) and the ureteropelvic junction (arrow) anteriorly (b). Coronal maximum- intensity projection reconstruction of another right kidney in an arterial phase showing an additional renal artery (c), which passes by the caval vein (*) anteriorly but by the ureter (arrow) posteriorly (d).
Table 2. Number of the crossing renal arteries and veins and their relations to the renal pelvis and ureters.
| Right side |
Left side |
|||||||||||||||
| V w Rn to the ureter |
V w Rn to the pelvis |
Wedge Stn |
V w/o A or P location |
V w Rn to the ureter |
V w Rn to the pelvis |
Wedge Stn |
V w/o A or P location |
|||||||||
| No CV | Pu CV | Ru CV | Pp CV | Rp CV | CVs (A and P) | V (S) | V w/o Rn to the pelvis | No CV | Pu CV | Ru CV | Pp CV | Rp CV | CVs (A and P) | V (S) | Vessel w/o Rn to the pelvis | |
| Arteries | 810 | 77 | 35 | 51 | 18 | 9+9 | 70 | 2 | 801 | 88 | 26 | 47 | 22 | 14+14 | 69 | 5 |
| Additional CV (Pu) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Additional CV (Ru) | – | – | 3 | – | – | 1 | – | – | – | – | 1 | – | – | 3 | – | – |
| Additional CV (Pp) | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | – | – | – |
| Additional CV (Rp) | – | – | 2 | – | – | – | – | – | – | – | 5 | – | – | – | – | – |
| Additional vessel (S) | – | 7 | 1 | 4 | 1 | 1 | 1 | – | – | 7 | – | 1 | – | – | 1 | – |
A, anterior; CV, crossing vessels; P, posterior; Pu, preureteral; Pp, prepelvic; S, superior; Rp, retropelvic; Rn, relation; Ru, retroureteral; Stn, situation; V, vessel; w, with; w/o, without.
Width of the renal pelvis in relation to the vascular situation
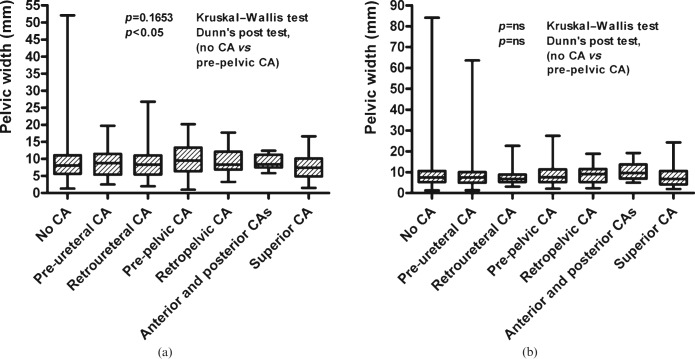
The differences in the width of the renal pelvis in relation to crossing arteries (Figure 3) were small on both sides, and were not significant on either side in the univariate analyses including all groups (p>0.05 on both sides, one-way ANOVA). Only in the group of patients with right anterior pre-pelvic crossing arteries was there a widening of the renal pelvis by 1.0 mm (9.8±4.4 mm; p>0.05) in comparison with the group of patients without crossing arteries (8.8±4.8 mm), a difference that was statistically significant in the post hoc Dunn test (p<0.05). The width of the ureters on the right was unrelated to the vascular situation (p>0.05; one-way ANOVA); on the left side, the width was smaller when there were anterior crossing vessels (p = 0.0107; one-way ANOVA). Crossing vessels were found with the same frequency in the groups with and without hydronephrosis (p>0.05; χ2 test). The findings were the same in children and adults.
Figure 3.
Width of the renal pelvis on the right side subject to the different groups of crossing arteries (CA) (a) on the right and (b) on the left side. ns, not significant.
Factors influencing hydronephrosis
In the logistical regression models, an independent influencing factor for the right kidney was, in addition to a cranial position of the kidney, hydronephrosis of the left kidney; an influencing factor for the left kidney, in addition to the presence of concrements, was hydronephrosis of the right kidney (Table 3). Independent of the definition of hydronephrosis, the width of the renal pelvis on the contralateral side was similarly significant in each case (Table 3). Crossing vessels did not play any role as influencing factors for the variable “hydronephrosis”.
Table 3. Independent predictors of hydronephrosis on both sides.
| Predictor | Odds ratio | Significance (p-value) | |
| Hydronephrosis on the right side | Hydronephrosis on the left side | 91.1 | <0.0001 |
| Level of the centre of the pelvis in relation to the spine (cranial position) | 4.8 | 0.028 | |
| Hydronephrosis on the left side | Hydronephrosis on the right side | 92.1 | <0.0001 |
| Parapelvine cysts | 17 | <0.0001 |
Factors influencing the width of the ureters
Positive independent influencing factors for the diameter of the ureters on the right side were multivariate stepwise regression models of the width of the renal pelvis on the right, concrements, the width of the renal parenchyma, cranial position of the kidney and diameter of the vena cava. On the left side the widths of the renal pelvis and the parenchyma were positive factors. The total number of renal arteries was a weak negative factor (p = 0.0007; Table 4).
Table 4. Independent predictors of the width of the kidneys, pelves and ureters on both sides.
| Predictor | Significance (p-value) | Regression coefficient β | |
| Width of the pelvis on the right side | Width of the pelvis on the left side | <0.001 | 0.454 |
| Female gender | <0.001 | 0.156 | |
| Height | 0.001 | 0.096 | |
| Age | 0.002 | 0.094 | |
| Width of the pelvis on the left side | Width of the pelvis on the right side | <0.001 | 0.472 |
| Calculi | 0.001 | 0.095 | |
| Rotation of the upper pole in dorsal position | 0.010 | 0.071 | |
| Parapelvine cysts | 0.023 | 0.062 | |
| Width of the ureter on the right side | Width of the pelvis | <0.001 | 0.238 |
| Calculi | 0.001 | 0.1 | |
| Width of the parenchyma | 0.002 | 0.094 | |
| Level of the centre of the pelvis in relation to the spine (cranial position) | 0.004 | 0.088 | |
| Diameter of the caval vein | 0.017 | 0.072 | |
| Width of the ureter on the left side | Width of the pelvis | <0.001 | 0.251 |
| Width of the parenchyma | 0.001 | 0.111 | |
| Number of crossing arteries | 0.009 | –0.082 |
Factors influencing the width of the renal pelvis
Positive independent influencing factors on the width of the renal pelvis on the right side were the width of the renal pelvis on the left, female gender, increasing age and height. On the left side, these factors were the width of the renal pelvis on the right side, concrements, parapelvic cysts and a greater dorsal rotation of the upper pole of the kidney (Table 4).
Discussion
This study shows that most arterial vascular variants have no verifiable influence on the width of the renal pelvis. Only pre-pelvic crossing arteries on the right side result in a weak and insignificant widening of the renal pelvis. A strong positive independent influencing factor for the width of the renal pelvis is the width of the renal pelvis on the contralateral side and for the development of hydronephrosis is hydronephrosis on the contralateral side. The other independent factors with respect to the width of the renal pelvis (i.e. age, height and female gender for the right side and concrements, parapelvic cysts and the rotation status of the kidney on the left side) and with respect to the development of hydronephrosis (i.e. the position of the kidney for the right side and concrements for the left side) are less significant. With respect to the width of the ureters, crossing arteries on the left side are a weak, but independent, negative influencing factor. Positive independent influencing factors are, for the right side, the width of the renal pelvis and the parenchyma, concrements, cranial position of the kidney and the diameter of the vena cava as a surrogate for the intravasal volume; for the left side, positive independent influencing factors are the width of the parenchyma and the renal pelvis. There is no correlation between width of the renal pelvis and crossing vessels.
Despite numerous examinations that place the frequency of extrinsic UPJ obstruction between 15% and 70% of all obstructions [5, 8, 13–16, 22–26], the question as to whether they could actually cause hydronephrosis or are found merely by coincidence in patients with an intrinsic UPJ obstruction remains unanswered [7, 12, 27]. This was because, for these studies, usually designed retrospectively, only patients with a manifest UPJ obstruction were included and not patients without UPJ obstruction for comparison. Angiographies and intraoperative observations of these symptomatic patients frequently led to the diagnosis of crossing vessels, which quickly gave rise to the logical step of assuming aetiological significance [28]. Angiography, which has been available for many decades, and later digital subtraction angiography, enables superior visualisation of the arteries, but not of their exact relation to the surrounding uncontrasted soft-tissue structures. Simultaneous contrasting of the renal system does make it possible to diagnose whether a vessel crosses ventrally or dorsally to the ureter, but the invasiveness of the examination and the exposure time required for alignment rule out using this method as a comparative means of diagnosing non-symptomatic patients. For a long time it was difficult to achieve a usable study design and this became possible only with the increasing use of spiral CT technology.
The larger width of the renal pelvis in patients with ventral crossing arteries on the right side found in this study supports the hypothesis that the renal collecting system can be compressed and angulated by a ventral crossing vessel, leading to outflow obstruction [8]. However, the clinical significance of this slight effect is doubtful at the least — neither crossing vessels lying anterior to the renal collecting system nor crossing vessels running anterior and posterior to the renal system are factors that cause the development of hydronephrosis, although it could be expected that especially the latter “pinching” situation would be associated with a measurable widening of the renal pelvis. Hydronephrosis as a strong independent predictor for hydronephrosis on the contralateral side, with odds ratios of >90 each, is more indicative of an intrinsic factor that is present in both UPJs. Another argument against the significance of crossing vessels is the incidence of crossing vessels at the UPJ described in the literature, as they actually coincide well with the frequencies of additional and accessory renal arteries between 10.4% and 56% indicated in the literature [29–34], but do not exceed them.
The main drawback of the present study is the fact that only 3.1% of the 1072 patients were children, while UPJ obstruction has a major role especially in foetuses, in the neonatal period [35, 36] and in childhood [25]. The reason for this omission is that children are rarely examined by CT scan owing to exposure considerations. If such examinations are carried out, various measures are taken to reduce exposure. Often, protocols are made with only a parenchymal contrast-medium phase or, if possible, low dosage protocols with high noise ratios are chosen, which were not suitable for this study. Thus, the results cannot be transferred to newborns and children. The results need to be verified for this age group, although the findings were the same in children and in adults. The frequency of widened pelves in the study population correlates well with the known prevalence of urinary tract obstruction in autopsy studies [37].
Conclusion
In summary, it can be stated that, on both sides, only hydronephrosis on the contralateral side is a strong independent predictor for the development of hydronephrosis; in general, for the width of the renal pelvis, only the width of the renal pelvis on the contralateral side is a strong independent predictor. Several other independent predictors such as age, height, gender, concrements and parapelvic cysts play a subordinate role as to the extent of their influence. Crossing vessels do not give rise to a widening of the renal pelves or ureters in adulthood. Only patients with a right anterior crossing vessel display a slight widening of the renal pelvis; this is significant in a univariate approach, but is not retained as an independent factor in multivariate regression models.
References
- 1.Johnston JH. The pathogenesis of hydronephrosis in children. Br J Urol 1969;41:724–34 [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Xu K, Fu B, Zhang J, Lang B, Ai X, et al. The retroperitoneal laparoscopic Hellström technique for pelvi-ureteric junction obstruction from a crossing vessel. BJU Int 2007;100:1335–8 [DOI] [PubMed] [Google Scholar]
- 3.Braun P, Guilabert JP, Kazmi F. Multidetector computed tomography arteriography in the preoperative assessment of patients with ureteropelvic junction obstruction. Eur J Radiol 2007;61:170–5 [DOI] [PubMed] [Google Scholar]
- 4.Sampaio FJ. Vascular anatomy at the ureteropelvic junction. Urol Clin North Am 1998;25:251–8 [DOI] [PubMed] [Google Scholar]
- 5.Rehman J, Landman J, Sundaram C, Clayman RV. Missed anterior crossing vessels during open retroperitoneal pyeloplasty: laparoscopic transperitoneal discovery and repair. J Urol 2001;166:593–6 [PubMed] [Google Scholar]
- 6.Sampaio FJ, Favorito LA. Ureteropelvic junction stenosis: vascular anatomical background for endopyelotomy. J Urol 1993;150:1787–91 [DOI] [PubMed] [Google Scholar]
- 7.Sampaio FJ. The dilemma of the crossing vessel at the ureteropelvic junction: precise anatomic study. J Endourol 1996;10:411–15 [DOI] [PubMed] [Google Scholar]
- 8.Stephens FD. Ureterovascular hydronephrosis and the “aberrant” renal vessels. J Urol 1982;128:984–7 [DOI] [PubMed] [Google Scholar]
- 9.Koff SA. Pathophysiology of ureteropelvic junction obstruction. Clinical and experimental observations. Urol Clin North Am 1990;17:263–72 [PubMed] [Google Scholar]
- 10.Satyapal KS, Haffejee AA, Singh B, Ramsaroop L, Robbs JV, Kalideen JM. Additional renal arteries: incidence and morphometry. Surg Radiol Anat 2001;23:33–8 [DOI] [PubMed] [Google Scholar]
- 11.Glodny B, Tröbinger MG, Hofmann KJ, Rehder P, Trieb T, Petersen J. A right accessory renal artery arising from a left additional common renal artery stem. Cardiovasc Intervent Radiol 2009;32:804–6 [DOI] [PubMed] [Google Scholar]
- 12.Conlin MJ. Results of selective management of ureteropelvic junction obstruction. J Endourol 2002;16:233–6 [DOI] [PubMed] [Google Scholar]
- 13.Van Cangh PJ, Wilmart JF, Opsomer RJ, Abi-Aad A, Wese FX, Lorge F. Long-term results and late recurrence after endoureteropyelotomy: a critical analysis of prognostic factors. J Urol 1994;151:934–7 [DOI] [PubMed] [Google Scholar]
- 14.Gupta M, Tuncay OL, Smith AD. Open surgical exploration after failed endopyelotomy: a 12-year perspective. J Urol 1997;157:1613–18 [PubMed] [Google Scholar]
- 15.Stern JM, Park S, Anderson JK, Landman J, Pearle M, Cadeddu JA. Functional assessment of crossing vessels as etiology of ureteropelvic junction obstruction. Urology 2007;69:1022–4 [DOI] [PubMed] [Google Scholar]
- 16.Nakada SY, Wolf JS, Jr, Brink JA, Quillen SP, Nadler RB, Gaines MV, Clayman RV. Retrospective analysis of the effect of crossing vessels on successful retrograde endopyelotomy outcomes using spiral computerized tomography angiography. J Urol 1998;159:62–5 [DOI] [PubMed] [Google Scholar]
- 17.Thomas R, Monga M, Klein EW. Ureteroscopic retrograde endopyelotomy for management of ureteropelvic junction obstruction. J Endourol 1996;10:141–5 [DOI] [PubMed] [Google Scholar]
- 18.Faerber GJ, Richardson TD, Farah N, Ohl DA. Retrograde treatment of ureteropelvic junction obstruction using the ureteral cutting balloon catheter. J Urol 1997;157:454–8 [PubMed] [Google Scholar]
- 19.Rouvière O, Lyonnet D, Berger P, Pangaud C, Gelet A, Martin X. Ureteropelvic junction obstruction: use of helical CT for preoperative assessment — comparison with intraarterial angiography. Radiology 1999;213:668–73 [DOI] [PubMed] [Google Scholar]
- 20.Khaira HS, Platt JF, Cohan RH, Wolf JS, Faerber GJ. Helical computed tomography for identification of crossing vessels in ureteropelvic junction obstruction — comparison with operative findings. Urology 2003;62:35–9 [DOI] [PubMed] [Google Scholar]
- 21.Poisel S, Spängler HP. The ramifications of the renal artery in relationship to the arterial blood supply of the renal parenchyma. Problem of the so-called renal segments. Acta Anat 1970;76:516–29 [PubMed] [Google Scholar]
- 22.Ericsson NO, Ruhde U, Livaditis A. Hydronephrosis associated with aberrant renal vessels in infants and children. Surgery 1961;50:687–90 [PubMed] [Google Scholar]
- 23.Lowe FC, Marshall FF. Ureteropelvic junction obstruction in adults. Urology 1984;23:331–5 [DOI] [PubMed] [Google Scholar]
- 24.Zeltser IS, Liu JB, Bagley DH. The incidence of crossing vessels in patients with normal ureteropelvic junction examined with endoluminal ultrasound. J Urol 2004;72:2304–7 [DOI] [PubMed] [Google Scholar]
- 25.Williams DI, Kenawi MM. The prognosis of pelviureteric obstruction in childhood: a review of 190 cases. Eur Urol 1976;2:57–63 [DOI] [PubMed] [Google Scholar]
- 26.Johnston JH, Evans JP, Glassberg KI, Shapiro SR. Pelvic hydronephrosis in children: a review of 219 personal cases. J Urol 1977;117:97–101 [DOI] [PubMed] [Google Scholar]
- 27.Carr MC, El-Ghoneimi A. Anomalies and surgery of the ureteropelvic junction in children. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th edn. Philadelphia, USA: Saunders-Elsevier, 2007:3359–82 [Google Scholar]
- 28.Hellstrom J, Giertz G, Lindblom K. Pathogenesis and treatment of hydronephrosis. J Belge Urol 1951;20:1–6 [PubMed] [Google Scholar]
- 29.Merklin RJ, Michels NA. The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries: a statistical analysis based on 185 dissections and review of the literature. J Int Coll Surg 1958;29:41–76 [PubMed] [Google Scholar]
- 30.Marshall AG. Aberrant renal arteries and hypertension. Lancet 1951;2:701–5 [DOI] [PubMed] [Google Scholar]
- 31.Derrick JR, Tyson DR. The association of aberrant renal arteries and systemic hypertension. Surgery 1960;48:907–12 [PubMed] [Google Scholar]
- 32.Davies ER, Sutton D. Hypertension and multiple renal arteries. Am Heart J 1966;71:285–6 [DOI] [PubMed] [Google Scholar]
- 33.Robertson PW, Hull DH, Klidjian A, Dyson ML. Renal artery anomalies and hypertension a study of 340 patients. Am Heart J 1967;73:296–307 [DOI] [PubMed] [Google Scholar]
- 34.Robertson PW, Klidjian A, Hull DH, Hilton DD, Dyson ML. The assessment and treatment of hypertension. New views on essential hypertention. Lancet 1962;22:567–72 [DOI] [PubMed] [Google Scholar]
- 35.Brown T, Mandell J, Lebowitz RL. Neonatal hydronephrosis in the era of sonography. AJR Am J Roentgenol 1987;148:959–63 [DOI] [PubMed] [Google Scholar]
- 36.Williams DI, Karlaftis CM. Hydronephrosis due to pelvi-ureteric obstruction in the newborn. Br J Urol 1966;38:38–44 [DOI] [PubMed] [Google Scholar]
- 37.Pais VM, Strandhoy JW, Assimos DG. Pathophysiology of urinary tract obstruction. : Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 9th edn. Philadelphia, USA: Saunders-Elsevier, 2007:1195–226 [Google Scholar]