Abstract
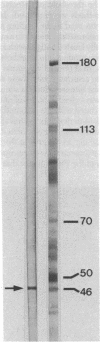
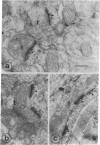
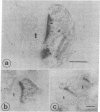
Antibodies against actin were used to corroborate the presence of actin as a major component protein of isolated brain postsynaptic densities. The same antibodies also were used as an immunohistochemical stain to study the distribution of actin in sections of intact brain tissue. This showed two major sites where actin is concentrated: smooth muscle cells around blood vessels and postsynaptic sites. In the postsynaptic area the highest concentration of actin occurs in postsynaptic densities and there also is intense staining in the surrounding cytoplasm, especially within dendritic spines. Antiactin staining was much weaker in other parts of neurons and in glial cells. The high concentration of actin in dendritic spines may be related to shape changes that these structures have been found to undergo in response to prolonged afferent stimulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berl S., Puszkin S., Nicklas W. J. Actomyosin-like protein in brain. Science. 1973 Feb 2;179(4072):441–446. doi: 10.1126/science.179.4072.441. [DOI] [PubMed] [Google Scholar]
- Blomberg F., Cohen R. S., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977 Jul;74(1):204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P., Horn G. Neuronal plasticity in the chick brain: morphological effects of visual experience on neurones in hyperstriatum accessorium. Brain Res. 1979 Feb 16;162(1):148–153. doi: 10.1016/0006-8993(79)90764-9. [DOI] [PubMed] [Google Scholar]
- Byers H. R., Fujiwara K. Stress fibers in cells in situ: immunofluorescence visualization with antiactin, antimyosin, and anti-alpha-actinin. J Cell Biol. 1982 Jun;93(3):804–811. doi: 10.1083/jcb.93.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Blomberg F., Berzins K., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977 Jul;74(1):181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss R. G., Brandon J. G., Globus A. Changes in morphology of dendritic spines on honeybee calycal interneurons associated with cumulative nursing and foraging experiences. Brain Res. 1980 Jun 16;192(1):49–59. doi: 10.1016/0006-8993(80)91007-0. [DOI] [PubMed] [Google Scholar]
- Coss R. G., Globus A. Spine stems on tectal interneurons in jewel fish are shortened by social stimulation. Science. 1978 May 19;200(4343):787–790. doi: 10.1126/science.644322. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. Hippocampal plasticity. Prog Brain Res. 1979;51:133–138. doi: 10.1016/S0079-6123(08)61301-1. [DOI] [PubMed] [Google Scholar]
- Fifková E., Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977 Apr;6(2):211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Flanagan M. D., Lin S. Comparative studies on the characteristic properties of two forms of brain actin separable by isoelectric focussing. J Neurochem. 1979 Mar;32(3):1037–1046. doi: 10.1111/j.1471-4159.1979.tb04591.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Zumbe A., Vassalli P. Actin and tubulin co-cap with surface immunoglobulins in mouse B lymphocytes. Nature. 1977 Oct 20;269(5630):697–698. doi: 10.1038/269697a0. [DOI] [PubMed] [Google Scholar]
- Globus A., Scheibel A. B. Synaptic loci on visual cortical neurons of the rabbit: the specific afferent radiation. Exp Neurol. 1967 May;18(1):116–131. doi: 10.1016/0014-4886(67)90093-3. [DOI] [PubMed] [Google Scholar]
- Gulley R. L., Reese T. S. Cytoskeletal organization at the postsynaptic complex. J Cell Biol. 1981 Oct;91(1):298–302. doi: 10.1083/jcb.91.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Lubit B. W., Schwartz J. H. Cytoplasmic actin in postsynaptic structures at the neuromuscular junction. J Cell Biol. 1981 Sep;90(3):789–792. doi: 10.1083/jcb.90.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Matus A. Monoclonal antibodies identify novel neural antigens. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2410–2414. doi: 10.1073/pnas.79.7.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol. 1978 Oct;79(1):173–183. doi: 10.1083/jcb.79.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski E. R., Rosenbaum J. L. Studies on the organization and localization of actin and myosin in neurons. J Cell Biol. 1979 Feb;80(2):356–371. doi: 10.1083/jcb.80.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBeux Y. J., Willemot J. An ultrastructural study of the microfilaments in rat brain by means of heavy meromyosin labeling. I. The perikaryon, the dendrites and the axon. Cell Tissue Res. 1975 Jun 27;160(1):1–36. doi: 10.1007/BF00219840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. I., Taff-Jones D. H. Morphology and molecular composition of isolated postsynaptic junctional structures. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):135–151. doi: 10.1098/rspb.1978.0097. [DOI] [PubMed] [Google Scholar]
- Matus A., Bernhardt R., Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci U S A. 1981 May;78(5):3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A., Mughal S. Immunohistochemical localisation of S-100 protein in brain. Nature. 1975 Dec 25;258(5537):746–748. doi: 10.1038/258746a0. [DOI] [PubMed] [Google Scholar]
- Matus A., Pehling G., Ackermann M., Maeder J. Brain postsynaptic densities: the relationship to glial and neuronal filaments. J Cell Biol. 1980 Nov;87(2 Pt 1):346–359. doi: 10.1083/jcb.87.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moring S., Ruscha M., Cooke P., Samson F. Isolation and polymerization of brain actin. J Neurobiol. 1975 Mar;6(2):245–255. doi: 10.1002/neu.480060210. [DOI] [PubMed] [Google Scholar]
- Schliwa M. Proteins associated with cytoplasmic actin. Cell. 1981 Sep;25(3):587–590. doi: 10.1016/0092-8674(81)90166-5. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Gallichio H. A., Jeffrey P. L., Livett B. G., Muller H. K., Cauchi M. N., Clarke F. M. Anti-actin stains synapses. Nature. 1976 Dec 16;264(5587):648–650. doi: 10.1038/264648a0. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Hard C. C. Actin co-caps with concanavalin A receptors. Nature. 1977 Oct 20;269(5630):695–697. doi: 10.1038/269695a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Apical dendritic spines of the visual cortex and light deprivation in the mouse. Exp Brain Res. 1967;3(4):337–352. doi: 10.1007/BF00237559. [DOI] [PubMed] [Google Scholar]
- Walters B. B., Matus A. I. Proteins of the synaptic junction. Biochem Soc Trans. 1975;3(1):109–112. doi: 10.1042/bst0030109. [DOI] [PubMed] [Google Scholar]
- Walters B. B., Matus A. I. Tubulin in postynaptic junctional lattice. Nature. 1975 Oct 9;257(5526):496–498. doi: 10.1038/257496a0. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Wallace R. W., Whitaker J. N., Cheung W. Y. Immunocytochemical localization of calmodulin and a heat-labile calmodulin-binding protein (CaM-BP80) in basal ganglia of mouse brain. J Cell Biol. 1980 Jan;84(1):66–76. doi: 10.1083/jcb.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]