Abstract
Objectives
The purpose of this study was to investigate factors associated with lymph node (LN) metastasis to identify which nasopharyngeal cancer (NPC) patients can undergo a reduction in the prophylactic radiation field. MRI of biopsy-proven NPC patients was evaluated to determine primary tumour extension and the existence of LN metastasis.
Methods
Sex, age, pathological type, T stage, primary tumour size, existence beyond the midline of the nasopharynx at the primary site and parapharyngeal extension of the primary tumour were assessed regarding their impact on the laterality of LN metastasis using the χ2 test.
Results
Of the 167 patients, 149 (89%) showed nodal involvement. The existence beyond the midline of the nasopharynx was significantly associated with the laterality of LN metastasis (p<0.0001). Most patients (82%) with primary tumour presence within the midline showed only ipsilateral LN metastasis or no LN metastasis. In addition, contralateral LN metastases were seen only at Level II and the retropharyngeal LN among most of other patients.
Conclusion
These results suggest that LN areas other than Level II and the retropharyngeal LN on the contralateral side could be omitted in patients with primary tumour presence within the midline and without the contralateral Level II or the retropharyngeal LN. Whether disease control is compromised by reducing the radiation field for subclinical diseases is a problem that should be solved in the future by prospective study.
Most patients with nasopharyngeal cancer (NPC) show cervical lymph node (LN) metastasis at the time of diagnosis, and the frequency is reported to be approximately 90% [1-3]. Some investigators have attributed the high frequency of LN metastasis to the abundance of lymphatic tissues in the posterior wall of the nasopharynx [4, 5]. In view of this fact, the bilateral cervical LN area has usually been included in the clinical target volume (CTV) on definitive radiotherapy (RT) for NPC [6, 7]. However, while NPC may occur at any age, it has a bimodal distribution with the first peak of occurrence in the 15–25 years age range [8]. There is concern that the radiation-induced second primary cancer or late complications such as carotid artery stenosis might increase in the future with an improvement of long-term survival outcome by advancements in therapy such as intensity-modulated radiotherapy (IMRT) or a new promising chemotherapy regimen in younger generations [9-11]. Moreover, mucositis due to a wide irradiation field is still a serious problem during the period of RT, even when RT alone is performed. Thus, it should be sufficiently addressed whether the adequate coverage of the entire bilateral cervical LN area in the CTV for potential subclinical diseases is absolutely necessary for all NPC patients or not.
IMRT has been increasingly used in the clinic and has shown clinical benefit in patients with head and neck cancer such as NPC. The optimal definition and the precise delineation of the CTV are absolutely necessary for IMRT, and those provide adequate coverage of the CTV while limiting the dose to the surrounding organs at risk (OAR). We have evaluated the relationship between tumour growth patterns and LN metastasis based on MRI in NPC patients [12, 13]. Our hypothesis of the current study is that if we could show the difference of frequency and regions of cervical LN metastasis according to patient and tumour characteristics, we could reduce the CTV for potential subclinical diseases in selected NPC patients. The present paper reports on the correlation between the laterality and regions of nodal metastasis and the characteristics of the patients and tumours in NPC patients by employing MRI, and pursues the possibility of the reduction of the cervical radiation field in selected patients.
Methods and materials
Patient selection
NPC patients who had been treated with RT with or without chemotherapy between November 1990 and April 2009 at our institution and who met the eligibility criteria were entered into this retrospective study. The eligibility criteria were as follows: biopsy-proven NPC (World Health Organization (WHO) Type I, II or III), evaluation by MRI with gadolinium enhancement before treatment and written informed consent before treatment.
MRI techniques and image assessment
MRI studies were performed with a 1.5 tesla unit (Signa; General Electric Medical Systems, Milwaukee, WI) before February 2009 and with a 3.0 tesla unit (Signa; General Electric Medical Systems) thereafter. The other MRI techniques were similar to those in our previous study [14]. MRI conducted before the treatment was evaluated by one experienced radiologist and two radiation oncologists specialising in head and neck cancer to determine tumour extension at the primary site and the existence of LN metastasis. All patients were restaged according to the 2002 American Joint Commission on Cancer (AJCC) staging classifications [15]. As for extension of the primary tumour, patients were classified according to two criteria. One criterion was the presence of lateral invasion of the nasopharynx, and the other was existence beyond the midline of the nasopharynx. The criterion chosen for the presence of lateral invasion of the nasopharynx was direct tumour extension through the pharyngobasilar fascia on MRI, i.e. parapharyngeal extension [15] (Figure 1), because some studies have shown that the incidence of LN metastasis was significantly higher in cases of parapharyngeal space invasion [4, 14]. When the primary tumour existed beyond the midline of the prevertebral fascia, this type of primary tumour was defined as existing beyond the midline of the nasopharynx (Figure 2). In addition, all patients were classified into three groups according to the maximal tumour diameter on axial imaging: small (≤20 mm), medium (>20 mm and <40 mm) and large (≥40 mm).
Figure 1.
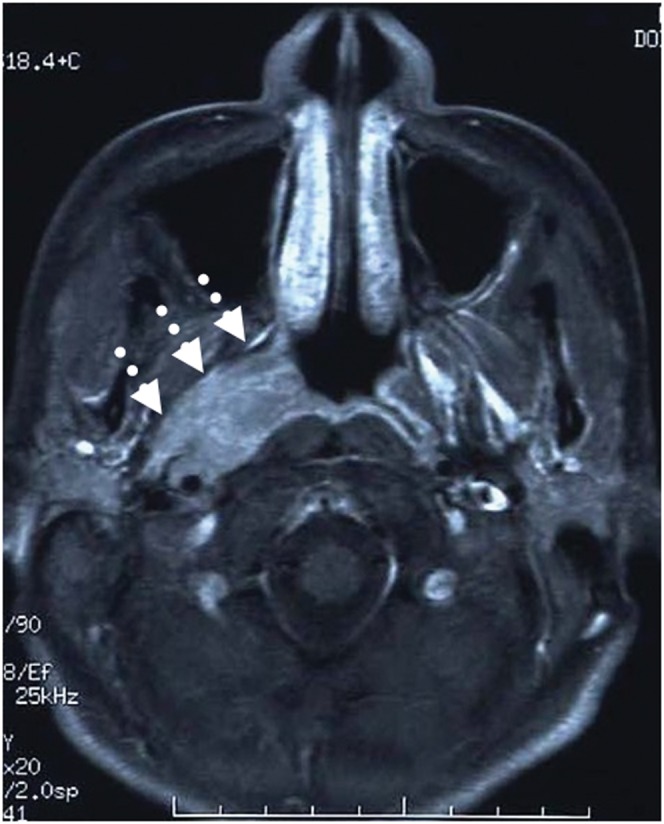
Axial contrast MRI of the nasopharynx in a patient with nasopharyngeal cancer showing tumour involvement in the right parapharyngeal space (arrows).
Figure 2.
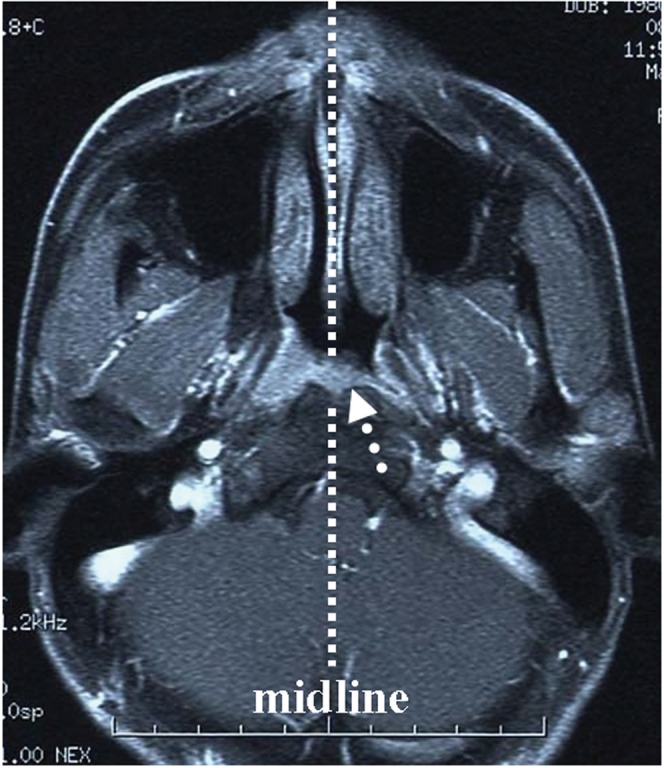
Axial contrast MRI of the nasopharynx in a patient with nasopharyngeal cancer showing tumour involvement beyond the midline of the nasopharynx (arrow).
Cervical LN metastasis was defined as nodes showing a minimal axial diameter of ≥10 mm [14]. In addition, the following lymph nodes were also regarded as metastasis even when the minimal axis diameter was ≤10 mm: necrotic lymph nodes with a visualised capsule, and lymph nodes showing a definite decrease in size on MRI after treatment. The retropharyngeal LN with a minimal axial diameter of ≥5 mm was considered metastatic [1]. The cervical nodes were subdivided into specific levels, as proposed by Gregoire et al [16]. When LNs located in the border of two regions crossed different axial planes, the LN status was recorded in both regions. When overlaps occurred in the same axial plane, assignment was performed according to the main body of LNs. LN metastasis was classified into three groups according to the side of LN metastasis: none, ipsilateral and bilateral. When LN metastasis existed only on the contralateral side of the primary tumour, it was defined as bilateral.
Study design and statistical analysis
All available charts were reviewed to assess patient and tumour characteristics. Sex, age at diagnosis (≤60 vs >60), the WHO pathological type (Type I vs Type II/III), T stage (T1 vs T2 vs T3 vs T4), primary tumour size (small vs medium vs large), existence beyond the midline of the nasopharynx at the primary site (yes vs no) and lateral invasion (none vs unilateral vs bilateral) were assessed regarding their impact on the laterality of LN metastasis using the χ2 test. p<0.05 was considered significant.
Results
Patient and tumour characteristics
A total of 167 patients were entered into this study. Of these, 149 (89%) showed nodal involvement. The characteristics of the patients and tumours are shown in Table 1. The distribution of metastatic node sites is shown in Table 2. There were no Level Ia or VI LN metastases, and Level Ib and parotid gland LN metastases were uncommon (2% and 0.5%, respectively).
Table 1. Patient and tumour pre-treatment characteristics.
| Characteristic | |
| Age (years) | 52 (11–83) |
| Sex | |
| Male | 125 (75%) |
| Female | 42 (25%) |
| T stage | |
| 1 | 61 (36%) |
| 2 | 50 (30%) |
| 3 | 20 (12%) |
| 4 | 36 (22%) |
| N stage | |
| 0 | 18 (11%) |
| 1 | 60 (36%) |
| 2 | 64 (38%) |
| 3 | 25 (15%) |
| Stage | |
| 1 | 7 (4%) |
| 2 | 43 (26%) |
| 3 | 60 (36%) |
| 4 A/B | 49 (29%) |
| 4 C | 8 (5%) |
| Pathological type | |
| WHO Type I | 34 (20%) |
| WHO Type II/III | 133 (80%) |
| Lateral invasion | |
| None | 64 (38%) |
| Unilateral | 71 (43%) |
| Bilateral | 32 (19%) |
| Beyond the midline of the nasopharynx | |
| Yes | 111 (66%) |
| No | 56 (34%) |
| Tumour size | |
| Small (≤20 mm) | 43 (26%) |
| Medium (>20 mm, ≤40 mm) | 78 (47%) |
| Large (>40 mm) | 46 (27%) |
The age is presented as the median value (range).
WHO, World Health Organization.
Table 2. Distribution of metastatic lymph nodes in 167 patients with nasopharyngeal cancer.
| Lymph node metastasis |
||||
| Nodes | None (%) | Ipsilateral (%) | Bilateral (%) | Total (%) |
| Retropharyngeal | 53 (32) | 62 (37) | 52 (31) | 68 |
| Parotid | 166 (99.5) | 1 (0.5) | 0 (0) | 0.5 |
| Level Ib | 164 (98) | 3 (2) | 0 (0) | 2 |
| Level I | 30 (18) | 61 (37) | 76 (45) | 82 |
| Level III | 110 (66) | 29 (17) | 28 (17) | 34 |
| Level IV | 135 (81) | 27 (16) | 5 (3) | 19 |
| Level V | 140 (84) | 19 (11) | 8 (5) | 16 |
Total values are the total percentage of the incidence of ipsilateral and bilateral lymph node metastasis.
Factors associated with lymph node metastasis
Correlations between the characteristics of the patients and tumours and the laterality of LN metastasis are shown in Table 3. The existence beyond the midline of the nasopharynx at the primary site was significantly associated with the incidence of LN metastasis (p<0.0001). Primary tumour presence within the midline showed a higher incidence only in ipsilateral LN metastasis than existence beyond the midline, with incidences of 68% and 25%, respectively (Table 3). On the other hand, primary tumour existence beyond the midline of the nasopharynx showed a higher incidence in bilateral LN metastasis than primary tumour presence within the midline of the nasopahrynx, with incidences of 66% and 18%, respectively (Table 3). As for the distribution of metastatic node sites in 56 patients with NPC within the midline of the nasopharynx, there was no bilateral LN metastasis at Level I, IV, V and VI, and in the parotid gland LN, and the incidence of bilateral LN metastasis at Level III was only 3%, as shown in Table 4. Two patients (3%) with contralateral LN metastasis at Level III also had contralateral LN metastasis at Level II and in the retropharyngeal LN. The bilateral LN metastases were seen with low frequency at Level II and in the retropharyngeal LN, with incidences of 16% and 10%, respectively (Table 4).
Table 3. Factors associated with lymph node metastasis.
| Lymph node metastasis |
||||
| Factors | None (%) | Ipsilateral (%) | Bilateral (%) | p-value |
| Age (years) | 0.37 | |||
| ≤60 | 12 (9) | 49 (38) | 67 (52) | |
| >60 | 6 (15) | 17 (44) | 16 (41) | |
| Sex | ||||
| Male | 11 (9) | 50 (40) | 64 (51) | |
| Female | 7 (17) | 16 (38) | 19 (45) | |
| T stage | 0.32 | |||
| 1 | 7 (12) | 27 (44) | 27 (44) | |
| 2 | 2 (4) | 21 (42) | 27 (54) | |
| 3 | 2 (10) | 8 (40) | 10 (50) | |
| 4 | 7 (19) | 10 (28) | 19 (53) | |
| Pathological type | 0.022a | |||
| WHO Type I | 8 (24) | 13 (38) | 13 (38) | |
| WHO Type II/III | 10 (7) | 53 (40) | 70 (53) | |
| Lateral invasion | 0.079 | |||
| None | 8 (13) | 29 (46) | 26 (41) | |
| Unilateral | 6 (8) | 31 (43) | 35 (49) | |
| Bilateral | 4 (12) | 6 (19) | 22 (69) | |
| Beyond the midline | <0.0001a | |||
| Yes | 10 (9) | 28 (25) | 73 (66) | |
| No | 8 (14) | 38 (68) | 10 (18) | |
| Tumour size | 0.11 | |||
| Small | 6 (14) | 18 (42) | 19 (44) | |
| Medium | 3 (4) | 31 (48) | 31 (48) | |
| Large | 9 (15) | 17 (29) | 33 (56) | |
aSignificant.
Table 4. Distribution of metastatic lymph nodes in 56 patients with nasopharyngeal cancer with the primary tumour within the midline of the nasopharynx.
| Lymph node metastasis |
||||
| Nodes | None (%) | Ipsilateral (%) | Bilateral (%) | Total (%) |
| Retropharyngeal | 25 (45) | 25 (45) | 6 (10) | 55 |
| Parotid | 56 (100) | 0 (0) | 0 (0) | 0 |
| Level Ib | 56 (100) | 0 (0) | 0 (0) | 0 |
| Level II | 15 (27) | 32 (57) | 9 (16) | 73 |
| Level III | 44 (79) | 10 (18) | 2 (3) | 21 |
| Level IV | 47 (84) | 9 (16) | 0 (0) | 16 |
| Level V | 47 (84) | 9 (16) | 0 (0) | 16 |
Total values are the total percentage of the incidence of ipsilateral and bilateral lymph node metastasis.
The WHO pathological type was also significantly associated with the incidence of LN metastasis (p = 0.022). Patients with WHO Type II/III showed a higher incidence of bilateral cervical LN metastasis than patients with WHO Type I, who inturn showed a higher rate of cervical LN metastasis absence than patients with WHO Type II/III. However, the incidences of patients who had ipsilateral cervical LN metastasis were similar between WHO Type I and Type II/III, with incidences of 38% and 40%, respectively (Table 3).
Age, sex, T stage and the primary tumour size were not associated with the incidence of LN metastasis. Lateral invasion of the nasopharynx at the primary site tended to be associated with the incidence of cervical LN metastasis, although not significantly (p = 0.079). The incidence of LN metastasis was not different between the no lateral invasion and unilateral invasion groups, but the bilateral invasion group showed a higher incidence of bilateral LN metastasis than other groups, as shown in Table 3.
Discussion
IMRT enables the delivery of higher radiation doses to the tumour while sparing OAR. Thus, as the reduction of high-dose radiation to OAR is one of the major purposes of IMRT, the necessity of adequately covering the entire bilateral cervical LN area in the CTV of all NPC patients is disputable. Indeed, despite the rapid dissemination of IMRT in the treatment of NPC, the optimal definition of the CTV has not been sufficiently addressed [17]. The definition of the CTV in IMRT for NPC advocated by various researchers was largely based on previous experience in conventional RT techniques such as parallel-opposed lateral fields, in which it is difficult to omit only the unilateral LN level from the radiation field. An increase in the treatment volume by either conventional RT or IMRT raises the probability of acute and long-term adverse effects. Many younger patients were present among the NPC patients [8, 18]. We are concerned about increased radiation-induced second primary cancer or late complications such as carotid artery stenosis with improvements in the long-term survival outcome brought about by advances in therapy such as IMRT or a new promising chemotherapy regimen [9-11]. In addition, a recent study pointed out the possibility that superior CTV coverage of IMRT results in an increase in the carotid artery dose compared with conventional three-field technique [19]. Vitolo et al [19] determined the radiation dose to the carotid artery in NPC patients treated with IMRT and compared it with the dose delivered by a conventional three-field technique. The median mean dose to the carotid arteries was 65.7 Gy with IMRT vs 58.4 Gy with conventional three-field technique (p<0.001), although with the application of dose constraints to the carotid arteries it was possible to reduce the mean carotid dose to 54 Gy in the IMRT replans. Therefore, it is reasonable to attempt to decrease the probability of carotid artery stenosis and other adverse effects by IMRT with a reduced CTV, if possible [20].
Lymphatic drainage of carcinomas of the head and neck has been associated with the primary tumour location and adjacent subsites to which the tumour has spread [21]. In the case of NPC, the lymphatic vessels of the nasopharynx drain in two general directions, lateral and medial [22]. Primary drainage involves the lateral pathway, which drains the lateral nasopharynx and flows into the lateral half of the upper internal jugular chain or into the lateral retropharyngeal LN [23]. These facts indicate that both retropharyngeal LN and cervical Level II nodes might be the first-echelon nodes in NPC [4]. A recent study using MRI data of 924 NPC patients has shown that LN metastases spread in an orderly fashion from higher level LN to lower level LN, and this result did not support prophylactic irradiation of Level IV and supraclavicular LN in patients who were negative for LN metastases [24].
In this study, the existence beyond the midline of the nasopharynx at the primary site was significantly associated with the laterality of cervical LN metastasis (p<0.0001). Primary tumour presence within the midline showed a significantly higher incidence only in ipsilateral LN metastasis than those existing beyond the midline, with incidences of 68% and 25%, respectively. In addition, more than 80% patients with primary tumour presence within the midline showed only ipsilateral LN metastasis or no LN metastasis. Our previous studies also support this result [12, 13]. In general, the CTV includes the retropharyngeal nodal regions, Levels Ib, II, III, IV and V in bilateral neck sites for NPC [8, 25, 26]. However, in this study, the incidences of contralateral LN metastasis existed only at Level II and in the retropharyngeal LN with low frequency among most patients with primary tumours within the midline of the nasopharynx. A few patients (3%) with contralateral LN metastasis at Level III also had contralateral LN metastasis at Level II and in the retropharyngeal LN. Therefore, these results suggest that the coverage of the ipsilateral cervical LN area, the contralateral Level II and retropharyngeal LN area could be sufficient for the CTV of NPC patients within the midline of the nasopharynx at the primary site and without the contralateral Level II and retropharyngeal LN metastasis. On the other hand, we think that when the contralateral Level II or retropharyngeal LN metastasis is shown, the levels lower than Level II and retropharyngeal LN could not be omitted from the CTV.
The WHO pathological type was also significantly associated with the incidence of cervical LN metastasis (p = 0.022). However, the incidence of patients who had ipsilateral cervical LN metastasis was similar between WHO Type I and Type II/III. Therefore, we think that the cervical radiation field cannot be reduced according to the WHO pathological type for NPC patients. T stage and the primary tumour size were not associated with the incidence of cervical LN metastasis. As presented in Table 1, 44% of T1 tumours and 44% of small (≤20 mm) tumours showed bilateral cervical LN metastasis. This result suggested that the bilateral cervical LN area could not be omitted from the CTV even in these patients because of the high rate of bilateral LN metastasis.
There are problems that limit the interpretation of findings in this study, e.g. a lack of opportunity for histological confirmation of the imaging findings, especially for LN metastasis. However, the definition of cervical LN metastasis in this study is a commonly used and reliable criterion. The combination of MRI and positron emission tomography/CT may provide a more accurate predictor of tumour-positive cervical nodes than the definition used in this study [17].
Conclusion
This study reported on the correlation between the laterality and regions of LN metastasis and the characteristics of the patients and tumours in 167 patients with NPC by employing MRI. Most patients (82%) with primary tumour presence within the midline showed only ipsilateral LN metastasis or no LN metastasis. In addition, contralateral LN metastases were seen only at Level II and the retropharyngeal LN in most of the other patients. These results suggest that the cervical radiation field could be reduced in patients within the midline of the nasopharynx at the primary site and without the contralateral Level II or retropharyngeal LN metastasis. However, whether disease control is compromised by reducing the radiation field for subclinical diseases is a problem that should be solved in the future by prospective study. On the other hand, the T stage and primary tumour size were not associated with the incidence of cervical LN metastasis. These results suggest that the bilateral cervical LN area could not be omitted from the CTV even in patients with an early T stage or small primary tumour because of the frequency of bilateral LN metastasis. This study will support the establishment of an optimal CTV according to NPC tumour and patient characteristics.
References
- 1.King AD, Ahuja AT, Leung SF, Lam WW, Teo P, Chan YL, et al. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck 2000;22:275–81 [DOI] [PubMed] [Google Scholar]
- 2.Ng WT, Lee AW, Kan WK, Chan J, Pang ES, Yau TK, et al. N-staging by magnetic resonance imaging for patients with nasopharyngeal carcinoma: pattern of nodal involvement by radiological levels. Radiother Oncol 2007;82:70–5 [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Li L, Hu C, Zhou Z, Ying H, Ding J, Feng Y. Patterns of level II node metastasis in nasopharyngeal carcinoma. Radiother Oncol 2008;89:28–32 [DOI] [PubMed] [Google Scholar]
- 4.Moss WT. The nasopharynx. In: Moss WT, Cox JD, editors. Radiation oncology, 6th edn. St Louis, MO: The C.V. Mosby Company, 1989: 198–214 [Google Scholar]
- 5.Perez CA. Nasopharynx. In: Perez CA, Brady LW, editors. Principles and practice of radiation oncology, 2nd edn. Philadelphia, PA: J.B. Lippincott Company, 1992: 617–43 [Google Scholar]
- 6.Ho JHC. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1978;4:183–98 [PubMed] [Google Scholar]
- 7.Lee AW, Lau KY, Hung WM, Ng WT, Lee MC, Choi CW, et al. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother Oncol 2008;87:204–10 [DOI] [PubMed] [Google Scholar]
- 8.Agulnik M, Epstein JB. Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol 2008;44:617–27 [DOI] [PubMed] [Google Scholar]
- 9.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83–8 [DOI] [PubMed] [Google Scholar]
- 10.Brown PD, Foote RL, McLaughlin MP, Halyard MY, Ballman KV, Collie AC, et al. A historical prospective cohort study of carotid artery stenosis after radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys 2005;63:1361–7 [DOI] [PubMed] [Google Scholar]
- 11.Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009;27:242–9 [DOI] [PubMed] [Google Scholar]
- 12.Wakisaka M, Mori H, Fuwa N, Matsumoto A. MR analysis of nasopharyngeal carcinoma: correlation of the pattern of tumor extent at the primary site with the distribution of metastasized cervical lymph nodes. Preliminary results. Eur Radiol 2000;10:970–7 [DOI] [PubMed] [Google Scholar]
- 13.Fuwa N, Ariji Y, Daimon T, Wakisaka M, Matsumoto A, Kodaira T, et al. Relationship between the growth pattern of nasopharyngeal cancer and the cervical lymph nodes based on MRI findings: can the cervical radiation field be reduced in patients with nasopharyngeal cancer? Br J Radiol 2006;79:725–9 [DOI] [PubMed] [Google Scholar]
- 14.van denBrekel MW, Stel HV, Castelijns JA, Nauta JJ, van derWaal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990;177:379–84 [DOI] [PubMed] [Google Scholar]
- 15.Cooper J, Flemming ID, Henson DE, for the American Joint Committee on Cancer Manual for staging of cancer, 6th edn. Philadelphia: JB Lippincott, 2002 [Google Scholar]
- 16.Gregoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol 2003;69:227–36 [DOI] [PubMed] [Google Scholar]
- 17.Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology 2008;249:203–11 [DOI] [PubMed] [Google Scholar]
- 18.Wang XS, Hu CS, Ying HM, Zhou ZR, Ding JH, Feng Y. Patterns of retropharyngeal node metastasis in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2009;73:194–201 [DOI] [PubMed] [Google Scholar]
- 19.Vitolo V, Millender LE, Quivey JM, Yom SS, Schechter NR, Jereczek-Fossa BA, et al. Assessment of carotid artery dose in the treatment of nasopharyngeal cancer with IMRT versus conventional radiotherapy. Radiother Oncol 2009;90:213–20 [DOI] [PubMed] [Google Scholar]
- 20.Lin S, Pan J, Han L, Zhang X, Liao X, Lu JJ. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys 2009;75:1071–78 [DOI] [PubMed] [Google Scholar]
- 21.Mukherji SK, Armao D, Joshi VM. Cervical nodal metastases in squamous cell carcinoma of the head and neck: what to expect. Head Neck 2001;23:995–1005 [DOI] [PubMed] [Google Scholar]
- 22.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972;29:1446–9 [DOI] [PubMed] [Google Scholar]
- 23.Sobin LH, Fleming ID. TNM Classification of malignant tumors, 5th edn. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80:1803–4 [DOI] [PubMed] [Google Scholar]
- 24.Tang L, Mao Y, Liu L, Liang S, Chen Y, Sun Y, et al. The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer 2009;115:680–8 [DOI] [PubMed] [Google Scholar]
- 25.Kodaira T, Tomita N, Tachibana H, Nakamura T, Nakahara R, Inokuchi H, et al. Aichi cancer center initial experience of intensity modulated radiation therapy for nasopharyngeal cancer using helical tomotherapy. Int J Radiat Oncol Biol Phys 2009;73:1129–34 [DOI] [PubMed] [Google Scholar]
- 26.Tham IW, Hee SW, Yeo RM, Salleh PB, Lee J, Tan TW, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy: The National Cancer Centre Singapore Experience. Int J Radiat Oncol Biol Phys 2009;75:1481–86 [DOI] [PubMed] [Google Scholar]


