Abstract
Objective
This study aimed to compare thin-section CT images from sarcoidosis patients who had either normal or elevated serum KL-6 levels.
Methods
101 patients with sarcoidosis who underwent thin-section CT examinations of the chest and serum KL-6 measurements between December 2003 and November 2008 were retrospectively identified. The study group comprised 75 sarcoidosis patients (23 male, 52 female; aged 19–82 years, mean 54.1 years) with normal KL-6 levels (152–499 U ml–1, mean 305.7 U ml–1) and 26 sarcoidosis patients (7 male, 19 female; aged 19–75 years, mean 54.3 years) with elevated KL-6 levels (541–2940 U ml–1, mean 802.4 U ml–1). Two chest radiologists, unaware of KL-6 levels, retrospectively and independently interpreted CT images for parenchymal abnormalities, enlarged lymph nodes and pleural effusion.
Results
CT findings in sarcoidosis patients consisted mainly of lymph node enlargement (70/75 with normal KL-6 levels and 21/26 with elevated KL-6 levels), followed by nodules (50 and 25 with normal and elevated levels, respectively) and bronchial wall thickening (25 and 21 with normal and elevated levels, respectively). Ground-glass opacity, nodules, interlobular septal thickening, traction bronchiectasis, architectural distortion and bronchial wall thickening were significantly more frequent in patients with elevated KL-6 levels than those with normal levels (p<0.001, p<0.005, p<0.001, p<0.001, p<0.001 and p<0.001, respectively). By comparison, there was no significant difference in frequency of lymph node enlargement between the two groups.
Conclusion
These results suggest that serum KL-6 levels may be a useful marker for indicating the severity of parenchymal sarcoidosis.
KL-6 is a mucin-like high molecular weight glycoprotein that is expressed on Type II pneumocytes and respiratory bronchiolar epithelial cells in the normal lung [1, 2]. Serum levels of KL-6 are elevated in various respiratory and non-respiratory conditions, including breast and pancreatic cancers [3, 4] and diabetes mellitus [5]. This observation has led to a focus on the use of KL-6 as a diagnostic and prognostic tool in respiratory diseases.
Serum and bronchoalveolar lavage fluid levels of KL-6, first described by Kohno et al [6] in 1988, were raised in patients with interstitial pneumonia [1, 2, 7]. Several investigators have also reported that KL-6 is a useful serum marker to confirm diagnosis and for long-term management in patients with diffuse pulmonary diseases, particularly interstitial lung diseases. Patients with idiopathic pulmonary fibrosis or non-specific interstitial pneumonia showed significantly elevated KL-6 levels [8-13].
Several studies indicate that the serum KL-6 level is elevated in patients with sarcoidosis [14-16]. However, no studies describing radiological findings comparing thin-section CT images between patients with elevated KL-6 levels and those with normal KL-6 levels have been published in the English language literature.
Thus, we aimed to retrospectively evaluate and compare pulmonary CT findings between patients with elevated KL-6 levels and those with normal KL-6 levels.
Methods and materials
Patients
101 patients with known serum KL-6 levels who underwent thin-section CT between December 2003 and November 2008 at our institutions, and in whom sarcoidosis was histologically and clinically diagnosed, were included in this study. Thin-section CT scans were performed within 3 days of KL-6 level measurements. Transbronchial lung biopsy and other tissue biopsies were performed within 2 weeks of the CT scan.
Our study included 75 sarcoidosis patients (23 men, 52 women; age range 19–82 years; mean age 54.1 years) with normal KL-6 levels (152–499 U ml–1; mean 305.7 U ml–1; Table 1) in whom transbronchial lung biopsy (n = 42), surgical lung biopsy (n = 1), lymph node biopsy (n = 12), muscle biopsy (n = 4), testis biopsy (n = 1), skin biopsy (n = 5) or clinical diagnosis (n = 10) had been performed. 26 sarcoidosis patients (7 men, 19 women; age range 19–75 years; mean age 54.3 years) with elevated KL-6 levels (521–2940 U ml–1; mean 802.4 U ml–1; Table 1) in whom transbronchial lung biopsy (n = 18), lymph node biopsy (n = 4), muscle biopsy (n = 1), liver biopsy (n = 1) or clinical diagnosis (n = 2) had been performed were also included in this study. The ethical review boards of the institutions that contributed cases to this study did not require approval or informed consent for the retrospective review of patient records and images.
Table 1. Comparison of thin-section CT findings.
| Finding | Normal KL-6 (n = 75) | Elevated KL-6 (n = 26) | p-Value |
| Consolidation | 3 (4.0) | 2 (7.7) | NS |
| Ground-glass opacity | 10 (13.3) | 16 (61.5) | <0.001 |
| Nodules | 50 (66.7) | 25 (96.2) | <0.005 |
| Interlobular septal thickening | 20 (26.7) | 25 (96.2) | <0.001 |
| Traction bronchiectasis | 7 (9.3) | 25 (96.2) | <0.001 |
| Intralobular reticular opacity | 3 (4.0) | 0 (0) | NS |
| Architectural distortion | 7 (9.3) | 26 (100) | <0.001 |
| Bronchial wall thickening | 25 (33.3) | 21 (80.8) | <0.001 |
| Lymph node enlargement | 70 (93.3) | 21 (80.8) | NS |
| Pleural effusion | 0 (0) | 0 (0) | NS |
Data in parentheses are percentages. NS, not significant.
Patients with idiopathic interstitial pneumonia were excluded from the study. Moreover, patients with all other known cases of interstitial pneumonia (i.e. connective tissue disease, pneumoconiosis, hypersensitivity pneumonitis or drug-induced pneumonitis), those diagnosed with concurrent infectious diseases by serological tests and by clinical and pathological findings, and those with malignancy or diabetes mellitus were excluded.
Sarcoidosis was diagnosed on the basis of histological findings and clinical history. The clinical findings in all patients were subsequently reviewed by two chest radiologists to ensure that all cases fulfilled the diagnostic criteria recommended by the American Thoracic Society and the European Respiratory Society [17].
CT examinations
Thin-section CT examinations were performed within 7 days of the chest radiographs. The CT examinations were performed with 1 mm collimation at 10 mm intervals from the apex of the lung to the diaphragm (n = 25; 19 with normal KL-6 levels and 6 with elevated KL-6 levels), or volumetrically with a multidetector CT system with 1 mm reconstruction (n = 76; 56 with normal KL-6 levels and 20 with elevated KL-6 levels). CT images were obtained at the end of inspiration and in a supine position. The scanning protocol consisted of the reconstruction of 1 mm collimation sections with a high spatial frequency algorithm at 10 mm intervals. Images were captured at window settings allowing the viewing of the lung parenchyma (window level −600 to −700 HU; window width 1200–1500 HU) and the mediastinum (window level 20–40 HU; window width 400 HU). All initial scans were evaluated.
Radiographic interpretation
Images were reviewed independently, in a random order, by two chest radiologists (with 21 and 9 years of experience in chest image interpretation). The observers were unaware of KL-6 levels and clinical information.
Chest radiographs were used to classify the patients as being Type 0 (normal chest radiograph), Type I (bilateral hilar lymphadenopathy), Type II (bilateral hilar lymphadenopathy with pulmonary infiltrates) and Type III (pulmonary infiltrates without lymphadenopathy).
CT images were assessed for the following radiological patterns: ground-glass opacity, consolidation, nodules, interlobular septal thickening, traction bronchiectasis, intralobular reticular opacity, architectural distortion, bronchial wall thickening, enlarged hilar/mediastinal lymph node(s) (>1 cm in diameter of the short axis) and pleural effusion. Areas of ground-glass opacity were defined as hazy increases in attenuation without obscuration of vascular markings [18, 19]. Areas of consolidation were defined as areas of increased attenuation causing obscuration of normal lung markings [18, 19]. Interlobular septal thickening was defined as abnormal widening of interlobular septa [19]. Intralobular reticular opacity was considered present when interlacing line shadows were separated by a few millimetres [18, 19]. Traction bronchiectasis was defined as irregular bronchial dilatation within the surrounding areas showing parenchymal abnormalities. Architectural distortion was considered present when bronchi, pulmonary vessels or interlobular fissures or septa were abnormally displaced [19].
Measurement of KL-6 levels
Serum KL-6 levels were measured within 3 days of the initial CT scans and before steroid treatment using a sandwich-type enzyme-linked electrochemiluminescence immunoassay kit (Picolumi KL-6; Sanko Junyaku, Tokyo). The recommended cut-off value was determined at 500 U ml–1 according to levels reported in healthy individuals [6]. The assay was performed by technicians unaware of the clinical information related to the samples.
Statistical analysis
Statistical analyses of the incidence of symptoms and laboratory data were conducted using Fisher's exact test and the χ2 test. Mean age comparison was conducted using Student's t-test. A p<0.05 was considered to be statistically significant.
Results
Radiographs
Patients were radiographically classified as being Type 0 (n = 17), Type I (n = 29), Type II (n = 38) or Type III (n = 17). The mean KL-6 value was 301±244 U ml–1 in stage 0, 333±259 U ml–1 in stage I, 1136±777 U ml–1 in stage II and 1331±775 U ml–1 in stage III. The serum KL-6 levels for Type II or III were significantly elevated compared with those in Type 0 and I (p<0.01).
CT patterns
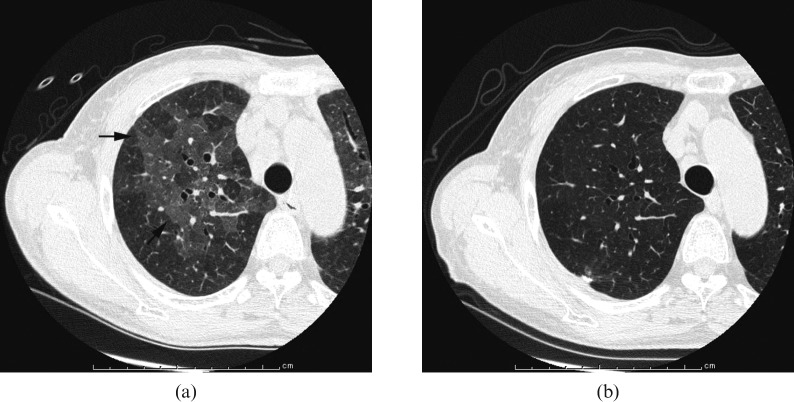
CT findings are summarised in Table 1. Of the 75 patients with normal KL-6 levels, nodules (n = 50, 66.7%) were observed most frequently, followed by bronchial wall thickening (n = 25, 33.3%), interlobular septal thickening (n = 20, 26.7%), ground-glass opacity (n = 10, 13.3%), architectural distortion (n = 7, 9.3%), traction bronchiectasis (n = 7, 9.3%), consolidation (n = 3, 4%) and intralobular reticular opacity (n = 3, 4%) (Figures 1–4).
Figure 1.
Images in a 69-year-old woman. (a) Transverse CT scan (1 mm section thickness) obtained at the level of right upper lobe shows ground-grass appearance (arrows; KL-6, 2940 U ml–1). (b) Transverse CT scan (1 mm section thickness) obtained, after surgical biopsy 4 years later, shows disappearance of ground-grass opacity (KL-6, 313 U ml–1).
Figure 4.
Images in a 65-year-old woman. (a) Transverse CT scan (1 mm section thickness) obtained at the level of division of right B6 shows consolidation in right middle lobe (arrow) and multiple nodules (arrowheads; KL-6, 400 U ml–1). (b) Transverse CT scan (1 mm section thickness) obtained 5 months later shows worsened consolidation (arrow) and unchanged nodules (arrowheads); however, the KL-6 level is not elevated (KL-6, 397 U ml–1).
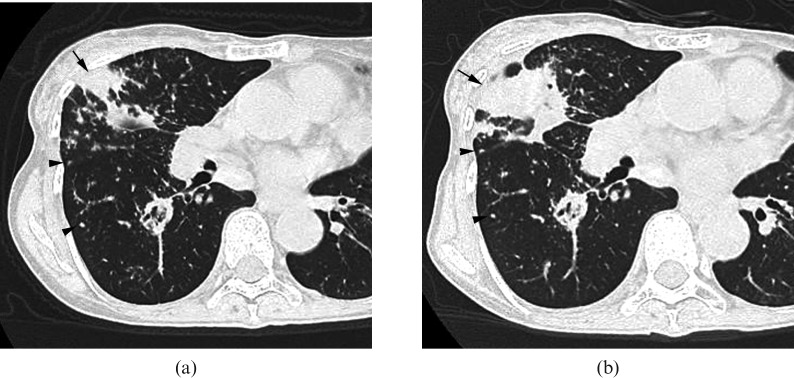
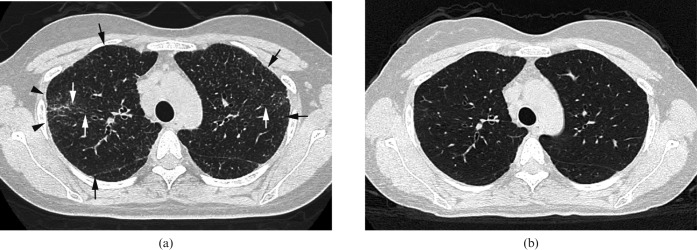
By contrast, of the 26 patients with elevated KL-6 levels, architectural distortion (Figures 2 and 3) was observed in all cases, followed by nodules (Figures 2–4), interlobular septal thickening and traction bronchiectasis (n = 25, 96.2%; Figures 2 and 3), bronchial wall thickening (n = 21, 80.8%; Figure 3), ground-glass opacity (n = 16, 61.5%; Figure 1) and consolidation (n = 2, 7.7%, Figure 4).
Figure 2.
Image in a 69-year-old man. (a) Transverse CT scan (1 mm section thickness) obtained at the level of left lower lobe shows a nodule in the periphery (arrow; KL-6, 333 U ml–1). (b) Transverse CT scan (1 mm section thickness) obtained 6 months later shows architectural distortion (KL-6, 581 U ml–1). Traction bronchiectasis is also present (arrows).
Figure 3.
Images in a 35-year-old woman. (a) Transverse CT scan (1 mm section thickness) obtained at the level of upper lobes shows multiple small nodules (arrows) and architectural distortion (arrowheads; KL-6, 1000 U ml–1). Traction bronchiectasis and bronchial wall thickening are also present (white arrows). (b) Transverse CT scan (1 mm section thickness) obtained 6 months later demonstrates disappearance of nodules, architectural distortion, traction bronchiectasis and bronchial wall thickening (KL-6, 285 U ml–1).
Ground-glass opacity, nodules, interlobular septal thickening, traction bronchiectasis, architectural distortion and bronchial wall thickening were significantly more frequent in patients with elevated KL-6 levels than in those with normal KL-6 levels (p<0.001, p<0.005, p<0.001, p<0.001, p<0.001 and p<0.001, respectively).
Lymph nodes and effusions
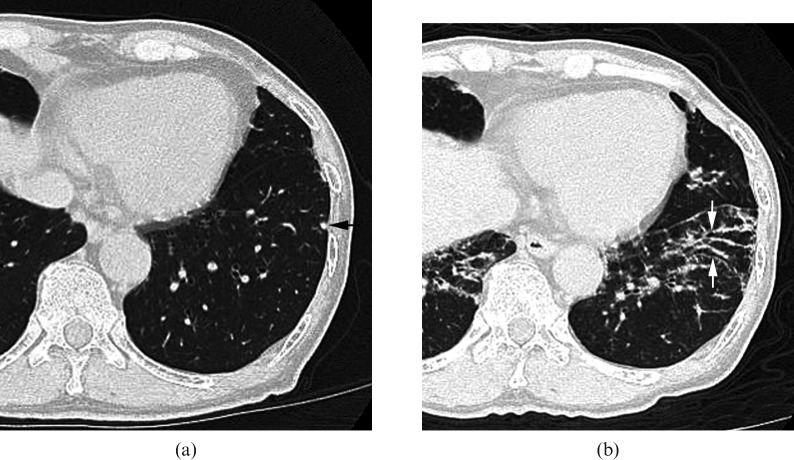
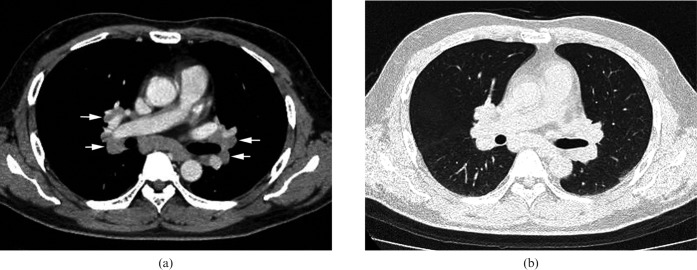
In 70 of 75 patients with normal KL-6 levels (93.3%) and in 21 of 26 patients with elevated KL-6 levels (80.8%), enlarged lymph nodes were observed at the pre-tracheal, paratracheal, tracheobronchial or subcarinal regions (Figure 5). There was no significant difference in frequency of lymph node enlargement between the two groups.
Figure 5.
Images in a 46-year-old male (KL-6, 239 U ml–1) (a) Transverse CT scan (1 mm section thickness) obtained at the level of the right pulmonary artery shows enlarged lymph nodes at subcarinal and bilateral hilar regions (arrows). (b) Transverse CT scan (1 mm section thickness) obtained at the level of the right pulmonary artery shows no abnormal parenchymal lesions.
Pleural effusions were not found in any patients in the study.
Follow-up study
Follow-up CT scans were obtained from 36 out of 101 patients at intervals of 1–61 months after initial CT examination (mean follow-up time 7 months). Of 14 patients with ground-glass attenuation (2 patients with normal KL-6 levels and 12 patients with elevated KL-6 levels), follow-up CT scans revealed complete clearing in 1 patient, a decrease in another patient, an increase in 1 patient and no change in 11 patients. In the patients with complete clearing and decreased ground-glass opacity, elevated KL-6 levels improved to within normal limits (Figure 1). In all of the patients with unchanged findings, initial KL-6 levels remained unchanged. By comparison, in the patient with an increase in ground-glass opacity, the KL-6 level worsened.
Of 19 patients with architectural distortion (2 with normal KL-6 levels and 17 with elevated KL-6 levels), follow-up CT scans showed a decrease in 4 patients, no change in 14 patients and an increase in 1 patient. In all patients with improved findings, KL-6 levels decreased (Figure 3); in the patient with worsened findings, the KL-6 level increased (Figure 2).
Of 33 patients with nodules (16 with a normal KL-6 level and 17 with elevated KL-6 levels), follow-up CT scans showed a decrease in 9 patients, no change in 19 patients and an increase in 5 patients. Of the 9 patients with improved findings, KL-6 levels improved in 7 patients (Figure 3) and remained unchanged in 2. Of the 19 patients with unchanged findings, KL-6 levels improved in 4 patients, remained unchanged in 14 and worsened in 1. By comparison, of the 5 patients with worsened findings, KL-6 levels remained unchanged in 4 and worsened in 1.
Of 22 patients with bronchial wall thickening (8 with normal KL-6 levels and 14 with elevated KL-6 levels), follow-up CT scans showed a decrease in wall thickening in 2 patients, no change in 19 patients and an increase in 1 patient. Of the patients with improved findings, KL-6 levels improved in 1 patient (Figure 3) and remained unchanged in 1. In all of the 19 patients with unchanged findings, initial KL-6 levels remained unchanged. In the patient with an increase in bronchial wall thickening, the KL-6 level worsened.
Of 3 patients with consolidation (2 with normal KL-6 levels and 1 with an elevated KL-6 level), follow-up CT scans remained unchanged in 2 and worsened in 1. In all of the patients with unchanged findings or worsened findings (Figure 4), the initial KL-6 level remained unchanged.
Of 28 patients with lymph node enlargement (15 with normal KL-6 levels and 13 with elevated KL-6 levels), follow-up CT scans showed a decrease in 1 patient and no change in 27 cases. In the patient with improved findings, the KL-6 level improved. Of the 27 patients with unchanged findings, KL-6 levels improved in 13 patients with ameliorated parenchymal abnormalities and worsened in 3 patients with exacerbated parenchymal findings. No change was observed in 11 patients with unchanged parenchymal abnormalities.
Discussion
KL-6 is a mucin-like high molecular weight glycoprotein found in 1988 by Kohno et al [6]. This group also developed a monoclonal antibody against KL-6, which reacts with a sialylated carbohydrate antigen strongly expressed in Type II alveolar pneumocytes and bronchiolar epithelial cells, but not on granulomatous tissue. Several studies demonstrate that KL-6 is elevated in the bronchoalveolar lavage fluid and serum of patients with various types of interstitial pneumonia. The elevation of serum KL-6 levels is considered to be associated with increased permeability of the alveolar capillary barrier [20]. It has also been reported that KL-6 induces chemotaxis of human fibroblasts in vitro, suggesting that it may have a pathological role in fibrosing lung disease [21]. Therefore, measurement of serum KL-6 is now widely accepted as a diagnostic marker for monitoring the activity of interstitial lung diseases, such as idiopathic interstitial pneumonia, and for the long-term management of various interstitial diseases [1, 2, 7-11, 13].
The clinical picture of sarcoidosis varies from that of a totally asymptomatic patient, with incidental radiographic findings of bilateral hilar lymphadenopathy, to a seriously dyspnoeic patient with acute respiratory distress syndrome. Alveolitis and subsequent granulomatous processes are important in the pathogenesis of sarcoidosis [22-24]. Bronchoalveolar lavage studies have revealed that the alveolitis in sarcoidosis is characterised by lymphocytic infiltration of T helper cells. The activated T cells release various cytokines such as interleukin-2 and interferon-gamma, which are believed to play many important roles in the pathogenesis of sarcoidosis. Active alveolitis is often accompanied by pulmonary tissue injury or clinical deterioration.
In 1989, Kohno et al [1] reported that serum KL-6 levels were elevated in 4 of 31 patients with sarcoidosis; however, further study was not performed. Kobayashi et al [16] investigated the use of serum KL-6 as a marker for sarcoidosis activity in 47 patients, and found that serum KL-6 levels were significantly elevated in radiographic Type II and Type III patients compared with Type 0 and Type I cases. These observations are similar to those in our study. However, it was difficult to ascertain whether the pulmonary lesions in these patients were characterised by interstitial, granulomatous or fibrous changes.
Hiroshige et al [25] presented a case report of a sarcoidosis patient with an elevated serum KL-6 level, where CT images showed panlobular ground-glass opacity with mosaic distribution. After steroid treatment the CT findings improved and serum KL-6 normalised.
However, no studies describing the radiological findings comparing thin-section CT images between patients with elevated KL-6 levels and those with normal KL-6 levels have been published in English. We retrospectively identified 101 patients with sarcoidosis who underwent chest thin-section CT examinations and had serum KL-6 measured.
Ground-glass opacity, traction bronchiectasis, architectural distortion, nodules, interlobular septal thickening and bronchial wall thickening were significantly more frequent in patients with elevated KL-6 levels than those with normal ones.
Pathologically, ground-glass opacity is considered to represent interstitial inflammatory infiltrate with marked thickening of alveolar septa by fibrous tissue [26]. Lynch et al [27] and Hiroshige et al [25] reported that ground-glass opacities correlated with active diffuse alveolitis.
Traction bronchiectasis and architectural distortion are considered to represent the organisation and fibrosis of the lungs around the bronchi [26, 28]. Our results showed that increased KL-6 levels in sarcoidosis patients whose CT scans consisted of ground-glass attenuation, architectural distortion or traction bronchiectasis may have been caused by an increase in KL-6 production by regenerating alveolar Type II pneumocytes and/or an enhanced permeability following the destruction of the air–blood barrier in the affected lungs.
Bronchial wall thickening, interlobular septal thickening and nodules along bronchi, interlobular septa and pleural regions were also significantly observed more frequently in patients with an elevated KL-6 level than in those with a normal KL-6 level. CT findings are considered to correspond to granulomas, with or without perigranulomatous fibrosis, formed in the connective tissue sheath around the bronchial walls, pulmonary vessels and airways [29]. Thus, our results might be due to perigranulomatous fibrosis with granulomas.
On the other hand, the present study showed that there was no significant difference in the frequency of consolidation between the two groups. Consolidation has revealed alveolitis with inflammatory exudates in alveolar spaces and/or accumulated granulomatous lesions [29-31]. In the present report, biopsy specimens corresponding to consolidation could not be obtained, and whether consolidation shows alveolitis or granulomatous lesions remains unclear. This result may possibly be due to a low frequency of consolidation in this study compared with that of previous studies [31]. Future work should confirm these findings by use of a greater sample size.
Finally, there were several limitations in our study. First, we undertook a retrospective study, and CT image interpretation was performed by consensus. Second, CT findings were not compared with pathological findings in all patients because some patients were asymptomatic and the diagnosis of sarcoidosis could be performed using clinical findings. Third, CT scans, measurement of KL-6 levels and histological specimens were not obtained on the same days. Fourth, CT images were obtained at several CT scans using different protocols. Furthermore, the relationship between the extent of abnormal findings and KL-6 levels was not evaluated.
In summary, thin-section CT findings of nodules, bronchial wall thickening, ground-glass opacity, traction bronchiectasis and architectural distortion were associated with elevated serum KL-6 levels. These results suggest that serum KL-6 levels may be a useful marker for indicating the severity of parenchymal sarcoidosis.
References
- 1.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity: sialylated carbohydrate antigen KL-6. Chest 1989;96:68–73 [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi J, Kitamura S. KL-6: A serum marker for interstitial pneumonia. Chest 1995;108:311–15 [DOI] [PubMed] [Google Scholar]
- 3.Ogawa Y, Ishikawa T, Ikeda K, Nakata B, Sawada T, Ogisawa K, et al. Evaluation of serum KL-6, a mucin-like glycoprotein, as a tumor marker for breast cancer. Clin Cancer Res 2000;6:4069–72 [PubMed] [Google Scholar]
- 4.Kohno N, Inoue Y, Hamada H, Fujioka S, Fujino S, Yokoyama A, et al. Difference in sero-diagnostic values among KL-6-associated mucins classified as cluster 9. Int J Cancer 1994;8 (Suppl):81–3 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Takamura K, Sakaue S, Ishii J, Yokouchi H, Nasuhara Y. Elevated serum KL-6 concentrations in patients with diabetes mellitus. J Diabetes Complications 2002;16:352–8 [DOI] [PubMed] [Google Scholar]
- 6.Kohno N, Akiyama M, Kyoizumi S, Hakoda M, Kobuke K, Yamakido M. Detection of soluble tumor-associated antigens in sera and effusions using novel monoclonal antibodies, KL-3 and KL-6, against lung adenocarcinoma. Jpn J Clin Oncol 1988;18:203–16 [PubMed] [Google Scholar]
- 7.Kohno N, Awaya Y, Oyama T, Yamakido M, Akiyama M, Inoue Y, et al. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am Rev Respir Dis 1993;148:637–42 [DOI] [PubMed] [Google Scholar]
- 8.Ishii H, Mukae H, Kadota J, Kaida H, Nagata T, Abe K, et al. High serum concentrations of surfactant protein A in usual interstitial pneumonia compared with non-specific interstitial pneumonia. Thorax 2003;58:52–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada F, Ando Y, Honda K, Tanoue S, Matsumoto S, Mori H. Comparison of pulmonary CT findings and serum KL-6 levels in patients with cryptogenic organizing pneumonia. Br J Radiol 2009;82:212–18 [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, et al. Prognostic value of circulation KL-6 in idiopathic pulmonary fibrosis. Respirology 2006;11:164–8 [DOI] [PubMed] [Google Scholar]
- 11.Lin FC, Chen YC, Chang SC. Clinical importance of bronchoalveolar lavage fluid and blood cytokines, surfactant protein D, and Kerbs von Lungren 6 antigen in idiopathic pulmonary alveolar proteinosis. Myyo Clin Proc 2008;83:1344–9 [DOI] [PubMed] [Google Scholar]
- 12.Hant FN, Ludwicka-Bradley A, Wang HJ, Li N, Elashoff R, Tashkin DP, et al. Scleroderma Lung Study Research Group. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol 2009;36:773–80 [DOI] [PubMed] [Google Scholar]
- 13.Satoh H, Kurishima K, Ishikawa H, Ohtsuka M. Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med 2006;260:429–34 [DOI] [PubMed] [Google Scholar]
- 14.Kitaichi N, Ariga T, Kase S, Yoshida K, Namba K, Ohno S. Usefulness of quantifying serum KL-6 levels in the follow-up of uveitic patients with sarcoidosis. Graefes Arch Clin Exp Ophthalol 2006;244:433–7 [DOI] [PubMed] [Google Scholar]
- 15.Janssen R, Sato H, Glutters JC, Bernard A, van Velzen-Blad H, du Bois RM, et al. Study of Clara cell 16, KL-6, and surfactant protein-D in serum as disease markers in pulmonary sarcoidosis. Chest 2003;124:2119–25 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi J, Kitamura S. Serum KL-6 for the evaluation of active pneumonitis in pulmonary sarcoidosis. Chest 1996;109:1276–82 [DOI] [PubMed] [Google Scholar]
- 17.Statement on sarcoidosis: joint statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736–55 [DOI] [PubMed] [Google Scholar]
- 18.Webb WR, Muller NL, Naidich DP. High-resolution computed tomography findings of lung disease. In: Aughenbaugh GL, ed. High-resolution CT of the lung, 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2001: 71–192 [Google Scholar]
- 19.Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327–31 [DOI] [PubMed] [Google Scholar]
- 20.Inoue Y, Barker E, Daniloff E, Hohno N, Hiwada K, Newman S. Pulmonary epithelial cell injury and alveolar-capillary permeability in berylliosis. Am J Respir Crit Care Med 1997;156:109–15 [DOI] [PubMed] [Google Scholar]
- 21.Hirasawa Y, Kohno N, Yokoyama A, Inoue Y, Abe M, Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am J Respir Cell Mol Biol 1997;17:501–7 [DOI] [PubMed] [Google Scholar]
- 22.Foley NM, Coral AP, Tung K, Hudspith BN, James DG, Johnson NM. Bronchoalveolar lavage cell counts as a predictor of short outcome in pulmonary sarcoidosis. Thorax 1989;44:732–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maycock RL, Bertrand P, Morrison CE, Scott JH. Manifestation of sarcoidosis: analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 1963;35:67–89 [DOI] [PubMed] [Google Scholar]
- 24.Israel HL. Prognosis of sarcoidosis. Ann Intern Med 1970;73:1038–9 [DOI] [PubMed] [Google Scholar]
- 25.Hiroshige S, Ando M, Okubo F, Ueno T, Fukami T, Takenaka R, et al. A case of pulmonary sarcoidosis demonstration Panlobular ground-glass opacity with mosaic distribution. Nihon Kokyuki Gakkai Zasshi 2009;47:212–17 [PubMed] [Google Scholar]
- 26.Remy-Jardin M, Giraud F, Remy J, Copin MC, Gosselin B, Duhamel A. Importance of ground-glass attenuation in chronic diffuse infiltrative lung disease: pathologic-CT correlation. Radiology 1993;189:693–8 [DOI] [PubMed] [Google Scholar]
- 27.Lynch DA, Webb WR, Gamsu G, Stubarg M, Golden J. Computed tomography in pulmonary sarcoidosis. J Comput Assist Tomogr 1989;13:405–10 [DOI] [PubMed] [Google Scholar]
- 28.Westcott JL, Cole SR. Traction bronchiectasis in end-stage pulmonary fibrosis. Radiology 1986;161:665–9 [DOI] [PubMed] [Google Scholar]
- 29.Nishimura K, Itoh H, Kitaichi M, Nagai S, Izumi T. Pulmonary sarcoidosis: correlation of CT and histopathologic findings. Radiology 1993;189:105–9 [DOI] [PubMed] [Google Scholar]
- 30.Nakano Y, Kurihara N, Miyamoto O, Takamatus K, Adachi N, Fujiwara H, et al. A case of sarcoidosis beginning with extensive ground-glass pattern on chest X-ray, accompanied with high fever and eosinophilia. Nihon Kyobu Shikkan Gakkai Zasshi 1989;27:98–106 [PubMed] [Google Scholar]
- 31.Abehsera M, Veleyre D, Grenier P, Jaillet H, Battesti JP, Brauner MW. Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. AJR Am J Roentgenol 2000;174:1751–7 [DOI] [PubMed] [Google Scholar]