Abstract
The article describes both the early development of oncology as a core discipline at the University of Heidelberg Hospital and the first steps towards ion beam treatment, from the pilot project carried out in co-operation with the Gesellschaft für Schwerionenforschung Darmstadt to the initial start-up of clinical service at the Heidelberg Heavy Ion Centre (HIT). We present an overview, based on data published in the literature, of the available clinical evidence relating the use of ion beam therapy to treat major indications in active particle centres. A rationale for the use of particle therapy in each of these indications is given. In view of the limited availability of data, we discuss the necessity to conduct clinical trials. We also look forward towards the next activities to be undertaken at the HIT.
Background
Every new project starts with a vision
As long ago as 1906, Vincenz Czerny established the idea of interdisciplinary cancer research, leading to the foundation of the Samaritan House and the concept of the “Institute for Experimental Cancer Research”. This institute would become the first hospital in Germany, and only the second worldwide, solely dedicated to cancer medicine and would pioneer new methods in both surgery and oncology. Vincenz Czerny became the father of radiation oncology at Heidelberg University. Cancer research is rooted deeply within Heidelberg University Hospital, and oncology continues to be one of the core disciplines in each of its departments. In the tradition of the original institute for experimental cancer research, DKFZ (The German Cancer Research Centre) was founded in 1966, providing a stimulating environment for departments attached to clinical medicine and those concentrating on related basic sciences. Radiation oncology as a speciality also continued to prosper at Heidelberg, and in 1987 this department was moved from its original home in the Samaritan House, on the old campus at Bergheim, to its new and more spacious home in the Kopfklinik on the new campus.
Upon establishing the clinical co-operation unit for radiotherapy (consisting of an interdisciplinary team of radiation oncologists, biologists, physicists, computer scientists and engineers) at the DKFZ, the department continued to push forward new treatment techniques, many made possible by technical developments; for example, intra- or extracranial stereotactic radiotherapy and intensity-modulated radiation therapy (IMRT) were established as routine clinical techniques from very early on. IMRT was introduced at Heidelberg in 1997, and for the past 10 years or so patients have routinely received this treatment for head and neck tumours and for prostate carcinomas. Today, the radiation oncology department provides care for almost 4000 patients each year, making use of 5 linear accelerators, a tomotherapy unit, a high-dose-rate (HDR)/pulsed-dose-rate (PDR) brachytherapy facility, 2 intraoperative treatment machines, a dedicated diagnostic division and 3 wards (containing 60 beds) for combination treatment and supportive care.
The idea of using particle radiation for treatment was actively fostered at Heidelberg. The physical properties of particle beams, which allow sharp dose gradients and hence relative dose escalation and minimised dose to normal organs, seemed especially appealing for the treatment of otherwise relatively radio-resistant tumours. Early data from institutions in the USA, such as the Lawrence Berkeley National Laboratory, seemed to clinically support this physical advantage [1-4]. However, this vision had to be pursued for quite some time before the pilot project for heavy-ion treatment was finally born.
First ideas and proposals to either national (1989) or European (1991) grant-awarding bodies were unsuccessful. Meanwhile, however, technical progress allowed the implementation of active, intensity-controlled scanning methods for particle beams at the Gesellschaft für Schwerionenforschung Darmstadt (GSI). The GSI is a research centre within the Helmholtz Association of German research institutes, located just 29 km south of Frankfurt and 70 km north of Heidelberg. Funded largely by the German federal government, and to a smaller extent by the State of Hessen, the GSI operates a large accelerator facility available to scientists in radiation biology, accelerator technology and particle physics. The Department of Radiation Oncology at Heidelberg University, together with the Clinical Cooperation Unit for Radiation Oncology at the DKFZ, the GSI and the Forschungszentrum Dresden–Rossendorf (FZD), who contributed knowledge relating to basic/applied materials research, nuclear/hadron physics and biomedical applications of positron emission tomography, successfully initiated the pilot project for clinical carbon-ion therapy in 1994. In 1997, the first patient finally received carbon-ion therapy with an active beam application at the GSI. The project was originally designed for basic research purposes and hence patient treatment was available for a total of only three treatment slots (each of 20 days) each year; nevertheless, almost 450 patients were treated within this project from 1997 to 2008, most of them patients with chordoma, chondrosarcoma or adenoidcystic carcinoma. As a result of this project, carbon-ion therapy was established in Germany as the treatment of choice for these indications whenever available. The project also convincingly demonstrated the feasibility of particle therapy for more routine clinical use.
In 2000, very soon after the first patient had been treated at Darmstadt, the collaborators presented a feasibility study for a dedicated hospital-based facility. Financial feasibility was established in 2001, an application made for federal funds and the project approved in 2003. Events progressed quickly, contracts were drawn up with the various companies contributing to the building and technical development of the Heidelberg Heavy Ion Centre (HIT) in 2003 and construction began in 2004.
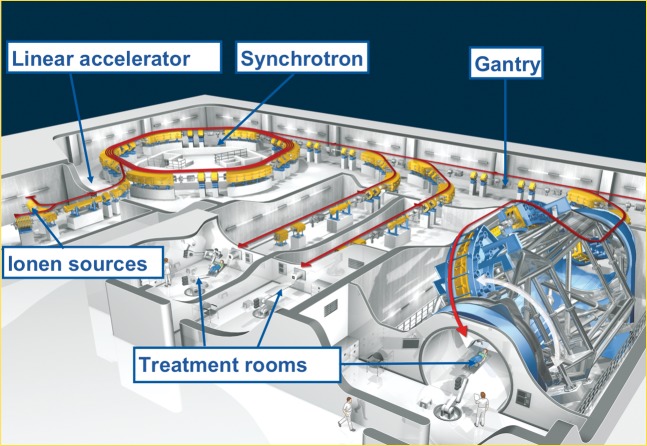
The HIT now has three treatment rooms: two with horizontal beam lines and a third with a gantry, another room has a horizontal beam application for quality assurance and experimental purposes (Figure 1). The accelerator system was successfully installed in October 2005, and construction of the world's first proton/heavy-ion gantry was completed in January 2007. Legal procedures, software developments and the adaptation of equipment allowed the formal handover of the facility to the Department of Radiation Oncology in November 2009. Routine patient treatment was started soon afterwards, on 15 November 2009, in the horizontal treatment room.
Figure 1.
The Heidelberg Heavy Ion Centre facility, Germany (copyright Stern).
All treatment rooms offer patient positioning on a robotically controlled treatment table; in addition, the horizontal treatment rooms have robotically controlled imaging units for position verification (Figure 2).
Figure 2.
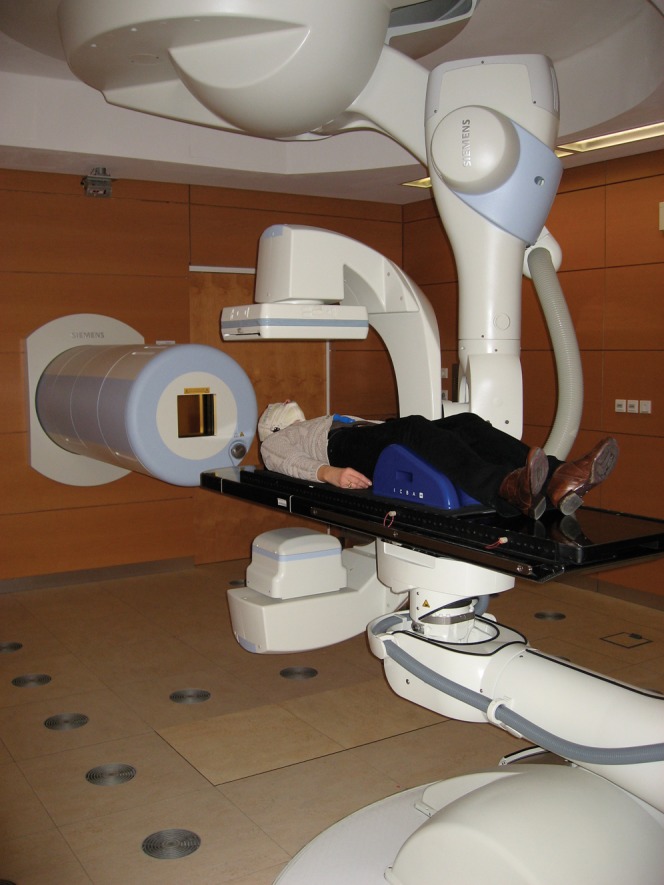
The Heidelberg Heavy Ion Centre's horizontal treatment room has facilities for robotically controlled patient positioning and imaging.
Clinical treatment is carried out in a daily 10 h routine, but research is ongoing to continue to develop and improve the accelerator technology, the software, the preparation of the carbon/proton gantry for clinical use and our understanding of radiobiology. As the carbon/proton beam is now permanently available, there is also an opportunity to broaden the potential spectrum of indications.
Radiotherapy has indeed come a long way from its beginnings in Heidelberg in 1906. More and more ion beam facilities are planned and already built worldwide. There are three more particle therapy centres being built or about to begin clinical treatment in Germany alone: one for proton and two for proton and carbon-ion treatments. Nevertheless, this technique is far from being widely available and so, out of necessity, there will have to be a degree of patient selection. Which patients should routinely receive particle therapy? Do all patients need particles? Can we select certain patients who will benefit?
The results of the Heidelberg–GSI co-operation show that carbon-ion treatment yielded excellent local control (LC) and overall survival (OS) rates for patients with chondrosarcoma (LC 89.8% at 4 years, OS 98.2% at 5 years) [5] or chordoma (LC 70% at 5 years, OS 88.5% at 5 years) [6]. Furthermore, the treatment was associated with only very mild side effects [no late toxicity: National Cancer Institute Common Toxicity Criteria (CTC) score >°3]. Combined treatment with carbon ions and IMRT also improved LC in adenoid cystic carcinoma when compared with IMRT alone, yielding a 77.5% LC at 4 years compared with matched controls of 24.6% LC [7].
Is the physical dose distribution that can be obtained by either proton or 12C radiation or the higher relative biological effect of heavy-ion radiation enough to justify the application of this technique for all tumours? Do we need randomised controlled trials to prove the obvious? Are the advantages so obvious that we can simply assume the superiority of this method? This discussion has been held throughout the decades, first when linear accelerators and modern radiotherapy techniques were introduced and again when IMRT became more widely available. Trials comparing IMRT and standard three-dimensional (3D) conformal radiotherapy are rare, and yet the use of IMRT for cancer of the head and neck, for example, has become the accepted standard of care. Hence, randomised trials sometimes give rise to animated discussions [8]. Moreover, some of the tumour types for which IMRT treatment has proven beneficial are rare, and patient numbers will never be high enough for randomised controlled trials. For some more common diseases, few patients have so far been treated with particle therapy and our “routine” clinical experience is limited. From our perspective, and also as an academic institution, we believe that novel paths need to be struck and treatment results evaluated prospectively. The comparison with medical oncology is that no new drug has ever been established without initial experience in Phase I and II trials. Sometimes, even single institution reports have led to the acceptance of approved drugs in new indications [9].
It is obvious that both technical and financial issues must be considered if particle therapy is to be used more widely, but it might be worth taking a look at the clinical evidence that has already been collected by working groups around the world. The body of evidence reviewed below on the use of particle therapy in selected indications was compiled by a MEDLINE search of original contributions from active particle centres.
Review of clinical evidence
Uveal melanoma
Proton therapy for uveal melanoma has been well described (Table 1), with reports of LC rates of >90% at 5 years. Hence, this technique is used for this disease as a method with expected outcomes equivalent to those of surgical enucleation and brachytherapy with 106Ru-applicators wherever available. Eye preservation rates range between 75% and 92% [9-18,20] when doses of around 60 GyE are used in four fractions. Gragoudas et al [13] tested 50 vs 60 GyE in a prospective Phase III trial and could not find a significant difference in LC. The field of view was, however, significantly higher at the lower dose, and hence this dose has since been established as standard. Even for larger uveal melanomas, high rates of eye and vision preservation were shown in a Japanese trial [19].
Table 1. Uveal melanoma.
| Report | Patient number | Radiotherapy | Local control rate | Eye preservation rate |
| Char et al [10] | 184 | Helium ions vs 125I | 95.4% at 5 years | Data not given |
| Castro et al [11] | 347 | Helium ions | 96% at 5 years | 81% at 5 years |
| Courdi et al [12] | 538 | Protons | 89.0% at 5 years | 88% at 5 years |
| Gragoudas et al [13] | 188 | Protons (50 GyE) | 97% at 5 years | 96% at 5 years |
| Protons (60 GyE) | 98% at 5 years | 95% at 5 years | ||
| Fuss et al [14] | 78 | Protons | 90.5% at 5 years | 75.2% at 5 years |
| Desjardins et al [15] | 1272 | 125I vs protons | 96.25% at 5 years | 92.8% at 5 years |
| 96% at 5 years | 88.8% at 5 years | |||
| Höcht et al [16] | 245 | Protons | 95.5% at 3 years | 87.5% at 3 years |
| Damato et al [17] | 88 | Protons | 96.7% at 4 years | 90.6% at 4 years |
| Dendale et al [18] | 1406 | Protons | 96% at 5 years | 92.3% at 5 years |
| Tsuji et al [19] | 57 | 12C | 97.4% at 3 years | 91.1% at 3 years |
Base of skull tumours: chordoma and chondrosarcoma
Chordoma and chondrosarcoma of the skull base have traditionally been a challenge for radiation oncologists. Treatment is primarily surgical but complete resection of tumours in the skull base is very often not achievable. Moreover, these tumours are considered comparatively radio-resistant and unfortunately are anatomically located next to critical and radio-sensitive structures. Techniques using conventional radiation have therefore shown disappointing LC rates of 17–50% for chordoma of the skull base. The best results have been achieved by the introduction of fractionated stereotactic radiotherapy (FSRT) [21]. These tumours are therefore an ideal target for particle therapy, which can provide superior dose distribution and, in case of 12C therapy, increased relative biological effectiveness (RBE).
Various working groups have been able to achieve LC rates of 46–74% at 5 years for chordoma [21-25] (Table 2) and of 78–98% for chondrosarcoma (Table 3) with the use of particle radiation. As these tumours are rare, however, pooled data are very often published for chordoma and chondrosarcoma.
Table 2. Chordoma.
| Author | Year | Patient number | Radiotherapy | Local control rate |
| Romero et al [22] | 1993 | 18 | Conventional radiotherapy | 17% at 5 years |
| Debus et al [21] | 2000 | 45 | FSRT | 50% at 5 years |
| Munzenrider and Liebsch [23] | 1999 | 519 | Protons/photons | 73% at 5 years |
| Castro et al [2] | 1994 | 223 | Helium ions | 63% at 5 years |
| Noel et al [24] | 2001 | 67 | Protons/photons | 71% at 3 years |
| Weber et al [25] | 2005 | 11 | Protons | 87.5% at 3 years |
| Schulz-Ertner et al [6] | 2007 | 67 | 12C | 70% at 5 years |
FSRT, fractionated stereotactic radiotherapy.
Table 3. Chondorsarcoma.
| Author | Year | Patient number | Radiotherapy | Local control rate |
| Munzenrider and Liebsch [23] | 1999 | 519 | Protons/photons | 98% at 5 years |
| Castro et al [2] | 1994 | 223 | Helium | 78% at 5 years |
| Noel et al [24] | 2001 | 67 | Protons/photons | 85% at 3 years |
| Weber et al [25] | 2005 | 18 | Protons | 100% at 3 years |
| Schulz-Ertner et al [6] | 2007 | 67 | 12C | 89.8% at 4 years |
The use of heavy ions, in particular, seems advantageous in the treatment of these tumours. This is supported by early data from the Lawrence Berkeley National Laboratory where the use of helium and neon ions provided LC rates of 78% for chondrosarcoma and 63% for chordoma [2]. Terahara et al [26] reported the achievement of slightly lower LCs by the use of a combination of photon and proton radiation (median dose 68.9 GyE) (59% at 5 years; 44% at 10 years) in 115 patients, but most of these treatments were carried out in the pre-3D era (i.e. between 1978 and 1993). Another analysis by colleagues from the Paul Scherrer Institute (Villigen, Switzerland) of 18 patients with chordoma and 11 patients with chondrosarcoma (total doses applied: 74 or 68 GyE protons) reported 3-year LCs of 87.5% and 100%, respectively [25]. Similar results were achieved by the application of 12C heavy ions: 54 patients with chondrosarcoma (median dose 60 GyE) achieved LCs of 96.2% and 89.8% at 3 and 4 years, respectively [5]. This treatment was accompanied by very mild toxicity (≥°3: 1.9%). During the same period, 96 patients with macroscopical remnant chordoma were also treated at the same institution (median dose 60 GyE). Again, LC rates of 80.6% and 70% at 3 and 5 years, respectively, were achieved by their treatment [6]. In this series, only two cases of °3 late toxicity were found (4.1%; one case of opticus-neuropathy, once necrosis of a fat implant). A retrospective comparison of LC rates with results from other working groups suggested a clear dose-dependency of LC, further underlining the advantage of particle therapy in the treatment of these tumours (Table 3).
Malignant salivary gland tumours: adenoid cystic carcinoma
Malignant salivary gland tumours are a rare and heterogenous group of tumours accounting for approximately 3–5% of head and neck cancers. High-grade tumours, such as mucoepidermiod carcinoma (35%) and adenoid cystic carcinoma (ACC) (25%), are the most common histological subtypes [27], characterised by a rather slow pattern of growth, perineural spread and high potential for haematogenous metastases. To date, the standard therapy for high-grade salivary gland carcinoma consists of complete surgical resection and adjuvant radiation in a high-risk situation [i.e. for R+ or close margin, perineural spread, neural infiltration, large tumours (T3/4) or nodal metastases] [27-29]. To achieve LC, radiation doses of >60 Gy are recommended [30-32]. It is worth noting that all tumour stages profit from post-operative radiotherapy [31,33,34].
LC was also significantly improved by the application of high-precision techniques, dose-escalation and high linear energy transfer radiation [7,33-36]. Both IMRT and FSRT provide better LC than conventional radiotherapy techniques, achieving 3-year progression-free survival (PFS) rates of 38% [37]. The highest LC rates achieved to date, at 75–100% [35,36], were achieved by neutron radiation, albeit at the cost of significant late toxicity.
One dose-escalation study [38], although carried out only in a small group of patients, suggests that treatment with heavy ions should be carried out at 70.2 GyE (3×3.9 GyE/week) or 64 GyE (4×4 GyE/week). Despite high doses per fraction, no CTC°3 late toxicities and very few °3 acute reactions occurred. LC at 5 years was still 100% even though various histological subtypes of salivary gland tumours were included in the trial. Pommier et al [39] treated 23 patients with ACC using protons at 75.9 GyE (median) in various fractionation schemes. Overall, they achieved LC rates at 5 years of 93%, but the authors noted several CTC°3 and one °5 late toxicity (a temporal lobe necrosis). Douglas et al [35] published a retrospective analysis of 159 patients with ACC who had been treated with neutrons (total dose: 19.2 Gy), but reported somewhat disappointing LC rates of only 57% at 5 years accompanied by significant late toxicity (14%>CTC°3). The results for the combined IMRT-12C treatment therefore compare favourably [7] with those for other treatments and include only very mild treatment-related side effects (°3 late toxicity in only one patient). IMRT at 54 Gy [2 Gy/fraction (Fx)] plus a 12C-boost of 18 GyE (3 GyE/Fx) yielded LC rates of about 78% at 4 years.
In view of the reported outcomes and low-toxicity profile, photon IMRT plus 12C-boost has been accepted as the standard treatment and method of choice for ACC in Germany. Table 4 gives an overview of treatment results in the larger patient series.
Table 4. Adenoid cystic carcinoma.
| Patient number | RT | LC rate at 5 years | Progression to T4 at 10 years | OS rate at 5 years | Late toxicity scores of 3 or 4 | |
| Chen et al [28] | 207 | No RT | 86% | 83% | ||
| Chen et al [31] | 140 | No RT | 80% | 0% | 85% | Not given |
| Photons (64 Gy) | 92% | 37% | ||||
| Douglas et al [35] | 159 | Neutrons (19.2 Gy) | 9.40% | |||
| R2 | 57% | |||||
| R1–2 | 100% | |||||
| Mendenhall et al [30] | 101 | RT (50–72.4 Gy) | 56% | 30% | 75% | 12.9% |
| RT + surgical excision | 94% | 62% | 90% | |||
| Garden et al [32] | 160 | Photons (60 Gy) | 96% | 81% | 33% | |
| Gurney et al [29] | Photons (60 Gy) | 94% | Not given | |||
| Münter et al [37] | 25 (large tumours) | IMRT (66 Gy) | 38% | 72% at 3 years | 0% | |
| Huber et al [38] | 75 | Neutrons (16 Gy) | 75% | 19% | ||
| Photons (64 Gy) | 32% | 4% | ||||
| Mixed: neutrons (8 Gy), photons (32 Gy) | 32% | 10% | ||||
| Mizoe et al [36] | 36 | 12C (48.6–52.8 Gy) | 50% | 0% | ||
| Schulz-Ertner et al [7] | 29 | Photons, IMRT + 12C (72 GyE) | 77.5% at 4 years | 75.8% at 4 years | 3.40% | |
| 34 | Photon + IMRT (66 Gy) | 24.6% at 4 years | 77.9% at 4 years | 5.90% | ||
| Pommier et al [39] | 23 | Photons (75.9 Gy) | 93% | 17% |
IMRT, intensity modulated radiotherapy; LC, local control; OS, overall survival; R1–2, microscopic residual tumour; R2, macroscopic residual tumour; RT, radiotherapy.
Non-small cell lung cancer
The dose dependency of LC in lung tumours has long been recognised [40]. International guidelines recommend doses of 60–70 Gy for fractionated (photon) radiotherapy; in clinical routine, however, patients often have already impaired pulmonary function before therapy. In extensive tumours, doses of 60–70 Gy are rarely achievable without compromising target volumes. Dose escalation using photon radiation is therefore possible for only small tumours: maximum reported doses per (single) fraction are 37 GyE (T1/T2) resulting in clinical outcomes comparable with those of surgical interventions. Treatment results for lung tumours have improved historically with the introduction of more conformal treatment techniques in the 1990s and with the application of IMRT for lung tumours at the beginning of the new millennium.
There are extensive data on, and much experience in, the treatment of early-stage lung cancer (T1/T2) with either protons [40-42] or 12C ions [43-46]. Various fractionation regimen have been evaluated. The facility at Chiba published LC rates for the treatment of T1/T2 tumours with doses between 79.2 GyE (8.8 GyE/Fx) and 95.4 GyE (5.3 GyE/Fx) of 79% at 5 years, with the only significant factor on multivariate analysis being total dose delivered [45-48]. Most of the local treatment failures occurred within the high-dose area, suggesting that still further dose escalation is necessary for improvement of treatment outcome. Treatment results from Loma Linda from a study of 68 patients [42] also suggested that OS rates were dose-dependent (27% at 51 GyE; 55% at 60 GyE).
LC rates following particle irradiation exceeded those achieved by fractionated photon radiotherapy. Total doses of >86.4 GyE (18 Fx) or 72 GyE (9 Fx) yield LC rates of >90% at 5 years [44], which apparently also translate into favourable OS rates.
Although almost all patients showed pulmonary changes on their follow-up CT after particle radiotherapy, only 9.9% (8/81) of patients had Radiation Therapy Oncology Group (RTOG) °2/°3-scored acute pulmonary reactions, which completely resolved. Hence pneumonitis rates following particle radiotherapy compare favourably with those commonly reported for photon radiotherapy (>15% at 12 months; 17–20% at 24 months). A Phase I dose-escalation trial reported one case of CTC°3 acute pneumonitis at their standard dose of 94 GyE [43].
Correct dose delivery in particle radiotherapy is, however, dependent on breathing motion and hence there are considerable range uncertainties. Complicated patient positioning, including the application of modern gating techniques, therefore necessitates the investigation of hypofractionated treatment regimes. Another trial from Chiba showed promising results after hypofractionated 12C radiotherapy (total dose 72 GyE at 8 GyE/Fx) with an LC rate of 94.7% (at 5 years) and cause-specific survival of around 50% at 5 years (55.2% for T1 and 42.9% for T2) [46]. When doses per fraction were further increased [49] (to 52.8/60 GyE in 4 Fx), excellent LC rates were maintained without increased toxicity. Reduction of pulmonary function parameters was marginal (forced expiratory volume 1–8%, vital capacity –7%) compared with –20% to 50% for standard photon techniques. These results were confirmed for protons by another working group [50].
The working group at Tsukuba, Japan, evaluated proton therapy in 51 patients with non-small cell lung cancer (NSCLC) of all stages (Table 5). Patients with Stage II/III disease also received elective nodal photon irradiation and a boost to positive nodes of up to 76 GyE (3 GyE/Fx). Patients with Stage I/II cancers showed survival rates of 55% and 23% at 2 and 5 years, respectively. Predictably, patients with Stage III/IV disease did less well, with OS rates of 62% and 0% at 2 and 5 years, respectively. Again, no RTOG °2/°3 late toxicities were seen although, as described previously, almost all patients (92%) showed radiogenic pulmonary changes in their CT scan [51]. These data indicate the potential benefits of particle therapy in NSCLC, and treatment-related toxicity (especially pneumonitis rates) of all reported regimen have been low. As LC rates are dose-dependent and high doses are needed for long-term tumour control, carbon-ion therapy may add benefit by offering increased RBE. This concept warrants further exploration, especially in patients unfit or unwilling to contemplate complex surgical procedures and subsequent morbidity.
Table 5. Non-small cell lung cancer.
| Patient number | Stage | Radiotherapy | LC rate | OS rate | Late toxicity according to RTOG score | |
| Koto et al [45] | T1 | 12C | T1: 64.4% at 5 years | |||
| Nishimura et al [47] | T2 | 12C[95.4 GyE (5.3 GyE/Fx)] | T2: 22% at 5 years | °3: 14.8%, °4/°5: 0% | ||
| Kadono et al [48] | 81 | 12C [79.2 GyE (8.8 GyE/Fx)] | 79% at 5 years | |||
| Nihei et al [43] | 37 | T1/T2 | Protons[70 GyE (3.5 GyE/Fx)] to 98 GyE (4.9 GyE/Fx) | 80% at 2 years | 82% at 2 years | >°2: 16.2% |
| Miyamoto et al [46] | 50 | T1/T2 | 12C [72 GyE (8 GyE/Fx)] | 94.7% at 5 years | T1: 89.4% at 5 yearsT2: 55.1% at 5 years | °3: 2% |
| Miyamoto et al [49] | 80 | T1/T2 | 12C [T1: 52.8 GyE (13.2 GyE/Fx)] | 97% at 5 years | 62% at 5 years | No °3 or higher |
| [T2: 60 GyE (15 GyE/Fx)] | 80% at 5 years | 25% at 5 years | ||||
| Protons | T1: 100% at 2 years | T1: 100% at 2 years | ||||
| Hata et al [50] | 21 | T1/T2 | Protons or photons + protons (III/IV) [50–60 GyE (in 10 Fx)] | T2: 90% at 2 years | T2: 47% at 2 years | No °3 or higher |
| Shioyama et al [51] | 51 | I–IV | Protons(70–78 GyE) | I/II: 55% at 2 years, 23% at 5 years III/IV: 62% at 2 years, 0% at 5 years | No °2/°3 or higher | |
| Bush et al [52] | 68 | T1/T2 | Protons[51 or 60 GyE (in 10 Fx)] | T1: 87% at 3 yearsT2: 49% at 3 years | 60 GyE: 55% at 3 years51 GyE: 27% at 3 yearsOverall: 74% at 3 years | No °2/°3 or higher |
Fx, fraction; LC, local control; OS, overall survival; RTOG, Radiation Therapy Oncology Group.
Oesophageal carcinoma
Radio-oncological treatment of oesophageal carcinoma usually consists of platinum-containing chemoradiotherapy. The Tsukuba working group developed a combined photon plus proton treatment regimen based on the idea of normal tissue sparing. Their radiation treatment consisted of photon radiation (48 Gy) and a proton boost (median 31.7 GyE). 40 patients received a median total dose of 76 Gy, 6 patients received protons only. The trial recruited patients of all tumour stages: 50% of the patients had T1 tumours, 85% had no evidence of nodal disease. In this series, LC at 5 years was 83% for T1 tumours and 29% for T2–4 tumours [53]. This difference proved statistically significant, further underlining the results seen in established (photon) regimen. However, the authors further noted a clear dose dependency in contrast to the results of the Intergroup Trial INT 0123 [54]. Cases of local relapse occurred mostly at the cranio-caudal field edges or out of field, suggesting changes in the applied target volume concept. Moreover, almost half of the patients (48%) developed therapy-related oesophageal ulcerations, which completely resolved in only one-third of these patients. The high complication rate was assumed to be due to the high doses per fraction (median 3 GyE, range 2.5–3.7 GyE). Current study protocols were adapted accordingly and updated results are pending.
The treatment of oesophageal carcinoma with carbon-ion therapy is therefore possible without major complications. However, this report needs to be taken as an initial treatment experience: target volume definition, and hence planning investigations, are demanding in high-precision techniques and the authors clearly state that the dose prescription also needs modification. One important message from this experience needs to be considered: the commonly accepted dose in oesophageal cancer has been derived from Grade V toxicities in the Minsky trial [54] occurring in the experimental arm, but <56 Gy, this report shows that the oesophagus can be subjected to higher doses with acceptable toxicity. In summary, particle therapy for oesophageal carcinoma needs further exploration.
Hepatocellular carcinoma
There is extensive literature on the treatment of hepatocellular carcinoma (HCC) with particle therapy. HCC is a very radio-resistant tumour, whereas the surrounding normal liver tissue is very radio-sensitive, hence conventional radiotherapy techniques can deliver doses sufficient to treat only small tumours.
Various trials using proton therapy in various fractionation schemes achieved impressive LC rates of 75–96% at 2 years and OS rates of 55–66% at 2 years (Table 6). Therapy-related side effects were generally mild: the trial by Bush et al [52] included only three cases of gastrointestinal bleeding (in all of these cases, the respective bowel part was directly adjacent to the tumour). Other trials found no late toxicity >°2 at all [57]. Even in the elderly population, good clinical results could be maintained without increased toxicity [58].
Table 6. HCC.
| Patients | Dose | Tumour diameter | Local control rate | Overall survival rate | |
| Bush et al [42] | 34 | Protons63 GyE (in 15 Fx) | 5.7 cm | 75% at 2 years | 55% at 2 years |
| Kawashima et al [55] | 30 | Protons[70 GyE (in 20 Fx)] | 4.5 cm | PFS: 96% at 2 years | 66% at 2 years |
| Kato et al [56] | 24 | 12C[49.5–79.5 GyE(3.3–5.3 GyE/Fx)] | 81% at 5 years | 25% at 5 years | |
| Mizumoto et al [57] | 3 | Protons[72.6 GyE (in 22 Fx)] | 86% at 3 years | 45.1% at 3 years | |
| Hata et al [58] | 21 | Protons[24 GyE (in 1 Fx)] | Not given | 100% | |
| Chiba et al [59] | 162 | Protons[72 GyE (in 16 Fx)] | 90% at 5 years | 23.5% at 5 years |
Fx, fraction; PFS, progression-free survival.
No significant change in liver function was found after 12C therapy [56] or when the liver tolerance of patients with liver cirrhosis was evaluated retrospectively after proton therapy [60]. Similar to surgical treatment, post-therapeutic hypertrophic changes were found in normal liver tissue after treatment. Hypertrophy varied between 19% and 51% and correlated significantly to the irradiated liver volume and initial functioning liver volume.
Even re-irradiation after prior proton radiotherapy (median dose 72 GyE) was tolerated reasonably well [61]. In view of liver reserves, however, the recommendation is that patients undergoing re-irradiation for HCC should not suffer from cirrhotic changes classified as worse than Child–Pugh A [61].
Probably the largest analysis of particle therapy in patients (n=162) with HCC was performed by Chiba and co-workers [59]. Control rates supported the excellent results, while acute and late side effects were negligible and did not influence the patients' prognosis.
Compared with photon therapy, particle therapy yields superior therapeutic outcomes. Only the most modern techniques (i.e. stereotactic radiosurgery) come close to providing the results offered by particle therapy [62,63], although data concerning photon radiotherapy for HCC are still more abundant than those for particle techniques. Future trials need to explore the role of particle therapy in this disease. As for NSCLC, carbon-ion therapy may be an equally good treatment option for patients who are not fit enough for surgical procedures.
Pancreatic carcinoma
The role of radiotherapy in the treatment of pancreatic carcinoma still needs to be determined, but this is another rather radio-resistant tumour, hence the possible benefits of particle therapy, with the advantage of higher RBE, seem obvious. Three Phase I/II trials for carbon-ion therapy have been initiated in Chiba, but these are still in follow-up and not yet completely published.
One dose-escalation (44.8–48 GyE in 16 Fx) trial is evaluating neoadjuvant 12C therapy for operable pancreatic carcinomas [64]. To date, the reported side effects have generally been mild, with no acute toxicities >°3. Only two Grade °3 toxicities have been found, and these were post-operative portal vein stenoses that could not safely be attributed to radiation therapy alone. LC rates at 1 and 2 years were both an impressive 100%, OS at 2 years was 62%. Resectability after heavy-ion therapy proved to be 68%, and actuarial OS was much higher (90.1%) [64]. A trial is now underway to evaluate hypofractionated carbon-ion therapy in a neoadjuvant setting (particle therapy with subsequent resection). In this study, which is still recruiting and has not published initial results, pre-operative particle therapy consists of 8 Fx over 2 weeks.
The group from Chiba investigated the application of 12C therapy (38.4–52.8 GyE in 12 Fx dose escalation) in locally advanced, inoperable pancreatic carcinoma. Among the 47 patients recruited to the trial, 7 Grade 3 acute reactions (6 patients with anorexia, 1 with cholangitis) and only 1 °3 late reaction were reported. The follow-up period for this trial is still short, hence only preliminary data are available: at 1 year, OS is 43% and LC at doses >45.6 GyE is 95% [65].
Early data from the University of California, Berkeley, comparing helium and photon irradiation in 49 patients reported superior LC rates for helium therapy, although increased LC did not translate into increased survival in this cohort [66]. Although the protocol applied combined chemoradiation, the treatment regimen consisted of a split-course radiation treatment, which would not be state of the art today. Furthermore, the treatment planning was not performed three-dimensionally (for either helium or photon treatments), and so care is needed when applying these results in a modern setting.
A final assessment of the applicability of particle therapy for pancreatic carcinoma is not possible on the basis of these results. The systematic approach to this question undertaken by the Japanese investigators could, however, clearly demonstrate the feasibility of this treatment for operable and locally advanced disease. The clinical results seen to date appear to be superior to those achieved in comparable photon trials, with a very favourable toxicity profile.
Carcinoma of the uterine cervix
Chemoradiation has long been established for the treatment of cervical carcinoma. Standard treatment includes external beam radiotherapy and/or brachytherapy. Further dose escalation, by the application of particle therapy, has been investigated in a number of studies. Kato and co-workers published two Phase I–II trials (involving 44 and 94 patients, respectively) on the treatment with carbon ions [66-68]. All patients were diagnosed with locally advanced carcinoma of the cervix (median diameter 6.5 cm). Treatment consisted of pelvic irradiation (16 Fx) followed by a boost to the primary tumour (8 Fx). Total doses varied between 52 and 72 GyE [67,68], and resulted in 5-year LC rates of 45% [68] and 79% [67], respectively. There were no serious acute treatment-related toxicities >°2, but two patients receiving doses >60 GyE to the intestine needed surgical interventions because of late gastrointestinal complications, as a result doses have since been limited to <60 GyE.
Proton therapy for carcinoma of the uterine cervix has been evaluated by Kagei et al [69] in patients receiving pelvic irradiation (photons at 44.8 GyE) plus 24 or 28 GyE proton boosts to the tumour. LC at 5 years and OS at 10 years in this trial were 100% and 89%, respectively, for IIB-scored tumours, and 61% and 40%, respectively, for IIIB/IVA tumours.
The clinical influence of oxygenation for photon therapy was clearly demonstrated by Dunst et al [70]. However, many pre-clinical investigations have found no therapy-relevant influence of tumour hypoxia for treatments involving high linear energy transfer radiation. Clinical data published by Kato et al [67] in advanced cervical carcinoma could now prove this thesis clinically.
Although we lack major trials to establish firmly the applicability of particle therapy in this indication, initial results are very promising. The independence of tumour oxygenation may be a major advantage offered by carbon-ion therapy that warrants further exploration.
Extracranial sarcoma and chondrosarcoma
Sarcomas and chordomas are relatively radio-resistant and, therefore, present ideal targets for particle therapy. However, no prospective studies have been published on the treatment of these tumours by particle therapy. Retrospective data indicate that high control rates can be achieved by a combination of photon and proton irradiation, especially in treatment of the primary situation rather than local relapses. At a median follow-up of 8.8 years, tumours in 12 of 14 patients were still locally controlled and doses >73 GyE led to continuing LC [71].
Post-operative proton therapy (median 72 GyE) for extracranial chordoma in 26 patients (15 pelvic tumours) resulted in an actuarial OS of 84% at 3 years and PFS of 77% [72]. When 30 patients with unresectable sacral chordoma were treated with carbon ions (52.8–73.6 GyE), LC and OS at 5 years were reported at 96% and 52%, respectively [73].
Prostate cancer
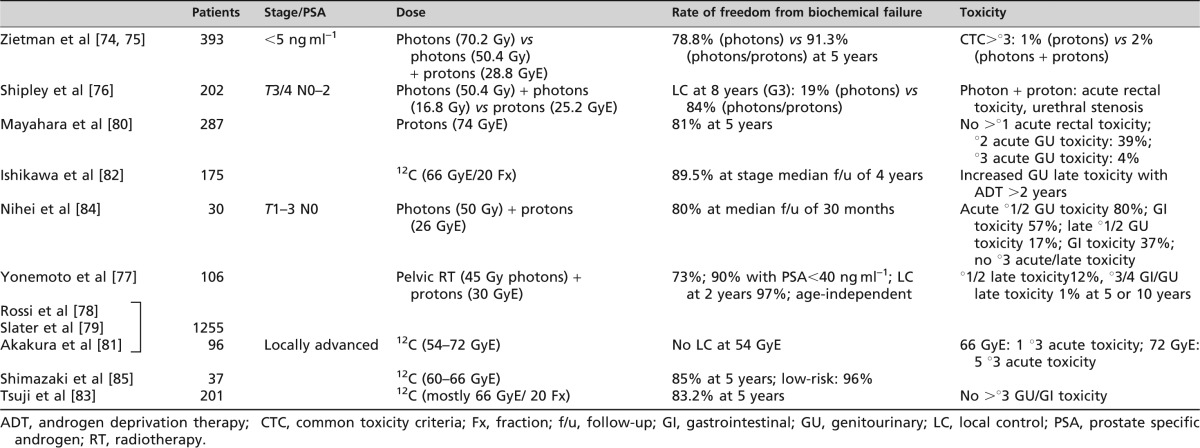
A variety of clinical data have been published on the use of particle therapy for prostate carcinoma, including the results of two Phase III trials [73-75]. Zietman et al [74,75] treated 393 patients with prostate carcinoma [prostate-specific antigen (PSA) <5 ng ml–1] with either photons alone or combined photon–proton therapy. The trial by Shipley et al [76] had a similar design but recruited 202 patients with Stage T3–4 N0–2 tumours. Both of these trials demonstrated superior freedom from biochemical relapse and LC rate in the proton-treated groups, albeit these groups were treated to higher total doses in both trials (Table 7). Yonemoto et al [77], Rossi et al [78] and Slater et al [79] published data on 1255 patients treated with a combined approach using 45 Gy photons to the pelvis followed by a 30 GyE proton boost to the prostate. This regimen also yielded very good results, with a freedom from biochemical relapse rate of 73% or even 90% in patients with initial PSA<90%. Another trial employed protons alone, but the follow-up for this study is not yet mature and hence only toxicity data are currently available [80]. No Phase III trial results are available for the treatment of prostate carcinoma with carbon ions, but there is a considerable amount of data for heavy-ion therapy of this disease. Doses of 54–72 GyE in various fractionations [80-82] have yielded similar results. However, 54 GyE 12C was found to be insufficient to obtain lasting LC, whereas doses of 72 GyE seemed to cause higher acute toxicity rates in a dose escalation trial [80]. Treatment-related toxicity was, however, generally very mild in all the trials (Table 7). CTC grade °3 late toxicities were very rare (1% at 5 and 10 years in the Loma Linda series), and hence both treatment outcomes and toxicity profiles are very much in favour of particle therapy. Biochemical relapse-free survival is dose-dependent; hence, the necessity for dose escalation with tolerable side effects has led to the routine use of IMRT for primary prostate cancer at many institutions. Further step-wise dose escalation will be limited largely by accompanying toxicity. As prostate cancer is a common disease, clinical trials will need to determine the significance of observed benefits in terms of relapse-free survival and toxicity in controlled clinical settings.
Table 7. Prostate carcinoma.
Summary and perspectives
In summary, irradiation with protons and heavy ions does indeed seem to have advantages over irradiation with photons. The larger series reviewed here have shown the benefits of this technology, and particle therapy has become an established treatment for uveal melanoma, for chordoma or chondrosarcoma of the skull base and for ACC. There are also promising data in support of the single-fraction treatment of early-stage NSCLC, HCC and prostate carcinoma. However, Phase III trials comparing photon and particle therapy or even proton and heavy-ion therapies are largely pending. This is primarily explained by the complexity and limited availability of particle therapy. In addition, a lot of these tumours have a comparatively low incidence, so systematic randomised controlled trials are not possible, even in the photon world.
So the question remains: do we need more trials, among them randomised controlled trials, to prove the clinical benefit of particle therapy? This discussion is repetitively revived whenever new treatment techniques become available, first with the introduction of linear accelerators and again when IMRT became more widely available. There is definitely good reason to apply more sophisticated new techniques (i.e. particle therapy), but it still needs to be proven that the advantages in dose distribution translate into measurable clinical benefits, such as improvement in OS or quality of life. Even for IMRT, which has gained more or less general acceptance, at least in head and neck and prostate cancer treatment, improvement in OS has only been proven retrospectively, and even then only for head and neck tumours [85]. Physicians, however, are required to make evidence-based decisions in almost every field of medicine. Should we deviate from this principle in adopting particle therapy without clinical evidence to support it? On the other hand, can randomised controlled trials be ethical when the dose distributions provided by particle therapy are obviously superior to those offered by other treatments?
New techniques also come with certain challenges: motion management and the radiobiological properties of particle beams, as well as fractionation patterns for this comparatively new technique, are still issues needing further investigation. In addition, more widespread introduction of particle therapy will increase financial pressure on the respective country's health system.
The Heidelberg approach will be to include most patients in prospective clinical trials. Although there is considerable evidence to support particle therapy, albeit mostly evidence base level 2b, the published data are very heterogeneous even within the same indications. For many tumours, there is evidence to support both proton and carbon-ion therapy and we need to determine which to choose for each individual patient if both are available. Chemoradiation has been established in the treatment of various tumours throughout the years, this has yet to be evaluated concomitantly with proton or heavy-ion therapy. Very little is known about the clinical interactions between systemic therapy and particle irradiation in terms of efficacy and toxicity. New indications could be opened up to treatment with particle beams, and hence there will need to be Phase I/II trials for these entities. We would, however, strongly emphasise the scientific aspiration to produce meaningful results for patients and physicians for the future.
In the tradition of Vincenz Czerny, international efforts may be necessary to achieve this goal, and hence various projects to this effect are welcome and currently in preparation.
References
- 1.Castro JR, Char DH, Petti PL, Daftari IK, Quivey JM, Singh RP, et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 1997;39:989–96 [DOI] [PubMed] [Google Scholar]
- 2.Castro JR, Linstadt DE, Bahary JP, Petti PL, Daftari I, Collier JM, et al. Experience in charged particle irradiation of tumors of the skull base: 1977–1992. Int J Radiat Oncol Biol Phys 1994;29:647–55 [DOI] [PubMed] [Google Scholar]
- 3.Linstadt D, Quivey JM, Castro JR, Andejeski Y, Phillips TL, Hannigan J, et al. Comparison of helium-ion radiation therapy and split-course megavoltage irradiation for unresectable adenocarcinoma of the pancreas. Final report of a Northern California Oncology Group randomized prospective clinical trial. Radiology 1988;168:261–4 [DOI] [PubMed] [Google Scholar]
- 4.Saunders W, Castro JR, Chen GT, Collier JM, Zink SR, Pitluck S, et al. Helium-ion radiation therapy at the Lawrence Berkeley Laboratory: recent results of a Northern California Oncology Group Clinical Trial. Radiat Res Suppl 1985;8:S227–34 [PubMed] [Google Scholar]
- 5.Schulz-Ertner D, Nikoghosyan A, Hof H, Didinger B, Combs SE, Jäkel O, et al. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys 2007;67:171–7 [DOI] [PubMed] [Google Scholar]
- 6.Schulz-Ertner D, Karger CP, Feuerhake A, Nikoghosyan A, Combs SE, Jäkel O, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys 2007;68:449–57 [DOI] [PubMed] [Google Scholar]
- 7.Schulz-Ertner D, Nikoghosyan A, Didinger B, Münter M, Jäkel O, Karger CP, et al. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer 2005;104:338–44 [DOI] [PubMed] [Google Scholar]
- 8.Nutting C, A'Hern R, Rogers MS, Sydenham MA, Adab F, Harrington K, et al. First results of a phase III multicenter randomized controlled trial of intensity modulated (IMRT) vs conventional radiotherapy (RT) in head and neck cancer (PARSPORT: ISRCTN48243537; CRUK/03/005). PORC ASCO 2009, abstract # LBA6006.
- 9.Casali PG, Messina A, Stacchiotti S, Tamborini E, Crippa F, Gronchi A, et al. Imatinib mesylate in chordoma. Cancer 2004;101:2086–97 [DOI] [PubMed] [Google Scholar]
- 10.Char DH, Quivey JM, Castro JR, Kroll S, Phillips T. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology 1993;100:1547–54 [DOI] [PubMed] [Google Scholar]
- 11.Castro JR, Char DH, Petti PL, Daftari IK, Quivey JM, Singh RP, et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 1997;39:989–96 [DOI] [PubMed] [Google Scholar]
- 12.Courdi A, Caujolle JP, Grange JD, Diallo-Rosier L, Sahel J, Bacin F, et al. Results of proton therapy of uveal melanomas treated in Nice. Int J Radiat Oncol Biol Phys 1999;45:5–11 [DOI] [PubMed] [Google Scholar]
- 13.Gragoudas ES, Lane AM, Regan S, Li W, Judge HE, Munzenrider JE, et al. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol 2000;118:773–8 [DOI] [PubMed] [Google Scholar]
- 14.Fuss M, Loredo LN, Blacharski PA, Grove RI, Slater JD. Proton radiation therapy for medium and large choroidal melanoma: preservation of the eye and its functionality. Int J Radiat Oncol Biol Phys 2001;49:1053–9 [DOI] [PubMed] [Google Scholar]
- 15.Desjardins L, Lumbroso L, Levy C, Mazal A, Delacroix S, Rosenwald JC, et al. Treatment of uveal melanoma with iodine 125 plaques or proton beam therapy: indications and comparison of local recurrence rates. J Fr Ophtalmol 2003;26:269–76 [PubMed] [Google Scholar]
- 16.Höcht S, Bechrakis NE, Nausner M, Kreusel KM, Kluge H, Heese J, et al. Proton therapy of uveal melanomas in Berlin. 5 years of experience at the Hahn–Meitner Institute. Strahlenther Onkol 2004;180:419–24 [DOI] [PubMed] [Google Scholar]
- 17.Damato B, Kacperek A, Chopra M, Campbell IR, Errington RD. Proton beam radiotherapy of iris melanoma. Int J Radiat Oncol Biol Phys 2005;63:109–15 [DOI] [PubMed] [Google Scholar]
- 18.Dendale R, Lumbroso-Le Rouic L, Noel G, Feuvret L, Levy C, Delacroix S, et al. Proton beam radiotherapy for uveal melanoma: results of Curie Institut–Orsay proton therapy center (ICPO). Int J Radiat Oncol Biol Phys 2006;65:780–7 [DOI] [PubMed] [Google Scholar]
- 19.Tsuji H, Ishikawa H, Yanagi T, Hirasawa N, Kamada T, Mizoe JE, et al. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a Phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys 2007;67:857–62 [DOI] [PubMed] [Google Scholar]
- 20.Egger E, Zografos L, Schalenbourg A, Beati D, Böhringer T, Chamot L, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 2003;55:867–80 [DOI] [PubMed] [Google Scholar]
- 21.Debus J, Schulz-Ertner D, Schad L, Essig M, Rhein B, Thillmann CO, et al. Stereotactic fractionated radiotherapy for chordomas and chondrosarcomas of the skull base. Int J Radiat Oncol Biol Phys 2000;47:591–6 [DOI] [PubMed] [Google Scholar]
- 22.Romero J, Cardenes H, la Torre A, Valcarcel F, Magallon R, Regueiro C, et al. Chordoma: results of radiation therapy in eighteen patients. Radiother Oncol 1993;29:27–32 [DOI] [PubMed] [Google Scholar]
- 23.Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999;175:57–63 [DOI] [PubMed] [Google Scholar]
- 24.Noel G, Habrand JL, Mammar H, Pontvert D, Haie-Meder C, Hasboun D, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base: the Centre de Protontherapie D'Orsay experience. Int J Radiat Oncol Biol Phys 2001;51:392–8 [DOI] [PubMed] [Google Scholar]
- 25.Weber DC, Ritz HP, Pedroni ES, Bolsi A, Timmermann B, Verwey J, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: the Paul Scherrer Institut experience. Int J Radiat Oncol Biol Phys 2005;63:401–9 [DOI] [PubMed] [Google Scholar]
- 26.Terahara A, Niemierko A, Goitein M, Finkelstein D, Hug E, Liebsch N, et al. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. Int J Radiat Oncol Biol Phys 1999;45:351–8 [DOI] [PubMed] [Google Scholar]
- 27.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986;8:177–84 [DOI] [PubMed] [Google Scholar]
- 28.Chen AM, Granchi PJ, Garcia J, Bucci MK, Fu KK, Eisele DW. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 2007;67:982–7 [DOI] [PubMed] [Google Scholar]
- 29.Gurney TA, Eisele DW, Weinberg V, Shin E, Lee N. Adenoid cystic carcinoma of the major salivary glands treated with surgery and radiation. Laryngoscope 2005;115:1278–82 [DOI] [PubMed] [Google Scholar]
- 30.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck 2004;26:154–62 [DOI] [PubMed] [Google Scholar]
- 31.Chen AM, Bucci MK, Weinberg V, Garcia J, Quivey JM, Schechter NR, et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: prognostic features of recurrence. Int J Radiat Oncol Biol Phys 2006;66:152–9 [DOI] [PubMed] [Google Scholar]
- 32.Garden AS, Weber RS, Ang KK, Morrison WH, Matre J, Peters LJ. Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer 1994;73:2563–9 [DOI] [PubMed] [Google Scholar]
- 33.Terhaard CHJ, Lubsen H, Van derTweel I, Hilgers FJM, Eijkenboom WMH, Marres HAM, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck 2004;26:681–92; discussion 692–3 [DOI] [PubMed] [Google Scholar]
- 34.Bittner N, Koh WJ, Laramore GE, Patel S, Mulligan MS, Douglas JG. Treatment of locally advanced adenoid cystic carcinoma of the trachea with neutron radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:410–14 [DOI] [PubMed] [Google Scholar]
- 35.Douglas JG, Koh WJ, Austin-Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg 2003;129:944–8 [DOI] [PubMed] [Google Scholar]
- 36.Mizoe JE, Tsujii H, Kamada T, Matuoka Y, Tsuji H, Osaka Y, et al. Dose escalation study of carbon ion radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004;60:358–64 [DOI] [PubMed] [Google Scholar]
- 37.Münter MW, Schulz-Ertner D, Hof H, Nikoghosyan A, Jensen A, Nill A, et al. Inverse planned stereotactic intensity modulated radiotherapy (IMRT) in the treatment of incompletely and completely resected adenoid cystic carcinomas of the head and neck: initial clinical results and toxicity of treatment. Radiat Oncol 2006;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber PE, Debus J, Latz D, Zierhut D, Bischof M, Wannenmacher M, et al. Radiotherapy for advanced adenoid cystic carcinoma: neutrons, photons or mixed beam? Radiother Oncol 2001;59:161–7 [DOI] [PubMed] [Google Scholar]
- 39.Pommier P, Liebsch NJ, Deschler DG, Lin DT, McIntyre JF, Barker FG, et al. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg 2006;132:1242–9 [DOI] [PubMed] [Google Scholar]
- 40.Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 1987;59:1874–81 [DOI] [PubMed] [Google Scholar]
- 41.Bush DA, Slater JD, Bonnet R, Cheek GA, Dunbar RD, Moyers M, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest 1999;116:1313–19 [DOI] [PubMed] [Google Scholar]
- 42.Bush DA, Slater JD, Shin BB, Cheek GC, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest 2004;126:1198–203 [DOI] [PubMed] [Google Scholar]
- 43.Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:107–11 [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto T, Yamamoto N, Nishimura H, Koto M, Tsujii H, Mizoe JE, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 2003;66:127–40 [DOI] [PubMed] [Google Scholar]
- 45.Koto M, Miyamoto T, Yamamoto N, Nishimura H, Yamada S, Tsujii H, et al. Local control and recurrence of stage I non-small cell lung cancer after carbon ion radiotherapy. Radiother Oncol 2004;71:147–56 [DOI] [PubMed] [Google Scholar]
- 46.Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, et al. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys 2007;67:750–8 [DOI] [PubMed] [Google Scholar]
- 47.Nishimura H, Miyamoto T, Yamamoto N, Koto M, Sugimura K, Tsujii H. Radiographic pulmonary and pleural changes after carbon ion irradiation. Int J Radiat Oncol Biol Phys 2003;55:861–6 [DOI] [PubMed] [Google Scholar]
- 48.Kadono K, Homma T, Kamahra K, Nakayama M, Satoh H, Sekizawa K, et al. Effect of heavy-ion radiotherapy on pulmonary function in stage I non-small cell lung cancer patients. Chest 2002;122:1925–32 [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol 2007;2:916–26 [DOI] [PubMed] [Google Scholar]
- 50.Hata M, Tokuuye K, Kagei K, Sugahara S, Nakayama H, Fukumitsu N, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys 2007;68:786–93 [DOI] [PubMed] [Google Scholar]
- 51.Shioyama Y, Tokuuye K, Okumura T, Kagei K, Sugahara S, Ohara K, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:7–13 [DOI] [PubMed] [Google Scholar]
- 52.Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology 2004;127:S189–93 [DOI] [PubMed] [Google Scholar]
- 53.Sugahara S, Tokuuye K, Okumura T, Nakahara A, Saida Y, Kagei K, et al. Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys 2005;61:76–84 [DOI] [PubMed] [Google Scholar]
- 54.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–74 [DOI] [PubMed] [Google Scholar]
- 55.Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005;23:1839–46 [DOI] [PubMed] [Google Scholar]
- 56.Kato H, Tsujii H, Miyamoto T, Mizoe JE, Kamada T, Tsuji H, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys 2004;59:1468–76 [DOI] [PubMed] [Google Scholar]
- 57.Mizumoto M, Tokuuye K, Sugahara S, Hata M, Fukumitsu N, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma with inferior vena cava tumor thrombus: report of three cases. Jpn J Clin Oncol 2007;37:459–62 [DOI] [PubMed] [Google Scholar]
- 58.Hata M, Tokuuye K, Sugahara S, Tohno E, Fukumitsu N, Hashimoto T, et al. Proton irradiation in a single fraction for hepatocellular carcinoma patients with uncontrollable ascites. Technical considerations and results. Strahlenther Onkol 2007;183:411–16 [DOI] [PubMed] [Google Scholar]
- 59.Chiba T, Tokuuye K, Matsuzaki Y, Sugahara S, Chugannji Y, Kagei K, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res 2005;11:3799–805 [DOI] [PubMed] [Google Scholar]
- 60.Ohara K, Okumura T, Tsuji H, Chiba T, Min M, Tatsuzaki H, et al. Radiation tolerance of cirrhotic livers in relation to the preserved functional capacity: analysis of patients with hepatocellular carcinoma treated by focused proton beam radiotherapy. Int J Radiat Oncol Biol Phys 1997;38:367–72 [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto T, Tokuuye K, Fukumitsu N, Igaki H, Hata M, Kagei K, et al. Repeated proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2006;65:196–202 [DOI] [PubMed] [Google Scholar]
- 62.Robertson JM, Lawrence TS, Dworzanin LM, Andrews JC, Walker S, Kessler ML, et al. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol 1993;11:1286–93 [DOI] [PubMed] [Google Scholar]
- 63.Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861–70 [DOI] [PubMed] [Google Scholar]
- 64.Yamada S, Kato H, Yamaguchi K, Kitabayashi H, Tsujii H, Saisyo H, et al. Carbon ion therapy for patients with localized, resectable adenocarcinoma of the pancreas. Proc ASCO GI 2005; Abstract #130. [Google Scholar]
- 65.Yamada S, Hara R. Carbon ion radiotherapy for pancreas cancer. Proceedings of the NIRS-MD Anderson symposium on clinical issues for particle therapy. NIRS M.D. Anderson Symposium, 3/2008. [Google Scholar]
- 66.Linstadt D, Quivey JM, Castro JR, Andejeski Y, Phillips TL, Hannigan J, et al. Comparison of helium-ion radiation therapy and split-course megavoltage irradiation for unresectable adenocarcinoma of the pancreas. Final report of a Northern California Oncology Group randomized prospective clinical trial. Radiology 1988;168:261–4 [DOI] [PubMed] [Google Scholar]
- 67.Kato S, Ohno T, Tsujii H, Nakano T, Mizoe JE, Kamada T, et al. Dose escalation study of carbon ion radiotherapy for locally advanced carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 2006;65:388–97 [DOI] [PubMed] [Google Scholar]
- 68.Matsushita K, Ochiai T, Shimada H, Kato S, Ohno T, Nikaido T, et al. The effects of carbon ion irradiation revealed by excised perforated intestines as a late morbidity for uterine cancer treatment. Surg Today 2006;36:692–700 [DOI] [PubMed] [Google Scholar]
- 69.Kagei K, Tokuuye K, Okumura T, Ohara K, Shioyama Y, Sugahara S, et al. Long-term results of proton beam therapy for carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 2003;55:1265–71 [DOI] [PubMed] [Google Scholar]
- 70.Dunst J, Kuhnt T, Strauss HG, Krause U, Pelz T, Koelbl H, et al. Anemia in cervical cancers: impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol Biol Phys 2003;56:778–87 [DOI] [PubMed] [Google Scholar]
- 71.Park L, DeLaney TF, Liebsch NJ, Hornicek FJ, Goldberg S, Mankin H, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys 2006;65:1514–21 [DOI] [PubMed] [Google Scholar]
- 72.Rutz HP, Weber DC, Sugahara S, Timmermann B, Lomax AJ, Bolsi A, et al. Extracranial chordoma: outcome in patients treated with function-preserving surgery followed by spot-scanning proton beam irradiation. Int J Radiat Oncol Biol Phys 2007;67:512–20 [DOI] [PubMed] [Google Scholar]
- 73.Imai R, Kamada T, Tsuji H, Yanagi T, Baba M, Miyyamoto T, et al. Carbon ion radiotherapy for unresectable sacral chordomas. Clin Cancer Res 2004;10:5741–6 [DOI] [PubMed] [Google Scholar]
- 74.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005;294:1233–9 [DOI] [PubMed] [Google Scholar]
- 75.Zietman AL. Correction: inaccurate analysis and results in a study of radiation therapy in adenocarcinoma of the prostate. JAMA 2008;299:898–9 [DOI] [PubMed] [Google Scholar]
- 76.Shipley WU, Verhey LJ, Munzenrider JE, Suit HD, Urie MM, McManus PL, et al. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys 1995;32:3–12 [DOI] [PubMed] [Google Scholar]
- 77.Yonemoto LT, Slater JD, Rossi CJ, Jr, Antoine JE, Loredo L, Archambeau JO, et al. Combined proton and photon conformal radiation therapy for locally advanced carcinoma of the prostate: preliminary results of a phase I/II study. Int J Radiat Oncol Biol Phys 1997;37:21–9 [DOI] [PubMed] [Google Scholar]
- 78.Rossi CJ, Jr, Slater JD, Yonemoto LT, Jabola BR, Bush DA, Levy RP, et al. Influence of patient age on biochemical freedom from disease in patients undergoing conformal proton radiotherapy of organ-confined prostate cancer. Urology 2004;64:729–32 [DOI] [PubMed] [Google Scholar]
- 79.Slater JD, Rossi CJ, Jr, Yonemoto LT, Bush DA, Jabola BR, Levy RP, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys 2004;59:348–52 [DOI] [PubMed] [Google Scholar]
- 80.Mayahara H, Murakami M, Kagawa K, Kawaguchi A, Oda Y, Miyawaki D, et al. Acute morbidity of proton therapy for prostate cancer: the Hyogo Ion Beam Medical Center experience. Int J Radiat Oncol Biol Phys 2007;69:434–43 [DOI] [PubMed] [Google Scholar]
- 81.Akakura K, Tsujii H, Morita S, Tsuji H, Yagashita T, Isaka S, et al. Phase I/II clinical trials of carbon ion therapy for prostate cancer. Prostate 2004;58:252–8 [DOI] [PubMed] [Google Scholar]
- 82.Ishikawa H, Tsuji H, Kamada T, Hirasawa N, Yanagi T, Mizoe JE, et al. Adverse effects of androgen deprivation therapy on persistent genitourinary complications after carbon ion radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2008;72:78–84 [DOI] [PubMed] [Google Scholar]
- 83.Tsuji H, Yanagi T, Ishikawa H, Kamada T, Mizoe JE, Kanai T, et al. Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys 2005;63:1153–60 [DOI] [PubMed] [Google Scholar]
- 84.Nihei K, Ogino T, Ishikura S, Kawashima M, Nishimura H, Arahira S, et al. Phase II feasibility study of high-dose radiotherapy for prostate cancer using proton boost therapy: first clinical trial of proton beam therapy for prostate cancer in Japan. Jpn J Clin Oncol 2005;35:745–52 [DOI] [PubMed] [Google Scholar]
- 85.Shimazaki J, Akakura K, Suzuki H, Ichikawa T, Tsuji H, Ishikawa H, et al. Monotherapy with carbon ion radiation localized prostate cancer. Jpn J Clin Oncol 2006;36:290–4 [DOI] [PubMed] [Google Scholar]