Abstract
The National Institute of Radiological Sciences in Chiba, Japan has offered carbon ion radiotherapy (CIRT) since 1994 using carbon ion beams generated by the heavy ion medical accelerator in Chiba (HIMAC). The total number of cases treated with the HIMAC exceeded 5000 in July 2009. Here, we present a retrospective analysis of CIRT for sacral chordoma. The study included 95 patients with medically unresectable sacral chordomas treated between 1996 and 2007. The median age of the patients was 66 years. Of all the patients, 84 had not been treated previously and 11 had a locally recurrent tumour following previous resection. The carbon ion dose ranged from 52.8 to 73.6 GyE (median 70.4 GyE) in a total of 16 fixed fractions over 4 weeks. The median clinical target volume was 370 cm3. The overall survival rate at 5 years for all 95 patients was 86%, and follow-up survival time was 42 months (range, 13–112 months). The 5-year local control rate was 88% and median time to local failure was 35 months (range, 13–60 months). Of the 95 patients, 91% remained ambulatory with or without a supportive device. Two patients experienced severe skin or soft tissue complications requiring skin grafts. 15 patients experienced severe sciatic nerve complications requiring continuing medication. CIRT appears effective and safe in the management of patients with sacral chordoma and offers a promising alternative to surgery.
The National Institute of Radiological Sciences (NIRS) began using photon beams for cancer therapy in 1961. Fast neutron therapy was used between 1975 and 1994, and proton therapy began in 1975. Building upon its accumulated skills and knowledge related to particle therapy, the NIRS launched heavy ion radiotherapy in June 1994 using carbon ion beams generated by the heavy ion medical accelerator in Chiba (HIMAC). The clinical trials were initiated by conducting Phase I/II dose-escalation studies on various types of tumours treated with carbon ion radiotherapy (CIRT). Their aim was to verify the safety of CIRT, to evaluate its anti-tumour effects and to identify the types of tumours eliminated more effectively by CIRT than by sophisticated conformal photon therapy.
All patients were treated according to clinical protocols (Phase I/II or Phase II) previously approved by the Ethics Committee of the NIRS. It has been our policy to use carbon ion beams to their best advantage, thereby reducing treatment time to less than that required for conventional treatment. In October 2003, the total number of patients treated with CIRT reached 1674, with a total of 1742 lesions. After completing these trials, CIRT was approved by the Japanese Ministry of Health in October 2003 as highly advanced medical technology (HAMT). In October 2006, the Health Insurance Law was partially changed and the name “HAMT” was changed to “Advanced Medicine”. This level of approval is an intermediate step to being approved as a treatment under the national health insurance system in Japan. In the near future, the treatment of some cancers with CIRT will be covered by the national health insurance system [1,2]. In July 2009, the number of cases treated with CIRT exceeded 5000. The HIMAC was the first medically dedicated heavy ion accelerator worldwide. Subsequently, two other facilities started to offer CIRT: in 1997 a facility opened at the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany; in 2004, another opened at the Hyogo Ion Beam Medical Centre in Hyogo, Japan; and finally in 2006 an accelerator opened at the Institute of Modern Physics Lanzhou in China [3]. In 2005, the NIRS succeeded in developing a synchrotron that is smaller than the HIMAC and designed to make CIRT more widely available [4].
The major cancers that have been treated with CIRT at NIRS are prostate cancer, bone and soft tissue sarcoma, head and neck cancer, recurring rectal cancer, lung cancer and hepatocellular carcinoma [1,2]. CIRT for bone and soft tissue sarcomas at NIRS was started in 1996 as a Phase I/II dose-escalation study for medically inoperable cases. Details of the study have been described in previous articles [1,2,5]. Until February 2000, only 64 tumours in 57 patients were treated. Following the trial, a Phase II fixed-dose clinical trial for medically inoperable cases and a HAMT trial with the same clinical eligibility criteria as the Phase II fixed-dose clinical trial were conducted between April 2000 and February 2009; 387 patients with 406 tumours were enrolled. The majority of these patients had tumours arising from the pelvis, spine, paraspinal and retroperitoneal regions (Table 1).
Table 1. Characteristics of patients with bone and soft tissue sarcomas treated in National Institute of Radiological Sciences trials.
| Characteristics | n |
| Number of tumours | 406 (387 patients) |
| Sex | |
| Male | 223 |
| Female | 164 |
| Tumour sites | |
| Pelvis | 305 |
| Spine/paraspine | 75 |
| Extremities etc. | 26 |
| Histology | |
| Bone | 303 |
| Chordoma | 134 |
| Chondrosarcoma | 60 |
| Osteosarcoma | 60 |
| Others | 49 |
| Soft tissue | 84 |
| Irradiation dose (GyE total in 16 fractions) | |
| 64.0 | 27 |
| 67.2 | 43 |
| 70.4 | 326 |
| 73.6 | 10 |
Carbon ion beams
The HIMAC has a 100 m linear accelerator and a synchrotron of about 40 m in diameter. It accelerates carbon ions to 800 MeV n–1, which is almost 80% of the speed of light. Beams of three different energies (290, 350 and 400 MeV n–1) are available for use as vertical beams (290 MeV n–1, 350 MeV n–1) or horizontal beams (290 MeV n–1, 400 MeV n–1) in treatment. The water-equivalent path length of all three energy beams ranges between 15 and 25 cm. For modulation of the Bragg peak to conform to a target volume, the beam lines for treatment are equipped with a pair of wobbler magnets, beam scatters, ridge filters, multileaf collimators and a compensation bolus. The ridge filter is designed to produce biologically equal effects along the spread-out Bragg peak (SOBP). The compensation bolus is fabricated for each patient to make the distal configuration of the SOBP similar to the irregular shape of any target volume. The collimator is used to define the lateral outline of the target volume [6-8].
Before the clinical studies began, radiobiological studies were carried out in vitro and with mouse skins to estimate the biological effectiveness values relative to the megavoltage of photons or fast neutrons. The relative biological effectiveness (RBE) of carbon ions varies with depth, therefore the point at which the RBE value is specified has to be reported. On the basis of linear energy transfer (LET) comparisons, this point was taken at NIRS to be in the distal part of the SOBP. Irrespective of the size of the SOBP, the RBE value of carbon ions for acute skin reaction was assessed to be 3.0. Several ridge fitters were designed to produce a physical dose gradient that makes the biological effect along the SOBP more uniform. These improvements were made on the basis of the biological response of human salivary gland tumour cells [6]. To express carbon doses in terms comparable with the megavoltage of photon beams, the biologically effective dose of carbon beams is measured in GyE [or cobalt gray equivalent (CGE)], defined as the absorbed carbon physical dose (Gy) multiplied by the RBE value. Although the RBE value may differ for the various types of tissue and time-dose fractionations employed, a single RBE value of 3.0 was used at NIRS to calculate GyE [6-8].
Chordoma
Between 1% and 4% of primary bone tumours are chordomas, with >50% of these being sacral chordomas [9]. Chordomas, which arise from notochordal remnants, have slower local growth and metastasise less frequently than other bone and soft-tissue malignant tumours. Surgery is the mainstay of treatment for sacral chordomas. Complete radical resection produces both longer continuous local control and an extended disease-free period compared with subtotal resection, but by the time symptoms first appear, chordomas are often already too large to be removed completely [10-14]. Chordoma has poor sensitivity to photon radiotherapy, although some studies have reported that photon radiation therapy delays recurrence after incomplete resection and can relieve symptoms caused by recurrences [15]. Sacral chordomas also have poor sensitivity to chemotherapy [9]; they are almost impossible to cure without surgery and there is little possibility of survival. Hence, chordoma is considered to be one of the most challenging mesenchymal tumours to treat effectively. CIRT has the physical advantage of good dose distribution. With high LET and high Bragg peak intensity, it is expected to be safer and more effective for the treatment of sacral chordomas than low-LET radiation, such as photon radiation.
Data from 30 patients with sacral chordoma treated with carbon ion radiotherapy were reported in 2004 [16]; a study of a further 38 patients in Phase I/II and II clinical trials observed for almost 5 years was described in 2010 [17]. This work has begun to establish the effectiveness of CIRT for sacral chordoma. Here, the results from 95 patients treated between 1996 and 2007 are evaluated.
Material and methods
Carbon ion radiotherapy for sacral chordoma
The specific technique of CIRT used at NIRS has been described in detail in previous publications [1-8]. Briefly, patients were positioned in a customised cradle and immobilised with a low-temperature thermoplastic sheet. The irradiated position was usually prone. A series of CT images of 5 mm slice thickness were acquired for treatment planning. Respiratory gating of both the acquired CT images and the therapy beam was performed. The motion of markers (lines) placed on the skin surface was observed on CT images before respiratory gating was applied [18]. Three-dimensional treatment planning for CIRT was performed using the HIPLAN software program (NIRS, Chiba, Japan) [19]. The planning target volume (PTV) included the clinical target volume (CTV) plus a 5 mm safety margin for positioning errors. Tumour extent was evaluated by MRI, CT and sometimes positron emission tomography. The CTV received at least 90% of the prescribed dose (Figure 1). However, in cases where the tumour was located very close to critical organs, such as the bowel and skin, the margin was reduced and consequently <90% of the dose was applied to some tumours. The cradle could be tipped ±20 degrees to ensure that the beam was pointed in the appropriate direction. The therapy was performed once a day, 4 days per week (Tuesday to Friday), at doses ranging from 52.8 to 73.6 GyE for a total of 16 fixed fractions over 4 weeks. Two to four irregularly shaped ports were applied. 91 patients were irradiated with 3 ports: from posterior to anterior, from left side to right side, and from right side to left side. In two patients, dose conformity was achieved using the patch technique, a method of irradiation that uses the special characteristics of carbon ion beams and divides the target into two segments to avoid excess dose irradiation to critical organs. One port was used in each session. At every treatment session, positioning was confirmed with a computer-aided, on-line positioning system and four doctors on shift checked all sessions each day.
Figure 1.
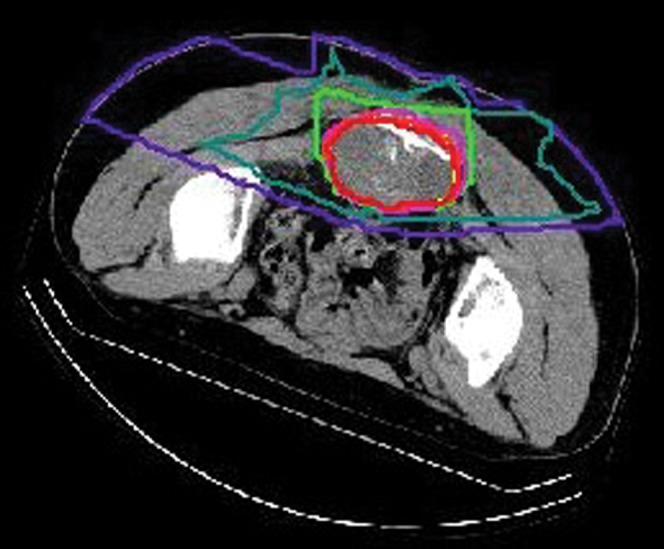
The dose distribution of carbon ion radiotherapy for sacral chordoma. The red line indicates 95% of the total dose.
The median CTV of the tumours in this study was 370 cm3 (range, 47–1468 cm3). 1 patient received a total dose of 54.8 GyE, 1 patient 64.0 GyE, 86 patients 70.4 GyE and 7 patients 73.6 GyE (Table 2).
Table 2. Characteristics of patients with chordoma.
| Characteristics | n |
| Number of patients | 95 |
| Sex | |
| Male | 68 |
| Female | 27 |
| Prior surgery | |
| Yes | 11 |
| No | 84 |
| Most cranial level of tumour | |
| L5 | 10 |
| S1 | 29 |
| S2 | 29 |
| S3 | 9 |
| S4 | 7 |
| Recurrence after resection | 11 |
| Irradiation dose (GyE total in 16 fractions) | |
| 52.8 | 1 |
| 64.0 | 1 |
| 70.4 | 86 |
| 73.6 | 7 |
| Total | 95 |
Patients
Between June 1996 and February 2007, a total of 95 patients with sacral chordoma received CIRT. 9 patients were registered in Phase I/II clinical trials; 29 patients in Phase II clinical trials; and 57 in HAMT trials. All patients signed an informed consent form approved by the local institutional review board. Details of eligibility for both trials have been described in previous articles[1,5]. The eligibility criteria applied were the same as those for the HAMT protocols:
tumour judged to be medically inoperable by the referring surgeon
tumour histopathologically diagnosed as chordoma
no distant metastasis at the time of initial referral for treatment
no prior radiation therapy at the same site
Karnofsky performance status score >60
tumour grossly measurable.
All tumours were pathologically confirmed as chordomas by our pathologists.
The median Karnofsky performance status score of the 68 males and 27 females was 80 (range, 70–90). The median age was 66 years (range, 30–85 years). Among the 95 patients, 84 had received no previous treatment, whereas 11 had tumours that had recurred after prior surgical resection. All the tumours originated in the sacrum. Their median diameter was 9 cm (range, 3–17 cm). The site distribution of spinal cord tumours was as follows: L5, 10 patients; S1, 29 patients; S2, 29 patients; S3, 9 patients; and below S4, 7 patients. More than 80% of the tumours were located at spinal cord levels higher than S2 (Table 2).
Statistical analysis
Patients were closely followed with physical, CT and MRI examinations at the end of their CIRT, and again 1–2 months after the completion of CIRT. Subsequent follow-ups were planned at least every 6 months at our hospital. For some patients (the elderly and those living in remote places), we depended on imaging films taken at local hospitals and medical reports from local doctors. The follow-up period was calculated from the initial date of carbon ion irradiation. Recurrence was defined as tumour regrowth, i.e. an increase in tumour volume observed in two consecutive MRI or CT scans. Modes of failure were defined as:
local failure—relapse within the PTV
marginal failure—relapse within 2 cm of the PTV
distant failure—tumour growth identified >2 cm from the PTV.
Local control and overall survival rates were calculated using the Kaplan–Meier method. The last follow-up date was February 2008. Radiation morbidity was classified according to the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer Acute/Late Radiation Morbidity Scoring System criteria in addition to the Late Effects of Normal Tissue/Subjective, Objective, Management and Analytic scoring system [20,21].
Results
All patients completed CIRT. 83 patients were alive and 12 patients had died at the time of the evaluation. The overall survival rate in all 95 patients at 5 years was 86% (Figure 2). Median follow up time was 42 months (range, 13–112 months). All survivors were followed-up for >1 year. The 5-year local control rate was 88% (Figures 2 and 3). Six patients had local recurrence after CIRT, and median time to local failure was 35 months (range, 13–60 months). Of the 95 patients, 91% remained ambulatory with or without a supportive device.
Figure 2.
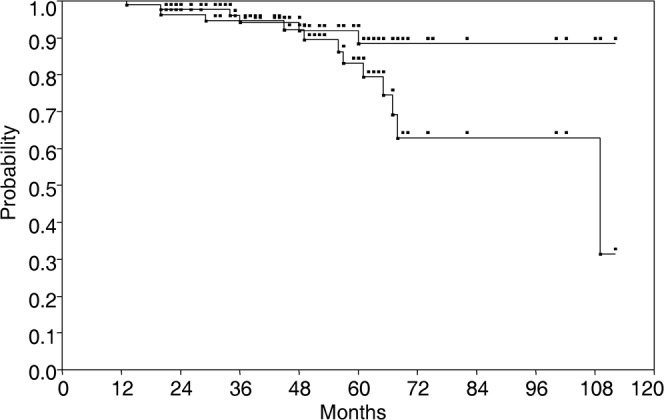
The overall survival (bottom line) and the local control rate (top line) in 95 patients with sacral chordoma treated with carbon ion radiotherapy.
Figure 3.
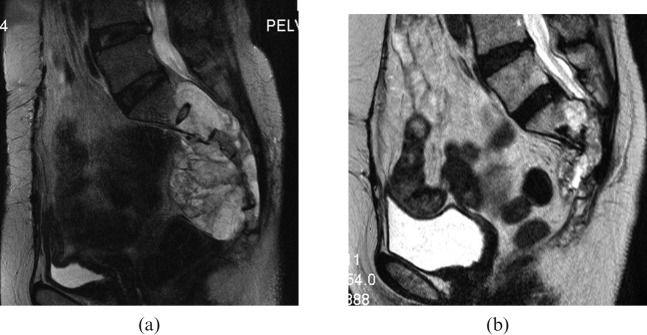
Sagittal T2 weighted MRI of a sacral chordoma (a) before carbon ion radiotherapy (CIRT), and (b) 4 years after CIRT the tumour shrank and the sacral bone deformity was shown.
Three patients had Grade 3 acute skin reactions and two had Grade 3 late skin reactions. Two patients treated with a total dose of 73.6 GyE experienced Grade 4 late skin and soft tissue complications requiring skin grafts on the buttocks. No patient required a colostomy as a result of toxicity from CIRT but one patient experienced transient Grade 1 rectal bleeding 20 months after CIRT. No other treatment-related surgical intervention (e.g. urinary diversion) was carried out. 15 patients, including 3 who were surgically treated before CIRT, required continuing medication for sciatic nerve neuropathy. 5 of these 15 patients received a total dose of 73.6 GyE.
Discussion
Sacral chordoma is one of the most challenging malignancies to treat [1]. Surgery is the mainstay but complete excision is sometimes difficult to achieve if the tumour volume is large and/or the tumour invades the upper levels of the sacrum (e.g. S1–2) and lumbar spine. The reported proportion of lesions for which complete tumour resection is achieved ranges from 20% to 70% [10-14]. The local control rate is approximately 60–80% in total excision cases, compared with 25–50% in subtotal resection cases [10-13]. Although >80% of tumours occurred at levels higher than S2 and all tumours were judged medically inoperable in our study, the 5-year local control rate was 88%. In the 38 Phase I and Phase I/II clinical trial patients receiving CIRT and observed over 56 months, the 5-year local control rate was 89%. This rate is similar to, or better than, those reported for patients treated by surgical resection.
Approximately 90% of the 95 patients given CIRT remained ambulatory. As already mentioned in a previous report, 30 of 38 patients in these clinical trials had no previous surgery [17]. Although the tumours of 26 of these 30 patients were located above the spinal cord level S2, approximately 90% remained ambulatory (with or without canes) almost 5 years after CIRT, and 50% needed no pain medication. No colostomies or urinary diversions were carried out as a consequence of CIRT [17]. Severe sciatic nerve neuropathy was observed in 15 of the 95 patients, which to some extent influenced their quality of life. We speculate that the main reason for this severe reaction is that all of these patients were irradiated above the S2 vertebra. On the other hand, 12 of 16 patients with S3–4 tumours had no symptoms after CIRT, even though referral surgeons had judged that these patients had medically inoperable tumours. The tumours in all 12 patients were controlled and their activities of daily living were the same before and after CIRT.
An analysis of dose–volume histograms of 44 sciatic nerves in 22 patients with sacral chordoma (receiving total doses ranging from 70.4 to 73.6 GyE and followed for >2 years) indicated that the length of irradiated sciatic nerves and dose of irradiation were possibly related to nerve injury. A length of >10 cm and a total dose of >70 GyE were possible thresholds for sciatic nerve injury (data not shown). DeLaney et al [22] at Massachusetts General Hospital (MGH), Boston, MA, reported Grade 3 sacral neuropathy in two patients who received 77.4 Gy RBE and no neural injuries in patients who received ≤70.2 Gy RBE. This result is in accord with their previous finding that the maximum tolerance dose to the cauda equina resulting in a 50% complication rate 5 years after treatment (TD50/5) was 72 GyE for males and 84 GyE for females [23]. This result would be difficult to apply to our results. MGH used fractional doses of <2 Gy RBE, whereas we used fractional doses of 4.4 and 4.6 GyE, corresponding to total doses of 70.4 and 73.6 GyE, respectively. The RBE of carbon ion beams for the sacral nerve or chordoma itself has not yet been elucidated. Furthermore, we speculate that other factors influence sciatic nerve symptoms in our cases. Among these factors, calcium deposits in nerve roots and defective remodelling of the sacral bone structure after CIRT were observed in patients with sciatic nerve injury and symptoms (Figure 4). Yanagi et al [24] used dose–surface histogram (DSH) to analyse the skin reaction of patients with bone and soft tissue sarcoma after CIRT. The area irradiated with >60 GyE (S60>20 cm2) on DSH was found to correlate significantly with late skin reactions that were worse than Grade 3. With regard to proton therapy, DeLaney et al [22] reported that recommended posterior skin dose was ≤66 Gy RBE.
Figure 4.
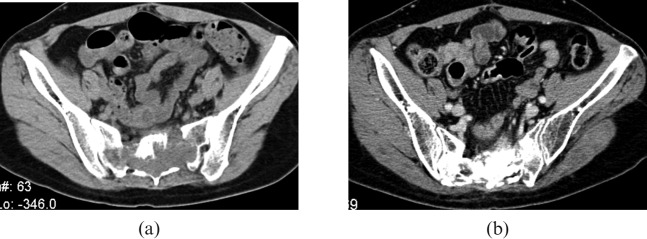
Contrast CT images of a sacral chordoma (a) before carbon ion radiotherapy (CIRT) and (b) 5 years after CIRT calcification deposited in the tumour bed.
Several reports have examined charged particle therapies for sacral chordoma, and the results for proton radiotherapy have been compared with those for CIRT. In 1993, Schoenthaler et al [25] at the Lawrence Berkley Laboratory, Berkeley, CA, reported a 5-year local control rate of 55% in 14 post-operative patients with sacral chordomas treated by charged neon and helium particles. This study further demonstrated a trend towards improved local control rate when neon (high LET) was used rather than helium (low LET). The authors recommended maximum debulking of tumour by radical surgery before the charged particle therapy. In 2004, Shulz-Ertner et al [26] reported the results of CIRT in 8 patients with sacral chordoma who were among 152 patients studied at GSI. The treatment sequence consisted of surgery, post-operative intensity-modulated radiotherapy and a carbon ion boost to the macroscopic residual tumour. The median photon dose was 50.4 Gy, and the carbon ion boost 18 GyE. Of the eight patients treated with combined photon radiotherapy to the sacrum and a carbon ion boost to the macroscopic tumour, one had local recurrence inside the irradiated field. DeLaney et al [22] reported results from a Phase II trial involving 50 spinal tumours, including 29 spinal chordomas, at MGH; high-dose photon/proton radiotherapy was part of the treatment regimen, which also included surgery. Before that trial, the results of a pilot study looking at the use of combined high-dose proton–photon radiation for axial skeleton tumours suggested that a primary target dose >77 Gy RBE and total resection of the visible tumour might improve local control rate [27]. In the Phase II trial, the median dose was 76.6 Gy RBE and none of the 23 patients with primary spinal chordoma had local failure. Detailed location-specific analysis of the primary chordomas was not presented in this study. The treatment of one primary sacral chordoma (of 5.5 cm in diameter) with 77.4 Gy RBE led to Grade 3 sacral neuropathy 5.5 years after proton therapy. Treatment of another primary sacral chordoma (of 8.4 cm in diameter) with 77.4 GyE was associated with erectile dysfunction within 3 years. Both tumours were controlled.
Rutz et al [28] reported results from the Paul Scherrer Institute (PSI) in Switzerland. Their treatment protocol involved either a combination of function-preserving surgery and spot-scanning proton therapy or a combination of function-preserving surgery, spot-scanning proton therapy and photon therapy. The study included 7 sacrococcygeal chordomas out of a total 26 spinal chordomas. Patients with residual tumour volume of <500 cm3 were enrolled. Median total dose was 72 CGE. This report did not present location-specific results, but the 3-year local control rate for all cases was 86%. The applicable dose constraint for centre cauda equina was 64 CGE, which was a lower dose than that applied at MGH [22]. All patients without local failure maintained their full independent status. They retained control over bladder, anal sphincter and ambulatory functions. This result was excellent but the follow-up period was short (35 months).
Regarding local control rate, the results from our study, assuming all our tumours were unresectable, are superior to those previously published, and indicate that CIRT for sacral chordoma is both a promising alternative to surgery and possibly the best charged particle therapy. Patients with sacral chordomas tend to be elderly at the time of diagnosis [1]. The median age in our study was 66 years, whereas in the 2006 MGH study, the average ages of patients with primary chordoma and with recurrent chordoma were 56 and 55 years, respectively [29]. The PSI study showed good results but, compared with our study, the patients were younger (median age, 49 years) and with smaller tumour volume [28]. Even for older patients, our study shows that the direct effects of treatment on patient activity may be acceptable.
There are some problems that need to be overcome if CIRT is to become more prevalent. The major issue is cost per treatment, which depends on manufacturing and operational costs [30]. To increase access to CIRT, the NIRS completed research and development on a compact charged particle therapy system in 2005. The NIRS expects that this system, which is about one-third the size of the HIMAC system and has one-third its manufacturing cost, will perform comparably. This type of accelerator was installed at Gunma University and launched on 16 March 2010. It is expected that this compact system will be installed at institutions throughout Japan.
Data from the present study indicate that CIRT has efficacy against sacral chordoma and limited toxicity. To prove the effectiveness and safety of CIRT, further investigations including clinical trials need to be conducted comparing CIRT with proton beam therapy and surgery.
Acknowledgement
This study was supported by the Research Project with Heavy Ions at NIRS-HIMAC of the NIRS.
References
- 1.Tsujii H, Kamada T, Baba M, Tsuji H, Kato H, Kato S, et al. Clinical advantages of carbon-ion radiotherapy. New J Phys 2008;10. Available from: http://www.iop.org/EJ/abstract/1367-2630/10/7/075009/ [Google Scholar]
- 2.Tsujii H, Mizoe JE, Kamada T, Baba M, Kato S, Kato H, et al. Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother Oncol 2004;73:S41–9 [DOI] [PubMed] [Google Scholar]
- 3.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol 2007;25:953–64 [DOI] [PubMed] [Google Scholar]
- 4.Noda K, Furukawa T, Fujisawa T, Iwata Y, Kanai T, Kanazawa M, et al. New accelerator facility for carbon ion radiotherapy. J Radiat Res 2007;48:A43–54 [DOI] [PubMed] [Google Scholar]
- 5.Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol 2002;20:4466–71 [DOI] [PubMed] [Google Scholar]
- 6.Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:201–10 [DOI] [PubMed] [Google Scholar]
- 7.Murakami T, Tsujii H, Furusawa Y, Ando K, Kanai T, Yamada S, et al. Medical and other application of high-energy heavy-ion beams from HIMAC. J Nucl Materials 1997;248:360–8 [Google Scholar]
- 8.Kanai T, Furusawa Y, Fukutsu K, Itsukaichi H, Eguchi-Kasai K, Ohara H. Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Radiat Res 1997;147:78–85 [PubMed] [Google Scholar]
- 9.Sundaresan N. Chordomas. Clin Orthop 1986;204:135–42 [PubMed] [Google Scholar]
- 10.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery 1999;44:74–9 [DOI] [PubMed] [Google Scholar]
- 11.Ozaki T, Hillmann A, Winkelmann W. Surgical treatment of sacrococcygeal chordoma. J Surg Oncol 1997;64:274–9 [DOI] [PubMed] [Google Scholar]
- 12.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H, et al. The surgical management of sacrococcygeal chordoma. Cancer 1999;85:878–83 [PubMed] [Google Scholar]
- 13.Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC., Jr Lumbosacral chordoma. Prognostic factors and treatment. Spine 1999;24:1639–45 [DOI] [PubMed] [Google Scholar]
- 14.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer 2000;88:2122–34 [DOI] [PubMed] [Google Scholar]
- 15.Catton C, O'Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V, et al. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol 1996;41:67–72 [DOI] [PubMed] [Google Scholar]
- 16.Imai R, Kamada T, Tsuji H, Yanagi T, Baba M, Miyamoto T, et al. Carbon ion radiotherapy for unresectable sacral chordomas. Clin Cancer Res 2004;10:5741–6 [DOI] [PubMed] [Google Scholar]
- 17.Imai R, Kamada T, Tsuji H, Sugawara S, Serizawa I, Tsujii H, et al. Effect of carbon ion radiotherapy for sacral chordoma: results of phase I–II and phase II clinical trials. Int J Radiat OncoI Biol Phys 2010;77:1470–6 [DOI] [PubMed] [Google Scholar]
- 18.Minohara S, Kanai T, Endo M, Noda K, Kanazawa M. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat OncoI Biol Phys 2000;47:1097–103 [DOI] [PubMed] [Google Scholar]
- 19.Endo M, Koyama-ito H, Minohara S, Miyahara N, Tomura H, Kanai T. HIPLAN—a heavy ion treatment planning system at HIMAC. J Jpn Soc Ther Radiol Oncol 1996;8:231–8 [Google Scholar]
- 20.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6 [DOI] [PubMed] [Google Scholar]
- 21.Anon LENT SOMA tables. Radiother Oncol 1995;35:17–60 [PubMed] [Google Scholar]
- 22.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Dean S, Yeap BY, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys 2009;74:732–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieters RS, Niemierko A, Fullerton BC, Munzenrider JE. Cauda equina tolerance to high-dose fractionated irradiation. Int J Radiat Oncol Biol Phys 2006;64:251–7 [DOI] [PubMed] [Google Scholar]
- 24.Yanagi T, Kamada T, Tsuji H, Imai R, Serizawa I, Tsujii H. Dose–volume histogram and dose–surface histogram analysis for skin reactions to carbon ion radiotherapy for bone and soft tissue sarcoma. Radiother Oncol 2010;95:60–5 [DOI] [PubMed] [Google Scholar]
- 25.Schoenthaler R, Castro JR, Petti PL, Baken-Brown K, Phillips TL. Charged particle irradiation of sacral chordomas. Int J Radiat Oncol Biol Phys 1993;26:291–8 [DOI] [PubMed] [Google Scholar]
- 26.Schulz-Ertner D, Nikoghosyan A, Thilmann C, Haberer T, Jäkel O, Karger C, et al. Results of carbon ion radiotherapy in 152 patients. Int J Radiat Oncol Biol Phys 2004;58:631–40 [DOI] [PubMed] [Google Scholar]
- 27.Hug EB, Fitzek MM, Liebsch NJ, Munzenrider JE. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys 1995;31:467–76 [DOI] [PubMed] [Google Scholar]
- 28.Rutz HP, Weber DC, Sugahara S, Timmermann B, Lomax AJ, Bolsi A, et al. Extracranial chordoma: outcome in patients treated with function-preserving surgery followed by spot-scanning proton beam irradiation. Int J Radiat Oncol Biol Phys 2007;67:512–20 [DOI] [PubMed] [Google Scholar]
- 29.Park L, DeLaney TF, Liebsch NJ, Hornicek FJ, Goldberg S, Mankin H, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys 2006;65:1514–21 [DOI] [PubMed] [Google Scholar]
- 30.Pijls-Johannesma M, Pommier P, Lievens Y. Cost-effectiveness of particle therapy: current evidence and future needs. Radiother Oncol 2008;89:127–34 [DOI] [PubMed] [Google Scholar]