Abstract
The aim of this study was to evaluate the influence of prognostic factors related to patient selection on survival outcomes. Survival outcomes were retrospectively analysed in a consecutive series of 67 newly diagnosed glioblastoma multiforme (GBM) patients who had received either conventional fractionated photon radiotherapy (CRT) or high-dose particle radiotherapy (HDT). In the CRT protocol, a total dose of 60.0–61.2 Gy was administered. In the HDT protocol, an average dose of approximately 30 GyE in a single session and additional fractionated photon irradiation of total dose 30 Gy were administered to patients receiving boron neutron capture therapy; and a total dose of 96.6 GyE was administered to patients receiving proton therapy. Most of the patients had received chemotherapy with nimustine hydrochloride (ACNU) alone or with ACNU, procarbazine and vincristine. The median overall survival (OS) and progression-free survival times for all patients were 17.7 months [95% confidence interval (CI), 14.6–20.9 months] and 7.8 months (95% CI, 5.7–9.9 months), respectively. The 1- and 2-year survival rates were 67.2% and 33.7%, respectively. For patients treated with HDT, the median OS was 24.4 months (95% CI, 18.2–30.5 months), compared with 14.2 months (95% CI, 10.0–18.3 months) for those treated with CRT. The Cox proportional hazards model revealed radiation modality (HDT vs CRT) and European Organisation for Research and Treatment of Cancer recursive partitioning analysis class to be the significant prognostic factors. Age, sex, pre-operative performance status, treatment with or without advanced neuroimaging, extent of surgery and regimen of chemotherapy were not statistically significant factors in predicting prognosis. The median OS was 18.5 months (95% CI, 9.9–27.1 months) in patients of 65 years and older, compared with 16.8 months (95% CI, 13.6–20.1 months) in those 64 years and younger (p=0.871). The positive effect of HDT treatment is unlikely to reflect patient selection alone. Randomised trials with strictly controlled inclusion criteria to ensure the comparable selection of patients are required to demonstrate conclusively that prolonged survival can be attributed to high-dose particle radiotherapies.
Glioblastoma multiforme (GBM) is a highly infiltrative primary malignant brain tumour in adults. The prognosis of GBM is generally extremely poor, and there has been little improvement in survival rates over the past three decades [1,2]. Several randomised trials have demonstrated the survival benefits of conventional fractionated photon radiotherapy at a total dose of 45–60 Gy; the median overall survival (OS) time (in these trials was 5.8–15.5 months [3-7]. Currently, conventional fractionated photon radiotherapy of approximately 60 Gy with concomitant and adjuvant use of temozolomide is recognised as the standard post-operative treatment for newly diagnosed GBM [8,9]. However, the 5-year survival rate with this standard therapy is <10% [9], suggesting that alternative therapeutic strategies are desperately needed.
Most dose escalation studies for radiotherapy have been designed as case series of a small number of selected patients who underwent additional stereotactic radiosurgery, fractionated proton beam radiation, intensity-modulated radiotherapy or another type of conformal radiotherapy [10-12]. Studies showing the better outcomes (median OS range, 9.5–25 months) suggested that a radiation dose of at least 90 Gy of hyperfractionated radiotherapy should be used for irradiation in order to accomplish local tumour control. There has, however, been no randomised controlled trial to provide evidence in support of these studies, nor has there been any trial using any form of high-dose radiotherapy that warrants a follow-up study [10,13]. Thus, controversy remains regarding both the efficacy of high-dose radiotherapy and the influence of strict patient selection on the outcomes achieved with this type of treatment. The high-dose irradiation of a small-volume target could minimise central recurrence and any adverse events that are dependent on radiation dose in such trials. However, recurrences often occur in target volumes that receive a relatively low-dose treatment. The 5-year survival rate with conventional-dose radiotherapy alone is reported at approximately 1% [9], suggesting that this treatment has limited success in most patients.
We recently reported the use of two different types of high-dose particle radiotherapy, boron neutron capture therapy (BNCT) and proton therapy (PT), in glioblastoma [14,15]. These radiotherapies for newly diagnosed GBM were administered on the basis of different selection criteria. Both showed an acceptable OS (i.e. 25.7 months in the BNCT group and 21.6 months in PT group), with acceptable adverse events [14,15]. Several different factors [e.g. age, pre-operative performance status (PS), tumour location, extent of surgery and use of conventional radiotherapy] have previously been shown to be prognostic of survival in patients with GBM [16-20]. Here, we aim to evaluate the influence of such patient selection-related factors on survival. Survival outcomes and prognostic factors were retrospectively analysed using our institutional consecutive series of newly diagnosed GBM patients who had received either conventional fractionated photon radiotherapy (CRT) or high-dose particle radiotherapy (HDT). We report the combined updated results for all patients treated by either form of particle therapy.
Materials and methods
We investigated 67 consecutive patients with newly diagnosed supratentorial GBM (Grade IV) who were treated at Tsukuba University Hospital, Japan, between January 1998 and August 2007. The patients were histopathologically diagnosed according to the classification system of the World Health Organization (WHO). Some of the survival data for patients who received PT or BNCT have been reported in earlier publications where they were assessed after different follow-up periods and using different survival analysis determinations [14,15].
Maximal safe resection was intended to remove all gadolinium-enhanced masses observed by MRI, i.e. the non-eloquent brain tissue surrounding a mass was targeted for removal with the aim of preserving neurological function in unresected areas of eloquent brain tissue. To this end, 5-aminolevulinic-acid-induced fluorescence guidance, neuronavigation and intraoperative monitoring were incorporated into the treatment. The navigation-guided fence-post procedure was carried out as previously reported [21] to treat the non-eloquent portions of tumours. In cases involving tumours that were located close to areas of eloquent tissue, we inserted silicon tubes along the boundary between the eloquent and non-eloquent tissue, as guided by MRI. The phrase “advanced neuro-imaging” is used to refer to all surgical interventions involving fluorescence guidance and/or neuronavigation.
The post-operative radiation schedule for patients with GBM at our facilities consisted of three protocols. As the standard radiotherapy, daily CRT (1.8–2.0 Gy) was administered five times per week, amounting to a total overall dose of 60.0–61.2 Gy. For selected patients, HDT, consisting of either BNCT or PT, was used. In the BNCT protocol, the gross tumor volume (GTV) and the clinical target volume (CTV)-1 were defined as the residual gadolinium-enhancing volume. CTV-2 and CTV-3 were defined as GTV plus a margin of 2 or 3 cm, respectively. An average dose of approximately 30 GyE in a single session and additional fractionated photon irradiation totalling 30 Gy were administered to the GTV. The detailed protocol for BNCT has been described elsewhere [14]. BNCT was administered to patients with a supratentorial unilateral tumour, located at no deeper than 7 cm from the brain surface, who had a Karnofsky performance status (KPS) of ≥50. In the PT protocol, CTV-1 was defined as in the BNCT protocol. However, CTV-2 was defined as GTV plus a margin of 1 cm, and CTV-3 was defined to include the surrounding oedema. Furthermore, in the PT protocol, a planning target volume (PTV) adopted was defined as the corresponding CTV plus a margin of 5 mm to allow for set-up error. Conventional photon radiotherapy (50.4 Gy in 28 fractions) was delivered to PTV-3 in the morning. In the first half of the protocol, additional concomitant boost proton radiotherapy (23.1 GyE in 14 fractions) was delivered to PTV-2 >6 h after this photon radiotherapy. Subsequently, in the latter half of the protocol, proton radiotherapy (23.1 GyE in 14 fractions) was delivered to PTV-1. As a result, the total dose was 96.6 GyE in 56 fractions for PTV-1, 73.5 GyE in 42 fractions for PTV-2 and 50.4 Gy in 28 fractions for PTV-3 [15].
The following criteria were used to select patients for PT: the presence of a supratentorial tumour lacking involvement of the brain stem or thalamus, a maximum post-operative tumour diameter of <4 cm, and a KPS of ≥60. BNCT was administered to 15 patients and PT to 17 patients. The combination therapy (PAV), consisting of procarbazine, nimustine hydrochloride (ACNU) and vincristine, was administered as the standard concomitant chemotherapy in combination with CRT. For patients at high risk of adverse events with PAV therapy (i.e. elderly patients or patients in poor neurological or general condition), ACNU alone was typically used as the concomitant chemotherapy. ACNU was also used in combination with HDT.
Statistical analysis
Statistical analysis was performed using SPSS software (version 11.0.1J; SPSS Inc., Chicago, IL; now an IBM company). OS and progression-free survival (PFS) were used to investigate the prognostic impact of the analysed variables. OS was defined as the time lapse from surgery until death or the final follow-up. PFS time was defined as the time lapse from surgery until the detection of progression or the final follow-up. Survival probabilities were calculated using the Kaplan–Meier method, and differences among patient groups were evaluated using the log-rank test. The Cox proportional hazards model was used to test the following prognostic factors in univariate and multivariate analyses: age (<65 vs ≥65 years), sex (female vs male), pre-operative PS (0–2 vs 3–4), European Organisation for Research and Treatment of Cancer (EORTC) recursive partitioning analysis (RPA) Class (III–IV vs V) [22], advanced neuro-imaging (with vs without), extent of surgery (complete resection vs other), chemotherapy (ACNU vs other) and radiation modality (HDT vs CRT).
Factors with a probability value of <0.05 on univariate analysis were selected for testing in the multivariate analysis. The results are expressed with relative risk and a 95% confidence interval (CI).
Results
The characteristics of the 67 patients are summarised in Table 1. A total of 34 men and 33 women, aged 31–84 years (median, 59 years), were included in the analysis. Surgical resection resulted in complete resection of the tumour in 13 patients (19%), in partial resection in 47 patients (70%) and biopsy in 7 patients (10%). 47 patients (70%) received chemotherapy with ACNU or other agents. 32 patients (48%) received HDT and 35 (52%) received CRT; consequently, all 67 patients received 1 of the 2 types of radiotherapy. There were 6 patients (9%) with a WHO PS of 0, 30 (45%) with a PS of 1, 12 (18%) with a PS of 2, 10 (15%) with a PS of 3 and 9 (13%) with a PS of 4. 9 patients (13%) were categorised as having the best GBM prognosis (Class III), whereas 21 (31%) were in Class IV and 37 (55%) in Class V, according to the EORTC-RPA classification system.
Table 1. Characteristics of 67 patients with glioblastoma multiforme.
| Characteristics | Number of patients (%) |
| Age | |
| Median 59.0 years | |
| Range 31–84 years | |
| Sex | |
| Male | 34 (51%) |
| Female | 33 (49%) |
| Side | |
| Left | 33 (49%) |
| Right | 27 (40%) |
| Midline or bilateral | 7 (10%) |
| Location | |
| Frontal lobe | 32 (48%) |
| Temporal lobe | 19 (28%) |
| Parietal lobe | 17 (25%) |
| Occipital lobe | 1 (1%) |
| Other | 6 (9%) |
| Advanced neuro-imaginga | |
| Yes | 31 (46%) |
| No | 36 (54%) |
| Extent of surgery | |
| Complete | 13 (19%) |
| Partial | 47 (70%) |
| Biopsy | 7 (10%) |
| Chemotherapy | |
| Nimustine hydrochloride | 45 (67%) |
| Other | 2 (3%) |
| None | 20 (30%) |
| Radiotherapy | |
| High dose | 32 (48%) |
| Conventional dose | 35 (52%) |
| WHO performance status | |
| 0 | 6 (9%) |
| 1 | 30 (45%) |
| 2 | 12 (18%) |
| 3 | 10 (15%) |
| 4 | 9 (13%) |
| EORTC-RPA classb | |
| III | 9 (13%) |
| IV | 21 (31%) |
| V | 37 (55%) |
EDRTC, European Organisation for Research and Treatment of Cancer; RPA, recursive partitioning analysis; WHO, World Health Organization.
aAdvanced neuro-imaging indicates the use of 5-aminolevulinic-acid-induced fluorescence guidance and/or neuro-navigation.
bRadiation Therapy Oncology Group RPA Class VI patients were included in EORTC-RPA Class V.
Nine patients were alive at the time of analysis, with a mean follow-up time of 21.4 (range, 1.0–71.2) months. The median OS for all patients was 17.7 (95% CI, 14.6–20.9) months. The 1- and 2-year survival rates were 67.2% and 33.7%, respectively. The median PFS time in this series was 7.8 (95% CI, 5.7–9.9) months. The 1- and 2-year PFS rates were 32.6 and 18.4%, respectively.
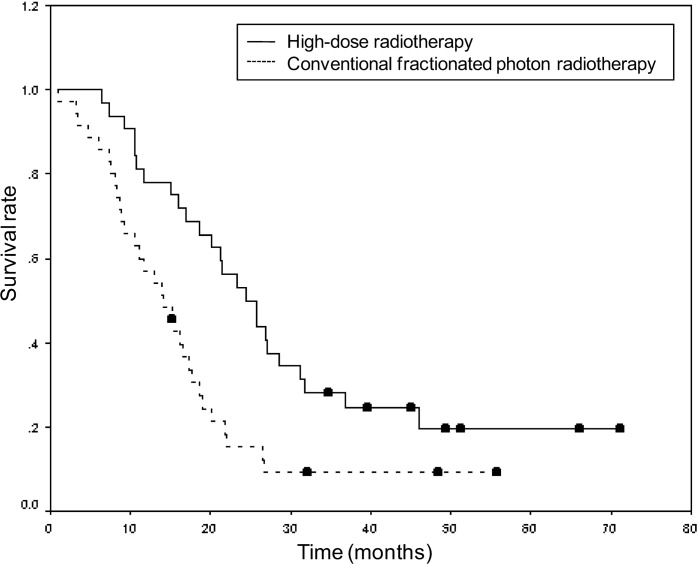
The univariate and multivariate analyses of the prognostic factors examined in this study are shown in Tables 2 and 3, respectively. The multivariate analysis revealed that radiation modality and EORTC-RPA class were significant prognostic factors. The median OS was 24.4 (95% CI, 18.2–30.5) months for patients treated with HDT, compared with 14.2 (95% CI, 10.0–18.3) months for those treated with CRT (Figure 1). Other previously reported prognostic factors, such as age, sex, pre-operative PS, treatment with or without advanced neuro-imaging, extent of surgery and chemotherapy regimen, were not statistically significant according to the multivariate analysis. The median OS was 18.5 (95% CI, 9.9–27.1) months in patients 65 years and older, compared with 16.8 (95% CI, 13.6–20.1) months in those aged 64 years and younger (p=0.871).
Table 2. Univariate analysis of prognostic factors for the survival of patients with glioblastoma multiforme.
| Variable | Hazard ratio (95% CI) | p-value |
| Age (<65 vs ≥65 years) | 0.954 (0.539–1.687) | 0.871 |
| Sex (female vs male) | 0.600 (0.351–1.023) | 0.061 |
| WHO performance status (0–2 vs 3–4) | 0.525 (0.295–0.936) | 0.029 |
| EORTC-RPA Class (III–IV vs V) | 0.502 (0.289–0.872) | 0.014 |
| Advanced neuro-imaging (with vs without) | 0.731 (0.430–1.244) | 0.248 |
| Extent of resection (complete vs others) | 0.735 (0.379–1.424) | 0.361 |
| Chemotherapy (ACNU vs others) | 0.632 (0.365–1.091) | 0.100 |
| Radiotherapy (high dose vs conventional dose) | 0.443 (0.258–0.762) | 0.003 |
ACNU, nimustine hydrochloride; CI, confidence interval; EORTC-RPA, European Organisation for Research and Treatment of Cancer recursive partitioning analysis; WHO, World Health Organization.
Table 3. Multivariate analysis of prognostic factors for the survival of patients with glioblastoma multiforme.
| Variable | Hazard ratio (95% CI) | p-value |
| WHO performance status (0–2 vs 3–4) | 0.634 (0.352–1.142) | 0.129 |
| EORTC-RPA Class (III–IV vs V) | 0.544 (0.310–0.954) | 0.034 |
| Radiotherapy (high dose vs conventional dose) | 0.495 (0.284–0.862) | 0.013 |
CI, confidence interval; EORTC-RPA, European Organisation for Research and Treatment of Cancer recursive partitioning analysis; WHO, World Health Organization.
Figure 1.
Kaplan–Meier estimates of overall survival according to radiation modality. The hazard ratio for death among patients treated with high-dose radiotherapy, as compared with that among patients treated with conventional fractionated photon radiotherapy, was 0.44 (95% confidence interval, 0.26–0.76; p<0.01).
Patients who were treated with HDT had a significantly better pre-operative PS than patients treated with CRT (p=0.025). Similarly, patients who were treated with HDT were more likely to have undergone complete resection than those treated with CRT (28.1% compared with 11.4%, respectively; p=0.078) and were more likely to be categorised in the better prognostic group (III–IV compared with V; p=0.059), but neither of these differences was statistically significant. Other clinical characteristics, i.e. age, sex, advanced neuro-imaging and chemotherapy regimen, did not differ between patients who underwent HDT and those treated with CRT (Table 4).
Table 4. Clinical characteristics of patients treated with high-dose particle radiotherapy compared with those who underwent conventional fractionated photon radiotherapy.
| Characteristics | Number of patients (%) |
p-value | |
| High dose | Conventional dose | ||
| Age | |||
| <65 years | 24 | 22 | 0.210 |
| ≥65 years | 8 | 13 | |
| Sex | |||
| Male | 14 | 20 | 0.198 |
| Female | 18 | 15 | |
| Advanced neuro-imaging | |||
| Yes | 14 | 17 | 0.441 |
| No | 18 | 18 | |
| Extent of surgery | |||
| Complete resection | 9 | 4 | 0.078 |
| Other | 23 | 31 | |
| Chemotherapy | |||
| ACNU | 21 | 24 | 0.501 |
| Other | 11 | 11 | |
| WHO performance status | |||
| 0–2 | 27 | 21 | 0.025 |
| 3–4 | 5 | 14 | |
| EORTC-RPA Class | |||
| III–IV | 18 | 12 | 0.059 |
| V | 14 | 23 | |
ACNU, nimustine hydrochloride; EORTC-RPA, European Organisation for Research and Treatment of Cancer recursive partitioning analysis; WHO, World Health Organization.
Discussion
The median OS for all patients was 17.7 months; a longer median OS of 24.4 months was seen in the HDT group, whereas the CRT group had an OS of 14.2 months. Receiving either HDT or CRT was also independently significant in determining prognosis. The survival data for the CRT patients in this study were comparable with those of reported previously for patients treated with the standard therapies. Patient selection (e.g. age, PS etc.) for the HDT group appeared not to be a major factor influencing survival time, nor did it negatively influence the survival time of the CRT patients.
It is generally accepted that the concomitant and adjuvant use of temozolomide with conventional fractionated photon radiotherapy can be effective in treating post-operative GBM with minimal additional toxicity, and a significant survival advantage has been demonstrated for this approach compared with the administration of radiotherapy alone. The median OS in a randomised controlled trial by Stupp et al [8] was 14.6 months with temozolomide plus radiotherapy, and 12.1 months with radiotherapy alone. The median OS of CRT patients observed in our study was comparable with that of patients in the temozolomide treatment group of Stupp et al's study, whereas the median OS of all patients in our study was longer. The patient characteristic data from the report by Stupp et al showed that patients in their temozolomide treatment group tended to be younger, to have better PS (0–1), and to belong to less high-risk (EORTC-RPA Class V) populations (Table 5). These findings suggest that the favourable survival data from all patients and from those who underwent HDT in this study were unlikely to reflect patient selection alone. However, the more extensive surgery (39% of patients in the EORTC-National Cancer Institute of Canada Clinical Trials Group (NCIC) study underwent complete resection [9] vs 19% in our study), more-moderate risk populations (53% of patients in the EORTC-NCIC study were EORTC-RPA Class IV [9] vs 31% in our study), aggressive salvage treatment and other indeterminate factors, such as surgical technique and administration of standard care, may have positively influenced the survival data in the EORTC-NCIC study. Additionally, small sample size and inconsistent and non-controlled patient characteristics may have affected our results and thus pose limitations on the findings of the present study.
Table 5. Comparison of patient characteristics in the conventional fractionated photon radiotherapy plus continuous daily temozolomide (CRT+TMZ) group of the European Organisation for Research and Treatment of Cancer National Cancer Institute of Canada Clinical Trials Group study [9] patients and all patients in our study.
| Characteristics | CRT+TMZ | This study |
| Number of patients | 287 | 67 |
| Age | ||
| Median | 56 years | 59 years |
| Range | 19–70 years | 31–84 years |
| Sex | ||
| Male | 185 (64%) | 34 (51%) |
| Female | 102 (36%) | 33 (49%) |
| Extent of surgery | ||
| Complete | 113 (39%) | 13 (19%) |
| Partial | 126 (44%) | 47 (70%) |
| Biopsy | 48 (17%) | 7 (10%) |
| WHO performance status | ||
| 0 | 113 (39%) | 6 (9%) |
| 1 | 136 (47%) | 30 (45%) |
| 2 | 38 (13%) | 12 (18%) |
| 3 | 0 (0%) | 10 (15%) |
| 4 | 0 (0%) | 9 (13%) |
| EORTC-RPA class | ||
| III | 42 (15%) | 9 (13%) |
| IV | 152 (53%) | 21 (31%) |
| V | 93 (32%) | 37 (55%) |
| Number of progressing tumours | 244 (85%) | 59 (88%) |
| Salvage surgery | 66a (27%) | 20 (34%) |
| Salvage chemotherapy | 142a (58%) | 54 (92%) |
EORTC-RPA, European Organisation for Research and Treatment of Cancer recursive partitioning analysis; WHO, World Health Organization.
aCalculated by the present authors.
In this study, the median OS of GBM patients increased from 15.2 (95% CI, 8.1–22.3) months for patients first treated between 1982 and 1997 to 17.7 (95% CI, 14.6–20.9) months for those first treated between 1998 and 2007, although this trend was not statistically significant (p=0.086). The 2-year relative survival rate also increased from 23.6% between for patients first treated 1982 and 1997 to 33.7% for those first treated between 1998 and 2007. Advances in surgical techniques, such as the introduction of fluorescence guidance in 1999 and of neuro-navigation in 2005, have been implemented at our institute alongside improvements in chemotherapeutic agents; however, no significant differences in the extent of surgery or chemotherapy regimen were observed between the two time periods (i.e. pre- and post-1998). There remains no reliable evidence that these new surgical approaches or chemotherapy regimen are having a positive effect on OS. It is possible, therefore, that the improvements in OS may not have been related to surgical approach but rather to changes made to the BNCT protocol (since 1998) and to the PT protocol (since 2001), when rotating gantries and regular daily fractionation became possible.
In the BNCT patient series, we observed some acute toxicities, such as mild erythema (commonly observed) and transient orbital swelling (observed in one patient; 6.7%), but no late toxicity was observed during the follow-up periods. In the PT patient series, acute toxicities such as radiation dermatitis (commonly observed), rash (observed in one patient; 5.9%) and headache (in five patients; 29.4%) were observed. As for late toxicity, radiation necrosis and leukoencephalopathy were each observed in one patient (5.9%) [14,15]. These data indicate that toxicity seems to be at an acceptable level for our HDT protocol, but incidence of late toxicities, such as radiation necrosis and leukoencephalopathy, in survivors needs to be monitored and clarified after a longer follow-up period.
Patient age has been reported to be a strong prognostic factor in the treatment of GBM. In the RPA of EORTC, being 50 years old or older is a significant prognostic factor in the categorisation of disease. In the present study, however, no correlation has been found between age and prognosis. Recently, there has been a significant improvement in the survival of elderly patients with GBM, thanks to the introduction of aggressive treatments [23-25]. In the present series, no statistically significant difference was observed in age-related survival probability. The mean age of PT and BNCT patients was 57 and 60 years, respectively. Aggressive treatment of elderly patients with GBM at our institute appears to have minimised any age-related difference in survival probability.
Patients who were treated with HDT had a better pre-operative PS and were more likely to have undergone complete resection than patients treated with CRT. It was not possible to separate the effects of HDT from the selection bias towards patients with a better prognosis, because the inclusion criteria for our HDT modalities, which are based on the characteristics of GBM patients, involve the restriction of PS and limitations to tumour size and location, both of which are correlated with the difficulty of resection. Even in patients categorised in the worst prognostic group (EORTC-RPA Class V), however, OS was significantly prolonged for those who had received HDT compared with those treated with CRT (p=0.007). Similarly, among patients who underwent partial resection or biopsy, the OS was also significantly prolonged for those who received HDT vs those treated with CRT (p=0.005). These results indicate that the survival benefits of HDT are unlikely to reflect patient selection alone.
Conclusions
It is generally accepted that both the size and the location of a tumour, as well as patient performance status, should be considered when evaluating the safety and efficacy of high-dose particle radiotherapies. In this study, patients selected to receive HDT showed longer survival times than those treated with CRT. Although HDT, compared with CRT, was factored out as a significant favourable prognostic factor, other major prognostic factors did not appear to be confounding. The results of this study suggest that the positive effects of HDT on patient survival are unlikely to reflect patient selection alone. Randomised trials with strictly controlled inclusion criteria to enforce the comparable selection of patients are required to demonstrate conclusively that prolonged survival is a result of these high-dose radiotherapies.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture Sports, Science and Technology of Japan (22591604).
References
- 1.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Relative survival rates and patterns of diagnosis analysed by time period for individuals with primary malignant brain tumour, 1973–1997. J Neurosurg 2003;99:458–66 [DOI] [PubMed] [Google Scholar]
- 2.Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC. Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: analysis of 625 cases. Br J Neurosurg 2007;21:496–500 [DOI] [PubMed] [Google Scholar]
- 3.Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 1978;49:333–43 [DOI] [PubMed] [Google Scholar]
- 4.Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 1980;303:1323–9 [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen K, Hagen S, Kollevold T, Torvik A, Holme I, Nesbakken R, et al. Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer 1981;47:649–52 [DOI] [PubMed] [Google Scholar]
- 6.Sandberg-Wollheim M, Malmstrom P, Stromblad LG, Anderson H, Borgstrom S, Brun A, et al. A randomized study of chemotherapy with procarbazine, vincristine, and lomustine with and without radiation therapy for astrocytoma grades 3 and/or 4. Cancer 1991;68:22–9 [DOI] [PubMed] [Google Scholar]
- 7.Andersen AP. Postoperative irradiation of glioblastomas. Results in a randomized series. Acta Radiol Oncol Radiat Phys Biol 1978;17:475–84 [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van denBent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96 [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Hegi ME, Mason WP, van denBent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66 [DOI] [PubMed] [Google Scholar]
- 10.Fitzek MM, Thornton AF, Rabinov JD, Lev MH, Pardo FS, Munzenrider JE, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg 1999;91:251–60 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Ino Y, Nakagawa K, Tago M, Todo T. High-dose conformal radiotherapy for supratentorial malignant glioma: a historical comparison. Lancet Oncol 2005;6:953–60 [DOI] [PubMed] [Google Scholar]
- 12.Iuchi T, Hatano K, Narita Y, Kodama T, Yamaki T, Osato K. Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys 2006;64:1317–24 [DOI] [PubMed] [Google Scholar]
- 13.Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93–05 protocol. Int J Radiat Oncol Biol Phys 2004;60:853–60 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Nakai K, Kageji T, Kumada H, Endo K, Matsuda M, et al. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol 2009;91:80–4 [DOI] [PubMed] [Google Scholar]
- 15.Mizumoto M, Tsuboi K, Igaki H, Yamamoto T, Takano S, Oshiro Y, et al. Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2010;77:98–105 [DOI] [PubMed] [Google Scholar]
- 16.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95:190–8 [DOI] [PubMed] [Google Scholar]
- 17.Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 1993;26:239–44 [DOI] [PubMed] [Google Scholar]
- 18.Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 1980;303:1323–9 [DOI] [PubMed] [Google Scholar]
- 19.Jeremic B, Milicic B, Grujicic D, Dagovic A, Aleksandrovic J. Multivariate analysis of clinical prognostic factors in patients with glioblastoma multiforme treated with a combined modality approach. J Cancer Res Clin Oncol 2003;129:477–84 [DOI] [PubMed] [Google Scholar]
- 20.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 1993;85:704–10 [DOI] [PubMed] [Google Scholar]
- 21.Yoshikawa K, Kajiwara K, Morioka J, Fujii M, Tanaka N, Fujisawa H, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol 2006;78:91–7 [DOI] [PubMed] [Google Scholar]
- 22.Mirimanoff RO, Gorlia T, Mason W, Van denBent MJ, Kortmann RD, Fisher B, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 2006;24:2563–9 [DOI] [PubMed] [Google Scholar]
- 23.Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med 2007;356:1527–35 [DOI] [PubMed] [Google Scholar]
- 24.Patwardhan RV, Shorter C, Willis BK, Reddy P, Smith D, Caldito GC, et al. Survival trends in elderly patients with glioblastoma multiforme: resective surgery, radiation, and chemotherapy. Surg Neurol 2004;62:207–15 [DOI] [PubMed] [Google Scholar]
- 25.Combs SE, Wagner J, Bischof M, Welzel T, Wagner F, Debus J, et al. Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys 2008;70:987–92 [DOI] [PubMed] [Google Scholar]