Abstract
MRI using T1 weighted, T2 weighted and gadolinium-enhanced sequences plays a central clinical role in diagnosis, characterisation, surveillance and therapeutic monitoring of gliomas. Such conventional MRI protocols provide high resolution multiplanar structural information, and substantially improved tissue characterisation compared with CT. However, the MRI signal lacks biological specificity, e.g. T2 weighted dependent signal abnormality is dominated by tissue water content, and contrast enhancement reflects a non-specific increase in blood-brain barrier permeability. This limits non-invasive glioma diagnosis, characterisation and therapeutic planning and assessment of active tumour load may be confounded by treatment-related effects. The complex features of glioma morphology and often subtle changes between MRI examinations are also frequently difficult to detect reliably by visual inspection of the images, even by an experienced radiologist. Moreover, the most widely used response criteria in clinical practice and therapeutic trials rely on linear measurements of enhancing tumour and are further challenged by the irregular shape and heterogeneous composition of gliomas. This contributes to the poor correlation of these criteria with hard clinical endpoints. While conventional MRI is widely available and provides essential anatomical information, the lack of pathology-specific biomarkers available from standard MRI sequences and methods of image analysis used limit overall diagnostic and prognostic efficacy of the examination.
Imaging plays a central role in diagnosis, characterisation, surveillance and therapeutic monitoring of intracranial tumours.
Contrast-enhanced CT has gradually been supplanted by MRI as the mainstay of clinical tumour imaging in many centres. Tumour hyperintensity on T2 dependent sequences [including spin echo and fluid-attenuated inversion-recovery (FLAIR)] reflects prolongation of transverse relaxation times related to increased tissue water content and ultrastructure, and areas of calcification or haemosiderin may show as foci of signal dropout. Pathological contrast enhancement following administration of intravenous gadolinium chelates reflects accumulation of paramagnetic compound in the interstitium, resulting from non-specifically increased blood–brain barrier permeability related to neovascularisation and necrosis.
Evaluation is by a radiologist’s visual inspection of images in a clinical context, and response evaluation in clinical trials has been traditionally based on linear measurements of enhancing tumour components.
Tumour characterisation
Neoplasm vs non-neoplastic lesions
Although some intracranial masses have sufficiently distinctive radiological features to allow confident imaging diagnosis, conventional structural imaging has limited specificity in distinguishing brain tumours from other non-neoplastic diseases that can present as space-occupying lesions [1]. For peripherally enhancing masses, the main differential diagnosis lies between high-grade and secondary brain tumours, inflammatory or demyelinating lesions and abscesses. Non-enhancing lesions may represent low-grade gliomas (LGGs), viral encephalitis and developmental anomalies, such as focal cortical dysplasia.
Glioma grading
Although pathological contrast enhancement is generally associated with more aggressive lesions, up to one-third of non-enhancing gliomas are malignant [2]. Certain subtypes of LGGs, notably gangliogliomas and pilocytic astrocytomas, some grade II oligodendrogliomas [3] and more rarely, low-grade astrocytomas [2], show enhancement. Contrast enhancement alone is therefore a limited differentiator between high-grade gliomas and LGGs in an individual patient.
Multiple regression analysis has been used to relate MRI features to pathological grade in astrocytomas. The degree and heterogeneity of contrast enhancement, oedema ± mass effect and necrosis/cyst formation were found to be related to higher tumour grade [4]. However, significant overlap for imaging characteristics between groups limits MRI as a definitive predictor of grade in clinical practice.
In LGGs undergoing malignant progression, change in imaging appearance frequently precedes clinical deterioration. The development and evolution of focal contrast enhancement is the most commonly used sign of tumour progression in clinical practice. It has proved a more reliable indicator of malignancy in gliomas than oedema, border definition, mass effect, necrosis and haemorrhage [5]. The point at which enhancement appears during the process of malignant transformation in a pre-existing low-grade lesion is uncertain.
Tumour subtyping
The distinction between oligodendrogliomas, notably those associated with 1p/19q translocation mutation, and astrocytomas has important implications for treatment response and prognosis. Oligodendrogliomas more frequently calcify, contain cystic elements, have better defined margins and more often occur in temporal locations than astrocytomas. Primary or de novo glioblastomas are associated with epidermal growth factor receptor amplification, and are associated with larger enhancing components relative to overall tumour volume and ill-defined margins compared with secondary glioblastoma arising from LGG [6,7].
However, the specificity of these findings on conventional MRI is too low to distinguish the above tumour subtypes reliably in an individual patient.
Prognostic measures
A study comparing MRI features to the hard endpoint of patient survival found that oedema and multifocality were poor prognostic indicators in high-grade gliomas, while non-contrast enhancing tumour was associated with longer survival [8]. Although not predicting grade, these findings have obvious clinical relevance.
Tumour delineation
Gliomas, in particular high-grade lesions, are heterogeneous in appearance and gene expression [8] with ill-defined boundaries. Breakdown of the blood–brain barrier leads to an increase in enhancement and vasogenic oedema [9]. The margins of active tumour have a limited correlation with contrast-enhancing components and T2 dependent oedema, which usually contains viable tumour cells.
This has implications for targeted biopsy where sampling may not include the most aggressive tumour component and planning for maximal safe surgical resection.
Imaging response criteria
The most widely used methods of defining tumour response in clinical trials rely on changes in linear measurement of enhancing tumour bulk.
WHO and Macdonald criteria
The World Health Organization (WHO) criteria were developed in 1979 to measure tumour response, and involves calculating the product of the largest diameter and its perpendicular length for each measurable lesion and summing the products. Macdonald et al [10] adapted these for brain tumours in 1990, suggesting steroid treatment and clinical deterioration should also be considered when establishing response. Although the Macdonald criteria have been widely adopted, they have been criticised for being ambiguous in defining the appropriate threshold for lesion size and lacking detail in how to apply the criteria [11].
RECIST criteria
The Response Evaluation in Solid Tumours (RECIST) criteria were introduced in an attempt to simplify measurements in solid tumours. They rely on a one-dimensional, rather than two- dimensional measurement and summing the longest diameters of lesions. A small number of studies have attempted to validate this unidimensional approach [12,13] and found comparable results when using the MacDonald criteria and RECIST criteria. The application of RECIST 1.0 criteria to brain neoplasia has been questioned; the method was designed for well-marginated solid tumours outside the central nervous system, whereas gliomas can be heterogeneous, infiltrating and partially cystic. The recently updated RECIST 1.1 criteria attempt to address this problem by providing guidance on the approach to partially necrotic tumours and discrete cystic lesions [14]. Tables 1 and 2 highlight key features and definitions related to the Macdonald and RECIST criteria.
Table 1. A comparison of the features of the main response criteria.
| WHO | Macdonald | RECIST 1.0 | RECIST 1.1 | |
| Measurements | 2D | 2D | 1D | 1D |
| Clinical parameters | No | Yes | No | Yes |
| Cystic areas included | N/Aa | Nob | No | Yesc |
1D, one-dimensional; 2D, two-dimensional; WHO, World Health Organization.
aSpecific guidance on cystic lesions is not provided.
bThe authors of the Macdonald criteria suggest their guidelines are suitable for heterogeneous lesions but not for discrete non-enhancing lesions.
cNon cystic lesions are preferable target lesions.
Table 2. A comparison of the definitions for disease progression and response set out by the MacDonald and RECIST 1.1 criteria.
| MacDonald | RECIST 1.1 | |
| Progressive disease | Tumour increased by ≥25% or new sites of diseaseNeurology worse with stable or increased glucocorticosteroid use | Sum of diameters of target lesions ≥20% and increase of ≥5 mmNew unequivocal malignant lesionsNew ↑ FDG uptake |
| Stable disease | Other criteria not met | Other criteria not met |
| Partial response | Enhancing tumour measurements decreased by ≥50% on consecutive studies one month apart. No new sites of diseaseStable or reduced glucocorticosteroid useNeurology stable or improved | Sum of diameters of target lesions decreased by ≥50% |
| Complete response | No enhancing tumour on consecutive studies one month apartNo glucocorticosteroid useNeurology stable or improved | Disappearance of all target lymph nodesPathological lymph nodes decrease to <10 mm in short axis |
FDG, fluorodeoxyglucose.
Challenges to tumour measurement and therapeutic evaluation
There are major limitations to linear measurement of enhancing tumour components in defining glioma progression and treatment response. First, gliomas are frequently irregular in shape and may change anisotropically or differentially, which limits meaningful linear measurement. In addition, visible contrast-enhancing components are not necessarily representative of active tumour volume; non-enhancing active tumour components and therapy-related changes in enhancement are well recognised. These factors are considered in more detail below.
Tumour shape and heterogeneity
Gliomas can have an irregular shape, particularly after surgery, and hence their volume is difficult to estimate from a linear measurement. Reproducibility of measurements is poor. Moreover, differential growth of tumour components and the structure of surrounding brain (notably white matter tracts) frequently causes them to grow anisotropically (i.e. more in one plane than another), which further confounds unidimensional measures of growth and response.
Multifocal enhancement and multiple small satellite lesions can make assessment difficult [11]. These are more common after treatment. Necrotic and cystic lesions have also caused ambiguity in determining appropriate tumour measurement. Although this specific issue is addressed in the new RECIST 1.1 criteria [14], it is worth noting that cystic and solid elements can be intimately related, particularly after treatment, making them difficult to measure.
Evaluation of non-enhancing tumour
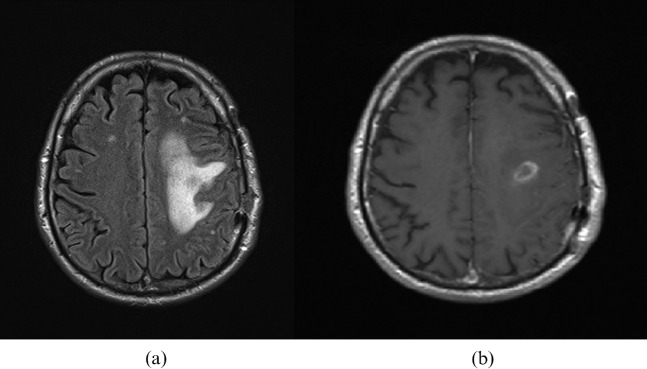
The challenge of measuring areas of non-enhancing tumour have been highlighted by The Response Assessment in Neuro-Oncology (RANO) Working Group, who suggest criteria for response assessment that include evaluation of non-enhancing areas of tumour [15]. This is thought to be particularly important for grade II and grade III gliomas, where the non-enhancing component may represent a sizeable proportion of the whole tumour and can be demonstrated by comparison of FLAIR and contrast-enhanced T1 weighted images (Figure 1). It can, however, be difficult to differentiate non-enhancing tumour from changes due to medical treatment, surgery or radiotherapy. The authors acknowledge these challenges, which prevent guidance on exact measures for response or progression of the non-enhancing components.
Figure 1.
Grade III astrocytoma. (a) Fluid-attenuated inversion-recovery image demonstrates the sizeable non-enhancing component of the tumour compared to (b) the modest enhancement demonstrated on the gadolinium-enhanced spin echo T1 weighted study.
Differential response
Heterogeneous biology within tumours may also be reflected in differential responses of different tumour components to treatment. Although complex patterns of tumour response on imaging are sometimes discussed for individual cases in clinical practice, there has been limited systematic examination of this in the literature. Differential treatment response has been described in transformed oligodendroglioma, and correlated with relative cerebral blood flow (rCBV) and local apparent diffusion coefficient (ADC) measures [16]. A study of patients treated with bevacizumab showed response in areas of necrotic tumour, while areas of solid tumour continued to grow [17]. These changes may not be captured reliably in summated measures of tumour bulk.
Pseudoprogression
It has long been known that radionecrosis may manifest as oedema and enhancement, which can be impossible to distinguish from progressive tumour using conventional MRI [18-20], and a range of non-specific enhancement patterns have been demonstrated [19]. Radiological pseudoprogression, where transient increases in apparent tumour size and enhancement are seen during and shortly after aggressive chemoradiotherapy regimens, is also increasingly recognised [21]. This phenomenon is more commonly seen in tumours with favourable MGMT (methylated O6-methyl guanine-DNA methyltransferase) methylation status, which ultimately show better treatment response. This issue has also been acknowledged by the RANO group, who suggest that within 12 weeks of chemoradiotherapy, progression can only be defined on imaging if there is new enhancement outside the radiation field [15].
Pseudoregression
Steroid treatment has been shown to decrease blood–tumour barrier permeability and regional cerebral blood volume [22]. Controlling for steroid treatment is therefore important when measuring response.
Anti-angiogenic agents specifically targeted to vascular endothelial growth factor are now being used to treat gliomas, and may have a complex effect upon vasculature [23], which in turn modulates contrast enhancement. There is concern the antivascular effects may cause pseudoregression, with decreased enhancement without actual tumour regression [11]. Therefore, contrast enhancement alone is not a suitable marker for tumour response in this context.
Validation of conventional MR endpoints
There is limited evidence that some MRI features correlate negatively with survival. Oedema is the most commonly cited negative predictor [8,24]. Again, the low specificity of these MR characteristics limits their use.
Studies assessing the conventional MRI measurements of brain tumour response described above, in general, have shown poor correlation between imaging response and survival [12,25]. However, one study has shown evidence of correlation between linear methods and overall survival at 2 months that could not be reproduced at 6 months [13]. The authors pointed to the short duration of response of current therapies as a possible explanation for these findings. Therefore, the intervals at which response is measured need careful consideration before assessments are made about the validity of imaging markers as predictors of survival.
The poor correlation of response criteria with hard endpoints is attributable to both the limitations in linear measures in defining irregular lesions and limited specificity of enhancement as a marker of active tumour outlined above. The subjective nature of deciding whether changes are treatment or tumour related is also likely to increase interobserver variability.
Conclusion
Conventional imaging with CT and MRI is widely available and provides useful structural information about gliomas but limited physiological detail. Response metrics based on linear measurements of enhancing tumour components are biologically non-specific and poorly reproducible, and provide limited prognostic power for outcome.
References
- 1.Al-Okaili RN, Krejza J, Wang S, Woo JH, Melhem ER. Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. Radiographics 2006;26:S173–89 [DOI] [PubMed] [Google Scholar]
- 2.Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002;59:947–9 [DOI] [PubMed] [Google Scholar]
- 3.White ML, Zhang Y, Kirby P, Ryken TC. Can tumor contrast enhancement be used as a criterion for differentiating tumor grades of oligodendrogliomas? AJNR Am J Neuroradiol 2005;26:784–90 [PMC free article] [PubMed] [Google Scholar]
- 4.Asari S, Makabe T, Katayama S, Itoh T, Tsuchida S, Ohmoto T. Assessment of the pathological grade of astrocytic gliomas using an MRI score. Neuroradiology 1994;36:308–10 [DOI] [PubMed] [Google Scholar]
- 5.Pierallini A, Bonamini M, Bozzao A, Pantano P, Stefano DD, Ferone E, et al. Supratentorial diffuse astrocytic tumours: proposal of an MRI classification. Eur Radiol 1997;7:395–9 [DOI] [PubMed] [Google Scholar]
- 6.Aghi M, Gaviani P, Henson JW, Batchelor TT, Louis DN, Barker FG. Magnetic Resonance Imaging Characteristics Predict Epidermal Growth Factor Receptor Amplification Status in Glioblastoma. Clin Cancer Res 2005;11:8600–5 [DOI] [PubMed] [Google Scholar]
- 7.Pope WB, Chen JH, Dong J, Carlson MR, Perlina A, Cloughesy TF, et al. Relationship between Gene Expression and Enhancement in Glioblastoma Multiforme: Exploratory DNA Microarray Analysis. Radiology 2008;249:268–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol 2005;26:2466–74 [PMC free article] [PubMed] [Google Scholar]
- 9.Pronin IN, Holodny AI, Petraikin AV. MRI of high-grade glial tumors: correlation between the degree of contrast enhancement and the volume of surrounding edema. Neuroradiology 1997;39:348–50 [DOI] [PubMed] [Google Scholar]
- 10.Macdonald D, Cascino T, Schold S, Cairncross J. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80 [DOI] [PubMed] [Google Scholar]
- 11.Sorensen AG, Batchelor TT, Wen PY, Zhang W-T, Jain RK. Response criteria for glioma. Nat Clin Pract Oncol 2008;5:634–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47 [DOI] [PubMed] [Google Scholar]
- 13.Shah GD. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro-Oncology 2006;8:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galanis E, Buckner JC, Maurer MJ, Sykora R, Castillo R, Ballman KV, et al. Validation of neuroradiologic response assessment in gliomas: Measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro-Oncology 2006;8:156–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol 2010;28:1963–72 [DOI] [PubMed] [Google Scholar]
- 16.Jager HR, Waldman AD, Benton C, Fox N, Rees J. Differential chemosensitivity of tumor components in a malignant oligodendroglioma: assessment with diffusion-weighted, perfusion-weighted, and serial volumetric MR imaging. AJNR Am J Neuroradiol 2005;26:274–8 [PMC free article] [PubMed] [Google Scholar]
- 17.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 2006;66:1258–60 [DOI] [PubMed] [Google Scholar]
- 18.de Wit MCY, de Bruin HG, Eijkenboom W, Sillevis Smitt PAE, van denBent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 2004;63:535–7 [DOI] [PubMed] [Google Scholar]
- 19.Nishimura R, Takahashi M, Morishita S, Sumi M, Uozumi H, Sakamoto Y. MR Gd-DTPA enhancement of radiation brain injury. Radiat Med 1992;10:109–16 [PubMed] [Google Scholar]
- 20.Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 2000;21:901–9 [PMC free article] [PubMed] [Google Scholar]
- 21.Brandsma D, Stalpers L, Taal W, Sminia P, van denBent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncology 2008;9:453–61 [DOI] [PubMed] [Google Scholar]
- 22.Ostergaard L, Hochberg FH, Rabinov JD, Sorensen AG, Lev M, Kim L, et al. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J Neurosurg 1999;90:300–5 [DOI] [PubMed] [Google Scholar]
- 23.Bullitt E, Reardon DA, Smith JK. A review of micro- and macrovascular analyses in the assessment of tumor-associated vasculature as visualized by MR. NeuroImage 2007;37:S116–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson MR, Pope WB, Horvath S, Braunstein JG, Nghiemphu P, Tso C-L, et al. Relationship between Survival and Edema in Malignant Gliomas: Role of Vascular Endothelial Growth Factor and Neuronal Pentraxin 2. Clin Cancer Res 2007;13:2592–8 [DOI] [PubMed] [Google Scholar]
- 25.Dempsey MF, Condon BR, Hadley DM. Measurement of Tumor “Size” in Recurrent Malignant Glioma: 1D, 2D, or 3D? AJNR Am J Neuroradiol 2005;26:770–6 [PMC free article] [PubMed] [Google Scholar]