Abstract
Invasion of tumour cells into the normal brain is one of the major reasons of treatment failure for gliomas. Although there is a good understanding of the molecular and cellular processes that occur during this invasion, it is not possible to detect the extent of the tumour with conventional imaging. However, there is an understanding that the degree of invasion differs with individual tumours, and yet they are all treated the same. Newer imaging techniques that probe the pathological changes within tumours may be suitable biomarkers for invasion. Imaging methods are now available that can detect subtle changes in white matter organisation (diffusion tensor imaging), tumour metabolism and cellular proliferation (using MR spectroscopy and positron emission tomography) occurring in regions of tumour that cannot be detected by conventional imaging. The role of such biomarkers of invasion should allow better delineation of tumour margins, which should improve treatment planning (especially surgery and radiotherapy) and provide information on the invasiveness of an individual tumour to help select the most appropriate therapy and help stratify patients for clinical trials.
Recent studies combining maximal resection, chemotherapy and radiotherapy have at last provided significant improvements in survival for patients with high-grade gliomas [1]. Despite these advances, most patients will still die from progressive disease. One of the major factors for treatment failure is the invasion of glioma cells into normal brain, a key feature of gliomas. These infiltrating tumour cells mean that surgical resection is rarely curative; even attempts at removing entire hemispheres have failed to halt tumour progression [2]. Radiation oncologists add a 2 cm margin to the apparent tumour to produce a clinical target volume (CTV) that encompasses these infiltrating cells. As this volume includes normal brain tissue that is sensitive to radiation injury, the total dose has to be reduced to within the tolerance limits of the normal brain [3]. This dose is insufficient to sterilise tumour cells, resulting in most tumours recurring within the high-dose treatment volume [4,5]. In addition, these infiltrating cells are predominantly migrating and not proliferating [6], so treatments that disrupt dividing cells (especially radiotherapy and chemotherapy) will have less effect on these cells.
Although tumour invasion is a key feature of gliomas, the degree of invasion is variable. Post-mortem studies show that between 20% and 27% of glioblastomas have limited invasion (i.e. infiltrating cells less than 1 cm from the edge of the gross tumour) [7,8]; 20% have more extensive invasion (i.e. invasion of more than 3 cm from the gross tumour) [8] with 8% showing disseminated spread [9]. It is clear that these groups should be treated differently and raises the question of whether these tumours should be considered as local disease (requiring aggressive local therapy) or diffuse disease (requiring systemic therapy) [10]. At present we cannot separate glioblastomas based on their extent of invasion. As a result we must treat all of them the same, despite the fact gliomas with limited invasion are likely to respond better to local therapies than those with diffuse invasion.
Attempts to better understand the molecular differences in more infiltrative tumours have suggested a number of genes that are upregulated in these tumours [11,12,13]. The problem with using this approach to determine the invasiveness of an individual tumour is that it requires tissue. As a result it cannot guide surgical treatment or local therapies at the time of resection. It is also unable to demonstrate the tumour margin. Development of imaging biomarkers for the non-invasive study of tumour invasion could potentially provide this information.
Problems with conventional imaging
With the advent of improvements in brain imaging the hope was that the margin of gliomas could be accurately determined. Biopsy and post-mortem studies, have shown that gliomas extend further than could be determined using conventional imaging techniques. For CT imaging, tumour cells extend beyond the area of CT enhancement and are frequently seen in regions of peritumoural oedema [14,15]. Tumour cells may be found in areas that appeared normal on CT in 20% of serial stereotactic biopsy specimens [16]. Tumour cells can be detected up to 6 cm from abnormal areas on CT [17].
The improved soft-tissue resolution of MR has failed to improve the identification of the tumour margin. Biopsy studies have shown that the tumour extends beyond the margin of T2 signal change in most glioblastomas [17,18], in some cases tumours extended up to 2.5 cm beyond the area of T2 signal change. Tumours can be identified in regions with a normal T1 signal in 16% of biopsies and have a normal T2 signal in 4% of biopsies [16]. It is clear from these studies that tumour cell invasion extends at least as far as the abnormal T2 signal in both high- and low-grade gliomas.
Studies have also focused on the border of the T2 weighted abnormality. In oligodendrogliomas the sharpness of the T2 weighted abnormality did not predict invasive behaviour, but did predict the presence of loss of heterozygosity of chromosomes 1p and 19q, which is a marker of good prognosis and response to chemotherapy [19]. In glioblastomas, tumours with a low T2 weighted border sharpness and a high ratio of the volume of the T2 weighted abnormality to the T1 weighted area correlated with increased expression of epidermal growth factor receptor (EGFR) [20], a molecular marker that is known to be associated with invasive behaviour [21].
If conventional imaging fails to identify invasion, novel methods based on our understanding of the biology of glioma invasion are needed to address this problem.
Biology of glioma invasion
Much of our understanding of the process of glioma invasion has come from the careful examination of post-mortem brains by Hans Scherer in the late 1930s. He showed that individual cells disperse predominantly along white matter tracts, with some spreading along blood vessels and along the ependymal and pial lining [7,22]. Spread along white matter tracts involves individual cells spreading within (intrafasicular), around (parafasicular) and between (interfibrillary) the axonal processes within the white matter. Little damage is caused at this stage, and the white matter tract remains intact. As the tumour develops and the number of tumour cells increases, the white matter tracts are destroyed by tumour. Scherer referred to this as neurophagic growth [22].
Glioma cell invasion of normal brain is a multistep process. One of the first stages is binding of tumours to the extracellular matrix (ECM) or other cells. Much of our understanding of this process comes from other cancer models and shows that the tumour binds via a number of receptor systems (especially integrin receptors) to matrix glycoproteins, such as fibronectin, laminin, vibronectin and collagen. In gliomas, however, these glycoproteins are only found on the basal membrane of blood vessels and the glial limitans, but are not found in white matter [23]. Although culture experiments suggest that glial tumours can secrete matrix proteins [24], the main matrix component surrounding neurons and glia are glycoaminoglycans, especially hyaluron. A number of adhesion molecules will bind to this [25].
Once tumours bind to the ECM it needs to create space to allow the cells to move in. The various proteases include the matrix metalloproteinases (MMPs) (especially MMP-2, MMP-9 and the membrane-bound MT1-MMP [26], the serine proteases (especially plasminogen activators [27]) and cysteine proteases (especially cathepsins [28]). Once there is space, the cells will migrate in to complete the process. Tumour invasion is accompanied by growth of vessels (angiogenesis) and all these processes are controlled by autocrine and paracrine communication between tumour cells, glial cells, endothelial cells and various immune cells [29].
Potential biomarkers of tumour invasion
Recent advances in imaging can now provide information that is not possible on anatomical "conventional" imaging. These techniques allow us to probe pathological changes within tumours. These methods provide information on cellularity (diffusion MR and MR spectroscopy), angiogenesis (perfusion MR), metabolism [various methods using positron emission tomography (PET) and MR spectroscopy] and cellular proliferation (both PET and MR spectroscopy) [30]. As all of these processes are involved in tumour invasion they can potentially be used as both direct and indirect markers of invasion.
Imaging white matter disruption
As has been previously discussed, gliomas preferentially spread along white matter tracts. This is in contrast with metastases, which tend to spread along vascular planes and form a tumour that is separate from the surrounding brain. Diffusion tensor imaging (DTI) is very sensitive in detecting disruption of white matter in regions that appear normal in a number of diseases [31-35]. Initial studies in brain tumours have shown a 30° deviation of corona radiata fibres in a patient with a low-grade glioma, suggesting that the fibres had been displaced but not infiltrated by the tumour [36]. Using directionally encoded colour maps, Mori et al [37] could differentiate tumour displacement of adjacent tracts from tumour infiltration in two patients with anaplastic astrocytomas. This method was further refined to identify four patterns of white matter involvement [38].
White matter disruption by the tumour: isotropic or near isotropic diffusion so that tract is not identifiable on fractional anisotropy (FA) or directionally encoded colour maps.
Tumour infiltrated white matter tracts: reduction of FA (>25%) with increased apparent diffusion coefficient (ADC) with abnormal colour hues not as a result of bulk movement.
Oedematous white matter tracts: reduction of FA (>25%) with increased ADC with normal direction and location (i.e. colour hues) on directionally encoded maps. There is some doubt if this differs much from the infiltrated tracts described above.
Displacement of white matter tracts: normal or mildly decreased (<25%) FA values compared with contralateral side, but alteration in either position or direction of fibres on directionally encoded colour maps.
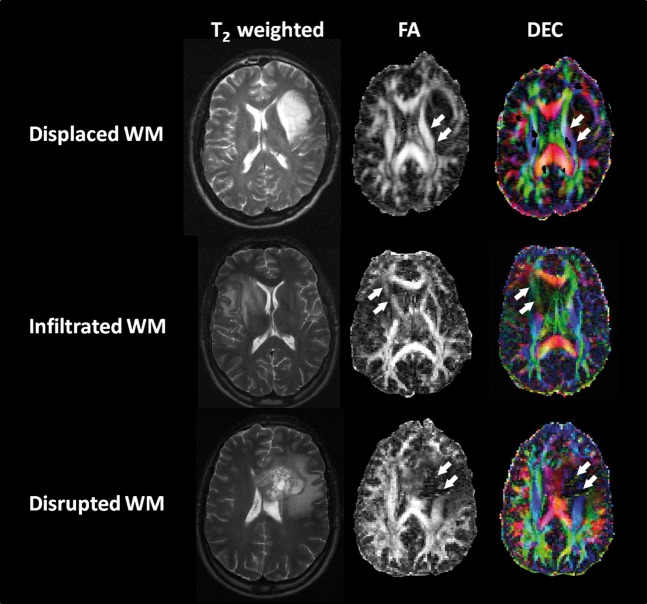
Examples of these patterns are demonstrated in Figure 1. At present, these studies lack histological confirmation, but using this information for intra-operative planning has allowed safe resection without worsening the neurological deficit [38].
Figure 1.
Example of the use of diffusion tensor imaging (DTI) to understand the effect of the tumour on white matter (WM) tracts. The upper row shows a tumour deviating a tract (arrows). In this case the fractional anisotropy (FA) values are similar to the contralateral side but the directionally encoded colour (DEC) maps shows the tract colour is different to the contralateral side. The middle row shows tumour invasion of a tract (arrows). The tract can still be seen but with reduced FA and hue on DEC. The lowest row shows tumour disruption (arrows), where no tract can be identified on either method.
Attempts have been made to differentiate the effects of invasive gliomas on white matter tracts from non-invasive tumours (e.g. meningiomas and metastases). The results appear mixed, with some studies showing a larger reduction in FA in the peritumoural region [39-42] while other studies only show significant increases in mean diffusivity (D) [43,44]. One study found no change in FA values but did find a decrease in the magnitude of the principal eigenvalue in the peritumoural tissue of gliomas [45]. Another study described visual differences in FA surrounding gliomas compared with metastases but failed to demonstrate changes in FA values [46] (an example of this visual difference is shown in Figure 2). Their study, however, failed to measure the FA in the peritumoural tissues.
Figure 2.
Visual differences in fractional anisotropy between a metastasis (upper row) and a glioblastoma. With the metastasis there is still intact white matter surrounding the tumour (arrows) whereas in the glioblastoma there is disruption of the white matter over a larger area. This difference, despite the marked oedema from both tumours, has been suggested as a result of tumour invasion.
Reduction of FA is not the only finding surrounding brain tumours. Longitudinal studies in a rat C6 tumour model (a model that does not infiltrate normal brain and acts more like a metastasis [47]) show an increase in anisotropy at the tumour margin, suggesting compression of surrounding white matter tracts [48]. This has been confirmed histologically in a rat model with C6 glioma cells engrafted into the spinal cord [49]. A similar finding has been reported at the edge of some glioblastomas and meningiomas in patients [41].
As it appears FA alone is too insensitive to identify occult white matter infiltration completely, various groups have developed new ways of analysing the tensor information. Zhou and Leeds [50] described a regional fibre coherence index that is based on the fibre orientation in a cluster of voxels around the voxel being investigated. The sum of these vectors are weighted by the proximity of the voxel to the one investigated. They found that, in some areas with low FA values, there was a low coherence index (i.e. surrounding voxels had random orientation of white matter fibres). Follow-up imaging revealed DTI abnormalities persisting up to 3 months later. In other areas they found a high index (i.e. fibres had similar orientations); in these regions anisotropy returned on follow-up. They suggested that a low coherence index represents tumour infiltration whereas a high index represents oedema. This finding was not confirmed with histology.
Lu et al [51] found that mean diffusivity (D) and FA in peritumoural regions were linearly related in a cohort of patients with non-invasive tumours (e.g. metastases and meningiomas). For gliomas this relationship is not linear. This difference could be quantified to produce a novel parameter called a tumour infiltration index derived from the difference of the expected FA (from the linear regression model) for a given D and the observed FA. They suggested that the differences between these two groups were due to tumour cell infiltration. This technique, however, could not differentiate between high- and low-grade gliomas. Morita et al [52] studied the peritumoural region in a number of tumours using lambda chart analysis method that plots the largest eigenvalues (i.e. λ1, called the longitudinal lambda, λL) vs the transverse lambda (the average of the two remaining eigenvalues, λ2 and λ3 is the transverse lambda, λT). The values for high-grade gliomas and other tumours (low-grade gliomas, metastases and meningiomas) were different. This would suggest reduced anisotropy in high-grade gliomas owing to tumour infiltration of the white matter.
The obvious question from these studies is whether the difference in diffusion around high-grade gliomas is actually as a result of tumour cell invasion. There is some evidence that this is indeed due to tumour. Follow-up studies have shown that DTI abnormalities can predict the presence of tumour [53,54] and can even predict the pattern of recurrence [55]. Multimodal imaging studies have shown that the reduction in anisotropy correlates with reduction in N-acetylaspartate (NAA) concentrations as measured with MR proton spectroscopy, suggesting it is due to the reduction in neuronal integrity [56]. A number of studies have tried to correlate image-guided biopsy material with DTI measurements. Pauleit et al [57] tried to correlate these peritumoural ADC measurements with histology determined by image-guided biopsies. Although the ADC from the peritumoural tissue was higher than the tumour tissue (1.23±0.21×10−3 mm2 s−1 vs 1.11±0.3×10−3 mm2 s−1) it was not significant. This study pooled the ADC values from tumour and peritumoural brain from a variety of tumour grades (and only included two glioblastomas) and is likely to be confounded by the variation of ADC values with tumour grade. Another study comparing diffusion tissue signatures [58,59] with tissue from image-guided biopsies avoided this problem by comparing the values with histology in individual patients [60]. Using this method it was possible to differentiate normal tissue from tumour or tumour-invaded brain with a sensitivity of 98% and specificity of 81%. One other study found FA values correlated better than ADC with tumour cellularity and infiltration [61].
The use of DTI to look at white matter disruption appears to be a sensitive marker of tumour margin. What is not certain is whether it tells us about invasiveness in an individual patient; however, some work suggests it might. Analysis of diffusion tissue signatures found that in 20% of the studied patients there was little DTI disruption of the surrounding white matter tracts [55]. The progression-free survival in this group of patients was markedly increased in this group, and follow-up imaging suggested they had limited invasiveness. This study was retrospective and contained a very heterogeneous group of tumours that had received a variety of treatments. Confirmation by a large prospective study is needed.
Tumour metabolism
Malignant tumours are hypermetabolic compared with the surrounding normal brain. There is an increase in glycolytic metabolism, increased protein synthesis and an increase in membrane synthesis to maintain the rapidly dividing malignant cells. There are various imaging methods that study these processes.
MR spectroscopy can differentiate between tumour and normal brain, and studies have attempted to determine tumour margins. The region of oedema surrounding a glioma has a similar spectroscopic pattern as the centre of the tumour with increased choline (Cho) peaks and reduced NAA compared with normal brain [62,63]. An example is shown in Figure 3. This was not seen in non-invasive tumours (such as meningiomas) where the spectra in regions of oedema resembled normal brain tissue [63]. Some of these areas of oedema identified on T2 weighted imaging have Cho/NAA ratios greater than 2, which is within the range seen with tumours [64]. In an image-guided biopsy study, Croteau et al [65] showed that the Cho/NAA and normalised Cho ratios correlated with the degree of tumour infiltration. Although spectroscopy was better than conventional MRI at defining the tumour margin, they were unable to differentiate between normal brain and mild tumour infiltration. Follow-up imaging has shown that these areas of spectroscopic abnormality could predict the later development of contrast enhancement [66]. More recent studies have shown that the spectroscopic abnormality is 20% larger than the volume of increased T2 signal and biopsies of this region show evidence of tumour invasion [67]. Other studies have detected increases in myoinositol (a spectroscopic marker of glia) and glutamate (implicated with anabolic pathways upregulated in tumours) in the contralateral hemisphere of glioblastomas [68]. This change was not seen in either low-grade gliomas or normal controls.
Figure 3.
An example of the proton spectra (echo time=30 ms) within and surrounding a World Health Organization grade III anaplastic astrocytoma. The first region is within normal brain and the normal spectra can be seen with high N-acetylaspartate (NAA) peaks and a lower choline peak. The second spectrum is taken from the peritumoural tissue where there is a much higher choline peak and a lower NAA peak. NAA is still detectable suggesting viable neurons are still present. Within the tumour there is a very high choline peak but NAA is undetectable.
Measuring the uptake of amino acids with PET imaging using either 11C-methionine (MET) or 18F-fluoroethyl-l-tyrosine (FET) has been shown to be a more sensitive method of tumour detection than 18F-fluorodeoxyglucose (FDG) PET [69]. Studies that fuse MR and methionine PET images have shown that the volume of increased methionine uptake is greater than the volume of gadolinium enhancement on T1 weighted MR, and, although smaller than the volume of increased T2 weighted signal, it extends beyond it in most cases [70]. Other studies have shown the region of increased amino acid uptake correlates well with areas of increased Cho/NAA [71] and DTI abnormalities in white matter tracts [72]. To see if methionine could better determine the margin of gliomas, Mosskin et al [73] compared methionine uptake with image-guided brain biopsies. They found that in a cohort of 38 patients that mainly had low-grade gliomas, the tumour extended beyond the area of methionine uptake in 5 out of 38 cases (13%), and that the methionine uptake overestimated the tumour size in a further 5 of 38 cases. In a later study comparing methionine uptake with unenhanced MRI, they found that the tumour extended beyond the area of methionine uptake in 2 out of 9 (22%) patients [74]. In other words, the methionine abnormality cannot accurately predict the tumour margins, suggesting that either the peripheral infiltrating cells do not take up methionine, or that other, reactive/inflammatory cells have increased uptake. Other biopsy studies suggest that a ratio of MET uptake (normalised to normal brain) of greater than 1.3 could detect tumour tissue with a sensitivity of 87% and specificity of 89% [75]. Interestingly, in low-grade gliomas, the infiltrating cells had a higher uptake than regions of solid tumour.
Cellular proliferation
Cellular proliferation is a cardinal feature of malignant tumours. In the brain, as the surrounding normal structures has a very low proliferation rate, attempts have been made to use these markers to better identify the tumour margin. 18F-fluorothymidine (FLT) is actively taken up into dividing cells and has an excellent contrast-to-background ratio [76-78]. The area of abnormality is larger than the abnormality seen on MR [77]. An example is shown in Figure 4. Studies measuring FLT uptake have shown it correlates well with tissue markers of proliferation [77,78,79]. A recent study comparing uptake of FLT to the appearance of tumour in image-guided biopsies has shown that FLT underestimates the extent of tumours in half of the cases [78]. This can be explained by the finding that dividing and infiltrating cells appear to be two distinct tumour phenotypes, and that the most invasive cells will not be dividing [6].
Figure 4.

An example of a 18F-fluorothymidine (FLT) positron emission tomography (PET) image overlying a contrast-enhanced T1 weighted image of a glioblastoma. Although the FLT uptake is largely in areas of contrast enhancement, the uptake extends into the surrounding brain. Histological analysis of image-guided biopsies, however, showed that the FLT uptake underestimated the region of tumour invasion in this case.
Perfusion changes owing to angiogenesis
Tumour growth and invasion is dependent on developing a suitable blood supply. Studies have shown that there is an increase in rCBV in regions adjacent to the tumour that appeared normal on conventional imaging [80]. Biopsies of these abnormalities have confirmed infiltrating tumour [81] and are not seen in non-infiltrating tumours (e.g. meningiomas) [82].
Molecular markers of gliomas
All of the methods so far discussed are indirect methods of detecting tumour cells. The advance of molecular imaging may allow the direct detection of these cells. It has already been mentioned that the expression of EGFR is an important molecular marker of de novo glioblastomas, which have the tendency to be more invasive [83]. A number of PET tracers are being developed to study these processes using both 18F- and 11C-labelled irreversible EGFR inhibitors [84,85], as well as 64Cu-labelled EGFR blocking drugs (e.g. Cetuximab) [86]. Animal studies suggest good identification of EGFR-positive tumours [86].
One of the key molecular components of the invasive behaviour is the production of MMPs. PET tracers of MMP activity are currently being developed using 125I- and 18F-labelled analogues of the non-peptidyl broad-spectrum MMP inhibitor CGS 27023A [87,88]. Animal studies suggest this preferentially detects activity of MMP-1, -2 and -9 [87], the MMPs with the most important activity in gliomas [89]. Such PET markers may help determine the degree of invasiveness in an individual glioma.
Conclusion
Tumour invasion is a key stage of gliomas that is poorly detected using conventional imaging methods. Using methods that look at changes in white matter structure (DTI), metabolism and proliferation we can begin to detect the edge of the tumour and get an idea of the degree of invasiveness for an individual patient. The utility of these methods in radiotherapy planning has already been shown; using DTI to plan radiotherapy may lead to reduction of planning target volumes of 35% and allow dose escalation by a mean of 7 Gy (range 4–14 Gy) for the same risk of normal tissue injury [90]. Similarly, Pirzkall et al [66] have shown that radiotherapy volumes derived from spectroscopy extended beyond the T2 signal abnormality in 60% of cases and should thus be used for planning radiotherapy. Further studies to show if these techniques could be used to improve radiotherapy planning are underway.
Acknowledgments
Stephen Price is funded by a Clinician Scientist Award from the National Institute for Health Research.
References
- 1.Stupp R, Mason WP, van denBent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96 [DOI] [PubMed] [Google Scholar]
- 2.Dandy WE. Removal of the right hemisphere for certain tumors with hemiplegia: preliminary report. J Am Med Assoc 1928;90:823–5 [Google Scholar]
- 3.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22 [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Fraass BA, Marsh LH, Herbort K, Gebarski SS, Martel MK, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys 1999;43:79–88 [DOI] [PubMed] [Google Scholar]
- 5.Oppitz U, Maessen D, Zunterer H, Richter S, Flentje M. 3D-recurrence-patterns of glioblastomas after CT-planned postoperative irradiation. Radiother Oncol 1999;53:53–7 [DOI] [PubMed] [Google Scholar]
- 6.Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME. Dichotomy of astrocytoma migration and proliferation. Int J Cancer 1996;67:275–82 [DOI] [PubMed] [Google Scholar]
- 7.Scherer HJ. The forms of growth in gliomas and their practical significance. Brain 1940;63:1–35 [Google Scholar]
- 8.Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg 1988;68:698–704 [DOI] [PubMed] [Google Scholar]
- 9.Parsa AT, Wachhorst S, Lamborn KR, Prados MD, McDermott MW, Berger MS, et al. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg 2005;102:622–8 [DOI] [PubMed] [Google Scholar]
- 10.Shapiro WR. Current therapy for brain tumors: back to the future. Arch Neurol 1999;56:429–32 [DOI] [PubMed] [Google Scholar]
- 11.Mariani L, McDonough WS, Hoelzinger DB, Beaudry C, Kaczmarek E, Coons SW, et al. Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res 2001;61:4190–6 [PubMed] [Google Scholar]
- 12.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Demuth T, Ross KR, et al. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J Neurooncol 2001;53:161–76 [DOI] [PubMed] [Google Scholar]
- 13.Mariani L, Beaudry C, McDonough WS, Hoelzinger DB, Kaczmarek E, Ponce F, et al. Death-associated protein 3 (Dap-3) is overexpressed in invasive glioblastoma cells in vivo and in glioma cell lines with induced motility phenotype in vitro. Clin Cancer Res 2001;7:2480–9 [PubMed] [Google Scholar]
- 14.Lilja A, Bergstrom K, Spannare B, Olsson Y. Reliability of computed tomography in assessing histopathological features of malignant supratentorial gliomas. J Comput Assist Tomogr 1981;5:625–36 [PubMed] [Google Scholar]
- 15.Selker RG, Mendelow H, Walker M, Sheptak PE, Phillips JG. Pathological correlation of CT ring in recurrent, previously treated gliomas. Surg Neurol 1982;17:251–4 [DOI] [PubMed] [Google Scholar]
- 16.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 1987;66:865–74 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 1992;34:463–9 [DOI] [PubMed] [Google Scholar]
- 18.Lunsford LD, Martinez AJ, Latchaw RE. Magnetic resonance imaging does not define tumor boundaries. Acta Radiol Suppl 1986;369:154–6 [PubMed] [Google Scholar]
- 19.Jenkinson MD, du Plessis DG, Smith TS, Joyce KA, Warnke PC, Walker C. Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain 2006;129:1884–91 [DOI] [PubMed] [Google Scholar]
- 20.Aghi M, Gaviani P, Henson JW, Batchelor TT, Louis DN, Barker FG., II Magnetic Resonance imaging characteristics predict epidermal growth factor receptor amplification status in glioblastoma. Clin Cancer Res 2005;11:8600–5 [DOI] [PubMed] [Google Scholar]
- 21.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res 2002;62:3335–9 [PubMed] [Google Scholar]
- 22.Scherer HJ. Structural development in gliomas. Am J Cancer 1938;34:333–51 [Google Scholar]
- 23.Rutka JT, Apodaca G, Stern R, Rosenblum M. The extracellular matrix of the central and peripheral nervous systems: structure and function. J Neurosurg 1988;69:155–70 [DOI] [PubMed] [Google Scholar]
- 24.Giese A, Rief MD, Loo MA, Berens ME. Determinants of human astrocytoma migration. Cancer Res 1994;54:3897–904 [PubMed] [Google Scholar]
- 25.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery 1996;39:235–50 [DOI] [PubMed] [Google Scholar]
- 26.Belien AT, Paganetti PA, Schwab ME. Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J Cell Biol 1999;144:373–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsatas D, Kaye H. The role of the plasminogen activation cascade in glioma cell invasion: a review. J Clin Neurosci 2003;10:139–45 [DOI] [PubMed] [Google Scholar]
- 28.Demchik LL, Sameni M, Nelson K, Mikkelsen T, Sloane BF. Cathepsin B and glioma invasion. Int J Dev Neurosci 1999;17:483–94 [DOI] [PubMed] [Google Scholar]
- 29.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 2007;99:1583–93 [DOI] [PubMed] [Google Scholar]
- 30.Price SJ. The role of advanced MR imaging in understanding brain tumour pathology. Br J Neurosurg 2007;21:562–75 [DOI] [PubMed] [Google Scholar]
- 31.Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, et al. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging 2002;23:433–41 [DOI] [PubMed] [Google Scholar]
- 32.Gillard JH, Papadakis NG, Martin K, Price CJS, Warburton EA, Antoun NM, et al. MR diffusion tensor imaging of white matter tract disruption in stroke at 3 T. Br J Radiol 2001;74:642–7 [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 2001;57:632–8 [DOI] [PubMed] [Google Scholar]
- 34.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–22 [DOI] [PubMed] [Google Scholar]
- 35.Filippi CG, Ulu AM, Ryan E, Ferrando SJ, van Gorp WW. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol 2001;22:277–83 [PMC free article] [PubMed] [Google Scholar]
- 36.Wieshmann UC, Symms MR, Parker GJ, Clark CA, Lemieux L, Barker GJ, et al. Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumour. J Neurol Neurosurg Psychiatry 2000;68:501–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori S, Frederiksen K, van Zijl PC, Stieltjes B, Kraut MA, Solaiyappan M, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol 2002;51:377–80 [DOI] [PubMed] [Google Scholar]
- 38.Witwer BP, Moftakhar R, Hasan KM, Deshmukh P, Haughton V, Field A, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg 2002;97:568–75 [DOI] [PubMed] [Google Scholar]
- 39.Price SJ, Burnet NG, Donovan T, Green HA, Pena A, Antoun NM, et al. Diffusion Tensor Imaging of Brain Tumours at 3T: A Potential Tool for Assessing White Matter Tract Invasion? Clin Radiol 2003;58:455–62 [DOI] [PubMed] [Google Scholar]
- 40.Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D. Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 2004;232:451–60 [DOI] [PubMed] [Google Scholar]
- 41.Tropine A, Vucurevic G, Delani P, Boor S, Hopf N, Bohl J, et al. Contribution of diffusion tensor imaging to delineation of gliomas and glioblastomas. J Magn Reson Imaging 2004;20:905–12 [DOI] [PubMed] [Google Scholar]
- 42.Talos IF, Zou KH, Kikinis R, Jolesz FA. Volumetric assessment of tumor infiltration of adjacent white matter based on anatomic MRI and diffusion tensor tractography. Acad Radiol 2007;14:431–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu S, Ahn D, Johnson G, Cha S. Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol 2003;24:937–41 [PMC free article] [PubMed] [Google Scholar]
- 44.van Westen D, Lätt J, Englund E, Brockstedt S, Larsson E-M. Tumor extension in high-grade gliomas assessed with diffusion magnetic resonance imaging: values and lesion-to-brain ratios of apparent diffusion coefficient and fractional anisotropy. Acta Radiol 2006;47:311–19 [DOI] [PubMed] [Google Scholar]
- 45.Wiegell MR, Henson JW, Tuch DS, Sorensen AG. Diffusion tensor imaging shows potential to differentiate infiltrating from non-infiltrating tumors. Proc Intl Soc Mag Reson Med 2003;11:2075 [Google Scholar]
- 46.Tsuchiya K, Fujikawa A, Nakajima M, Honya K. Differentiation between solitary brain metastasis and high-grade glioma by diffusion tensor imaging. Br J Radiol 2005;78:533–7 [DOI] [PubMed] [Google Scholar]
- 47.Farrell CL, Stewart PA, Del Maestro RF. A new glioma model in rat: the C6 spheroid implantation technique permeability and vascular characterization. J Neurooncol 1987;4:403–15 [DOI] [PubMed] [Google Scholar]
- 48.Lope-Piedrafita S, Garcia-Martin ML, Galons JP, Gillies RJ, Trouard TP. Longitudinal diffusion tensor imaging study of a rat brain glioma model. Proc Intl Soc Mag Reson Med 2005;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inglis BA, Neubauer D, Yang L, Plant D, Mareci TH, Muir D. Diffusion tensor MR imaging and comparative histology of glioma engrafted in the rat spinal cord. AJNR Am J Neuroradiol 1999;20:713–16 [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou XJ, Leeds NE. Assessing glioma cell infiltration using a fiber coherence index: a DTI study. Proc Intl Soc Mag Reson Med 2005;13:365 [Google Scholar]
- 51.Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI. Diffusion-tensor mr imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 2004;232:221–8 [DOI] [PubMed] [Google Scholar]
- 52.Morita K, Matsuzawa H, Fujii Y, Tanaka R, Kwee IL, Nakada T. Diffusion tensor analysis of peritumoral edema using lambda chart analysis indicative of the heterogeneity of the microstructure within edema. J Neurosurg 2005;102:336–41 [DOI] [PubMed] [Google Scholar]
- 53.Price SJ, Pena A, Burnet NG, Pickard JD, Gillard JH. Detecting glioma invasion of the corpus callosum using diffusion tensor imaging. Br J Neurosurg 2004;18:391–5 [DOI] [PubMed] [Google Scholar]
- 54.Zhou XJ, Leeds NE, Poonawalla AH, Weinberg J. Assessment of tumour cell infiltration along white-matter fiber tracts using diffusion tensor imaging. Proc Intl Soc Mag Reson Med 2003;11:2238 [Google Scholar]
- 55.Price SJ, Jena R, Burnet NG, Carpenter TA, Pickard JD, Gillard JH. Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur Radiol 2007;17:1675–84 [DOI] [PubMed] [Google Scholar]
- 56.Stadlbauer A, Nimsky C, Gruber S, Moser E, Hammen T, Engelhorn T, et al. Changes in fiber integrity, diffusivity and metabolism of the pyramidal tract adjacent to gliomas: a quantitative diffusion tensor fiber tracking and MR spectroscopic imaging study. AJNR Am J Neuroradiol 2007;28:462–9 [PMC free article] [PubMed] [Google Scholar]
- 57.Pauleit D, Langen KJ, Floeth F, Hautzel H, Riemenschneider MJ, Reifenberger G, et al. Can the apparent diffusion coefficient be used as a noninvasive parameter to distinguish tumor tissue from peritumoral tissue in cerebral gliomas? J Magn Reson Imaging 2004;20:758–64 [DOI] [PubMed] [Google Scholar]
- 58.Price SJ, Pena A, Burnet NG, Jena R, Green HA, Carpenter TA, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol 2004;14:1909–17 [DOI] [PubMed] [Google Scholar]
- 59.Pena A, Green HAL, Carpenter TA, Price SJ, Pickard JD, Gillard JH. Enhanced visualization and quantification of magnetic resonance diffusion tensor imaging using the p:q tensor decomposition. Br J Radiol 2006;79:101–9 [DOI] [PubMed] [Google Scholar]
- 60.Price SJ, Jena R, Burnet NG, Hutchinson PJ, Dean AF, Pena A, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 2006;27:1969–74 [PMC free article] [PubMed] [Google Scholar]
- 61.Stadlbauer A, Ganslandt O, Buslei R, Hammen T, Gruber S, Moser E, et al. Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 2006;240:803–10 [DOI] [PubMed] [Google Scholar]
- 62.Sijens PE, Oudkerk M. 1H chemical shift imaging characterization of human brain tumor and edema. Eur Radiol 2002;12:2056–61 [DOI] [PubMed] [Google Scholar]
- 63.Di Costanzo A, Scarabino T, Trojsi F, Popolizio T, Catapano D, Giannatempo GM, et al. Proton MR spectroscopy of cerebral gliomas at 3 T: spatial heterogeneity and tumour grade and extent. Eur Radiol 2008;18:1727–35 [DOI] [PubMed] [Google Scholar]
- 64.McKnight TR, dem Bussche MH, Vigneron DB, Lu Y, Berger MS, McDermott MW, et al. Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg 2002;97:794–802 [DOI] [PubMed] [Google Scholar]
- 65.Croteau D, Scarpace L, Hearshen D, Gutierrez J, Fisher JL, Rock JP, et al. Correlation between magnetic resonance spectroscopy imaging and image- guided biopsies: semiquantitative and qualitative histopathological analyses of patients with untreated glioma. Neurosurgery 2001;49:823–9 [DOI] [PubMed] [Google Scholar]
- 66.Pirzkall A, Li X, Oh J, Chang S, Berger MS, Larson DA, et al. 3D MRSI for resected high-grade gliomas before RT: tumor extent according to metabolic activity in relation to MRI. Int J Radiat Oncol Biol Phys 2004;59:126–37 [DOI] [PubMed] [Google Scholar]
- 67.Stadlbauer A, Buchfelder M, Doelken M, Hammen T, Ganslandt O. Magnetic resonance spectroscopic imaging for visualization of the infiltration zone of glioma. Cen Eur Neurosurg 2011;72:63–9 [DOI] [PubMed] [Google Scholar]
- 68.Kallenberg K, Bock HC, Helms G, Jung K, Wrede A, Buhk JH, et al. Untreated glioblastoma multiforme: increased myo-inositol and glutamine levels in the contralateral cerebral hemisphere at proton mr spectroscopy1. Radiology 2009;253:805–12 [DOI] [PubMed] [Google Scholar]
- 69.Derlon JM, Chapon F, Noel MH, Khouri S, Benali K, Petit-Taboue MC, et al. Non-invasive grading of oligodendrogliomas: correlation between in vivo metabolic pattern and histopathology. Eur J Nucl Med 2000;27:778–87 [DOI] [PubMed] [Google Scholar]
- 70.Miwa K, Shinoda J, Yano H, Okumura A, Iwama T, Nakashima T, et al. Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: insight from a PET and MR fusion image study. J Neurol Neurosurg Psych 2004;75:1457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stadlbauer A, Prante O, Nimsky C, Salomonowitz E, Buchfelder M, Kuwert T, et al. Metabolic imaging of cerebral gliomas: spatial correlation of changes in O-(2-18F-fluoroethyl)-L-tyrosine PET and proton magnetic resonance spectroscopic imaging. J Nucl Med 2008;49:721–9 [DOI] [PubMed] [Google Scholar]
- 72.Stadlbauer A, Pölking E, Prante O, Nimsky C, Buchfelder M, Kuwert T, et al. Detection of tumour invasion into the pyramidal tract in glioma patients with sensorimotor deficits by correlation of 18F-fluoroethyl-L-tyrosine PET and magnetic resonance diffusion tensor imaging. Acta Neurochir (Wien) 2009;151:1061–9 [DOI] [PubMed] [Google Scholar]
- 73.Mosskin M, Bergstrom M, Collins VP, Ehrin E, Eriksson L, von Holst H, et al. Positron emission tomography with 11C-methionine of intracranial tumours compared with histology of multiple biopsies. Acta Radiol Suppl 1986;369:157–60 [PubMed] [Google Scholar]
- 74.Mosskin M, Ericson K, Hindmarsh T, von Holst H, Collins VP, Bergstrom M, et al. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol 1989;30:225–32 [PubMed] [Google Scholar]
- 75.Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 2004;10:7163–70 [DOI] [PubMed] [Google Scholar]
- 76.Choi SJ, Kim JS, Kim JH, Oh SJ, Lee JG, Kim CJ, et al. [18F]3-deoxy-3-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 2005;32:653–9 [DOI] [PubMed] [Google Scholar]
- 77.Jacobs AH, Thomas A, Kracht LW, Li H, Dittmar C, Garlip G, et al. 18F-Fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 2005;46:1948–58 [PubMed] [Google Scholar]
- 78.Price SJ, Fryer TD, Cleij MC, Dean AF, Joseph J, Salvador R, et al. Imaging regional variation of cellular proliferation in gliomas using 3’-deoxy-3’-[18F]fluorothymidine positron-emission tomography: an image-guided biopsy study. Clin Radiol 2008;64:52–63 [DOI] [PubMed] [Google Scholar]
- 79.Chen W, Cloughesy T, Kamdar N, Satyamurthy N, Bergsneider M, Liau L, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med 2005;46:945–52 [PubMed] [Google Scholar]
- 80.Henry RG, Vigneron DB, Fischbein NJ, Grant PE, Day MR, Noworolski SM, et al. Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol 2000;21:357–66 [PMC free article] [PubMed] [Google Scholar]
- 81.Price SJ, Green HAL, Dean AF, Joseph J, Hutchinson PJ, Gillard JH. Correlation of relative cerebral blood volume with cellularity and proliferation in high grade gliomas: an image-guided biopsy study. AJNR Am J Neuroradiol 2011;32:501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehmann P, Vallee JN, Saliou G, Monet P, Bruniau A, Fichten A, et al. Dynamic contrast-enhanced T2*-weighted MR imaging: a peritumoral brain oedema study. J Neuroradiol 2009;36:88–92 [DOI] [PubMed] [Google Scholar]
- 83.Diedrich U, Lucius J, Baron E, Behnke J, Pabst B, Zoll B. Distribution of epidermal growth factor receptor gene amplification in brain tumours and correlation to prognosis. J Neurol 1995;242:683–8 [DOI] [PubMed] [Google Scholar]
- 84.Mishani E, Abourbeh G, Rozen Y, Jacobson O, Laky D, Ben D, I, et al. Novel carbon-11 labeled 4-dimethylamino-but-2-enoic acid [4-(phenylamino)-quinazoline-6-yl]-amides: potential PET bioprobes for molecular imaging of EGFR-positive tumors. Nucl Med Biol 2004;31:469–76 [DOI] [PubMed] [Google Scholar]
- 85.Abourbeh G, Dissoki S, Jacobson O, Litchi A, Ben DR, Laki D, et al. Evaluation of radiolabeled ML04, a putative irreversible inhibitor of epidermal growth factor receptor, as a bioprobe for PET imaging of EGFR-overexpressing tumors. Nucl Med Biol 2007;34:55–70 [DOI] [PubMed] [Google Scholar]
- 86.Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging 2007;34:850–8 [DOI] [PubMed] [Google Scholar]
- 87.Kopka K, Breyholz HJ, Wagner S, Law MP, Riemann B, Schroer S, et al. Synthesis and preliminary biological evaluation of new radioiodinated MMP inhibitors for imaging MMP activity in vivo. Nucl Med Biol 2004;31:257–67 [DOI] [PubMed] [Google Scholar]
- 88.Breyholz HJ, Wagner S, Levkau B, Schober O, Schafers M, Kopka K. A 18F-radiolabeled analogue of CGS 27023A as a potential agent for assessment of matrix-metalloproteinase activity in vivo. Q J Nucl Med Mol Imaging 2007;51:24–32 [PubMed] [Google Scholar]
- 89.Beliveau R, Delbecchi L, Beaulieu E, Mousseau N, Kachra Z, Berthelet F, et al. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Ann N Y Acad Sci 1999;886:236–9 [DOI] [PubMed] [Google Scholar]
- 90.Jena R, Price SJ, Baker C, Jefferies SJ, Pickard JD, Gillard JH, et al. Diffusion tensor imaging: possible implications for radiotherapy treatment planning of patients with high-grade glioma. Clin Oncol 2005;17:581–90 [DOI] [PubMed] [Google Scholar]