Abstract
Although brain tumours are rare compared with other malignancies, they are responsible, in many cases, for severe physical and cognitive disability and have a high case fatality rate (13% overall survival at 5 years). Gliomas account for over 60% of primary brain tumours and usually present with one or more symptoms of raised intracranial pressure, progressive neurological deficit, seizures, focal or global cognitive decline. The diagnosis is made by a combination of imaging and histological examination of tumour specimen. Contrast-enhanced MRI is the gold standard imaging modality and provides highly sensitive anatomical information about the tumour. Advanced imaging modalities provide complementary information about brain tumour metabolism, blood flow and ultrastructure and are being increasingly incorporated into routine clinical sequences. Imaging is essential for guiding surgery and radiotherapy treatments and for monitoring response to, and progression of, therapy. However, changes in imaging over time may be misinterpreted and lead to incorrect assumptions about the effectiveness of treatments. Thus, the disappearance of contrast enhancement and resolution of oedema after anti-angiogenesis treatments is seen early while conventional T2 weighted/FLAIR sequences demonstrate continual tumour growth (pseudoregression). Conversely imaging may suggest lack of efficacy of treatment e.g. increasing tumour size and contrast enhancement following chemoradiation for malignant gliomas (pseudoprogression), which then stabilise or resolve after a few months of continued treatment and that paradoxically may be associated with a better outcome. These factors have led to a re-evaluation of the role of standard sequences in the assessment of treatment response spurning interest in the development of quantitative biomarkers.
Brain tumours are relatively rare when compared with breast, lung, prostate and colorectal cancer but cause considerable suffering and have a high case fatality ratio. They can occur at any age and are the most common solid tumour in children. They are the second leading cause of death from neurological disease in the UK (second only to stroke). The crude UK annual incidence for primary tumours is 15.3/100 000 and for secondary tumours 14.3/100 000 patients [1] and is slightly higher in men than in women, and in white people than in black people.
Tumour types
The most common site for brain tumours is the supratentorial compartment and the most common histological types are those of neuro-epithelial origin (gliomas), followed by meningiomas, pituitary tumours and others. They have been classified into distinct pathological groups by the World Health Organization (WHO) and are graded in ascending order of malignancy according to certain histological features [2] (Table 1).
Table 1. Abridged World Health Organization (WHO) classification of brain tumours.
| WHO grade | |
| 1. Tumours of neuro-epithelial tissue | |
| a. Astrocytic tumours | |
| i. Pilocytic astrocytoma | I |
| ii. Pilomyxoid astrocytoma | II |
| iii. Subependymal giant cell astrocytoma | I |
| iv. Pleomorphic xanthoastrocytoma | II |
| v. Diffuse astrocytoma | II |
| vi. Anaplastic astrocytoma | III |
| vii. Glioblastoma | IV |
| viii. Gliomatosis cerebri | III |
| b. Oligodendroglial tumours | |
| i. Oligodendroglioma | II |
| ii. Anaplastic oligodendroglioma | III |
| c. Mixed gliomas | |
| i. Oligoastrocytoma | II |
| ii. Anaplastic oligoastrocytoma | III |
| d. Ependymal tumours | |
| i. Subependymoma | I |
| ii. Myxopapillary ependymoma | I |
| iii. Ependymoma | II |
| iv. Anaplastic ependymoma | III |
| e. Choroid plexus tumours | |
| i. Choroid plexus papilloma | I |
| ii. Atypical chroid plexus papilloma | II |
| iii. Choroid plexus carcinoma | III |
| f. Other neuro-epithelial tumours | |
| i. Astroblastoma | n/a |
| ii. Chordoid glioma of the third ventricle | IV |
| g. Neuronal and mixed neuronal–glial tumours | |
| i. Dysplastic gangliocytoma of the cerebellum (Lhermitte–Duclos disease) | I |
| ii. Desmoplastic infantile astrocytoma/ganglioglioma | I |
| iii. Dysembryoplastic neuro-epithelial tumour | I |
| iv. Gangliocytoma | I |
| v. Ganglioglioma | I or II |
| vi. Anaplastic ganglioglioma | III |
| vii. Central neurocytoma | II |
| viii. Extraventricular neurocytoma | II |
| ix. Cerebellar liponeurocytoma | I or II |
| x. Papillary glioneuronal tumour | |
| xi. Rosette-forming glioneuronal tumour of the fourth ventricle | |
| xii. Paraganglioma of the filum terminale | I |
| h. Tumours of the pineal region | |
| i. Pineocytoma | II |
| ii. Pineal parenchymal tumour of intermediate differentiation | n/a |
| iii. Pineoblastoma | IV |
| i. Embryonal tumours | |
| i. Medulloblastoma | IV |
| ii. Central nervous system (CNS) primitive neuro-ectodermal tumour | |
| iii. CNS neuroblastoma | |
| iv. CNS ganglioneuroblastoma | |
| v. Medulloepithelioma | |
| vi. Ependymoblastoma | |
| vii. Atypical teratoid/rhabdoid tumour | |
| 2. Tumours of peripheral nerves | |
| a. Schwannoma | I |
| i. Cellular | |
| ii. Plexiform | |
| iii. Melanotic | |
| b. Neurofibroma | I |
| i. Plexiform | |
| c. Perineurioma | |
| i. Perineurioma NOS | I–II |
| ii. Malignant perineurioma | III |
| d. Malignant peripheral nerve sheath tumour (MPNST) | III |
| i. Epitheloid MPNST | |
| ii. MPNST with mesenchymal differentiation | |
| iii. Melanotic MPNST | |
| iv. MPNST with glandular differentiation | |
| 3.Tumours of meninges | |
| a. Tumours of meningothelial cells | |
| i. Meningioma | I-III |
| b. Mesenchymal tumours | I–IV |
| c. Primary melanocytic tumours | |
| d. Tumours of uncertain histogenesis | |
| 4. Lymphomas and haematopoietic neoplasms | |
| 5. Germ cell tumours | |
| 6. Tumours of the sellar region | |
| 7. Metastatic tumours |
NOS, not otherwise specified.
The remainder of this paper will discuss the diagnosis and treatment of gliomas only. Low-grade gliomas (LGGs) (WHO grades I and II) usually present in children and young adults, while high-grade gliomas (HGGs) (WHO grades III and IV) occur in late middle age and elderly people. Pilocytic astrocytomas are the most frequently encountered tumour in childhood and, in contrast to adult tumours, are more frequently infratentorial. Other typical locations include the optic nerve and hypothalamus.
Prognosis
Most intrinsic brain tumours are incurable and the outcome is determined by a combination of tumour and patient factors. The most important prognostic factors in the survival of patients with gliomas are the patient age at diagnosis, functional status and histological grade. The prognosis of gliomas, as defined by median survival, varies from just over 1 year (WHO grade IV glioblastoma multiforme) to greater than 10 years (WHO grade II oligodendroglioma). There is increasing evidence that molecular markers may be helpful in refining prognostic categories, e.g. deletion of chromosomes 1p/19q is a favourable prognostic marker in oligodendrogliomas [3].
Clinical features
There are no clinical features that are pathognomic of a brain tumour, and as a consequence the early symptoms are non-specific. Neurological symptoms and signs reflect tumour location and growth rate rather than tumour histology. In the majority of cases, patients present with a combination of generalised and focal symptoms usually manifest as one or more of four clinical syndromes:
raised intracranial pressure
progressive neurological deficit
partial and generalised seizures
cognitive and behavioural decline.
Children with posterior fossa tumours usually present with a combination of raised pressure, ataxia and brainstem symptoms and signs. Adult patients with supratentorial LGGs present typically with seizures, whereas patients with malignant gliomas more often present with a progressive neurological deficit or raised intracranial pressure.
Raised intracranial pressure
Brain tumours increase intracranial pressure by a direct mass effect, by provoking cerebral oedema or by producing obstructive hydrocephalus. The most common presenting symptom of raised intracranial pressure is headache, which occurs as the first symptom in 25% of patients [4]. Because the brain does not contain pain-sensitive structures, headache has been attributed to local swelling and distortion of pain-sensitive nerve endings in blood vessels and meninges. There are no specific features suggestive of a brain tumour headache, but a patient whose headache wakes him/her in the early hours of the morning, particularly if associated with vomiting and visual obscurations (transient fogging of vision usually on rapid changes in posture), should always be scanned. Signs of raised pressure include papilloedema and sixth nerve palsies, but most patients present at an earlier stage when the history is rather non-specific. It is therefore reassuring to know that less than 1% of patients presenting with isolated headache have a brain tumour. All patients presenting with non-migrainous headaches should have careful examination of fundi and visual fields as hemianopias may not be symptomatic.
Progressive neurological deficit
Focal neurological symptoms due to brain tumour are typically subacute and progressive. However, some tumours present as a stroke or transient ischaemic attack, and scans may initially suggest an infarct. Hemispheric tumours produce contralateral weakness, sensory loss, dysphasia, dyspraxia and visual field loss depending on their location. Posterior fossa tumours cause ataxia, cranial nerve palsies (particularly third and sixth) and raised pressure, usually by obstructing the fourth ventricle. Mid-brain tumours may present with Parinaud's syndrome (vertical gaze palsy, convergence-retraction nystagmus and light-near dissociation). Cerebellopontine angle tumours cause progressive unilateral deafness, facial sensory loss and ataxia.
Seizures
These are the presenting symptom in 25–30% of patients with tumours and are present at some stage in 40–60% of patients [4]. Most patients present with secondarily generalised tonic–clonic seizures although there may be a preceding history of partial seizures whose significance was not fully appreciated at the time. LGGs, in particular, are associated with seizures and frequently remain the only symptom for many years. Conversely, malignant gliomas have a lower frequency of seizures, presumably because of their more rapid growth and destructive characteristics. Tumours that are superficially located in the frontal and temporal lobes are more likely to cause seizures.
Cognitive and behavioural decline
This is an uncommon early presentation of brain tumours, typically seen with large subfrontal meningiomas or gliomas, occurring in about 20% of patients at diagnosis without focal neurological symptoms. Personality changes may be quite subtle initially and may present as an inability to cope at work.
Imaging of brain tumours
The diagnosis of a brain tumour is first suggested after conventional contrast-enhanced CT or MRI. MRI of brain tumours is undoubtedly the gold standard non-invasive technique for the diagnosis, pre-surgical planning and post-therapeutic monitoring of brain and spinal tumours but, it has still not replaced the biopsy for accurate histological grading of a tumour. MRI techniques that are now routinely available include thin (<2 mm) slices to provide high-resolution images, fast spin echo sequences to reduce imaging time (particularly useful for confused and agitated patients) and fat saturation to improve tissue visualisation. The resolution is still not sufficiently high enough to be able to detect areas of distant tumour infiltration, which renders gliomas as incurable today as they were over 100 years ago. Functional MRI is being increasingly used in the planning of surgery for tumours in eloquent areas of the brain to enable radical surgery to be carried out with less morbidity. Intra-operative MRI is now standard practice in some centres and diffusion tractography can provide information on white matter tracts, but whether these approaches can prolong survival has yet to be shown.
Contrast-enhanced MRI is routine and enhancement is frequently seen in HGG (grades III and IV), whereas dynamic contrast enhancement techniques are increasingly used to provide data for diagnosis and surgical guidance. However, there are still significant limitations particularly around the lack of specificity, e.g. distinguishing neoplasia from inflammation and infection and, more problematically, the use of imaging to assess treatment response (see below).
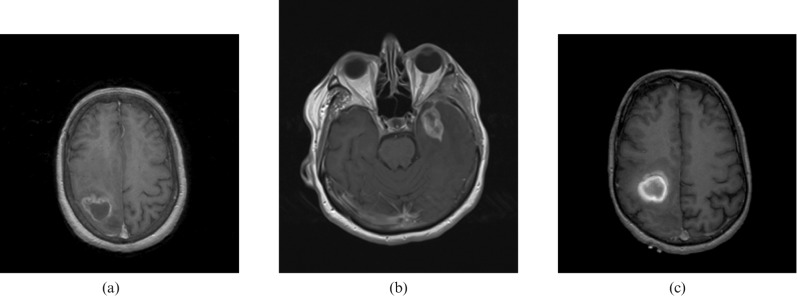
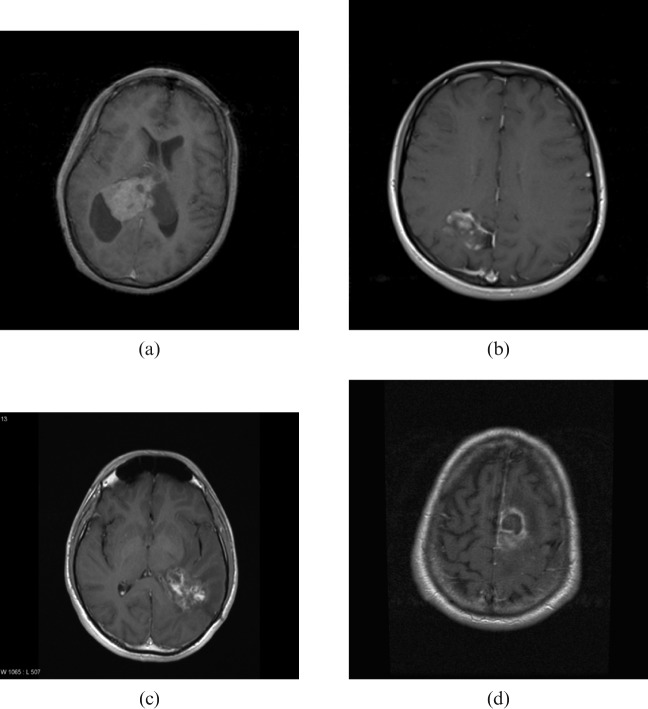
Imaging is highly sensitive for a brain tumour but not particularly specific. Perilesional contrast enhancement, a radiological sign of blood–brain barrier breakdown, is typically seen in malignant tumours, e.g. HGGs (Figure 1a), primary central nervous system lymphomas (Figure 1b) and metastases (Figure 1c) as well as in benign tumours, e.g. meningiomas (Figure 2a), pilocytic astrocytomas (Figure 2b) and non-neoplastic processes, e.g. tumefactive multiple sclerosis (Figure 2c) and bacterial abscesses (Figure 2d). The diagnostic accuracy of conventional imaging even in the best centres is only 80–90%, so surgical biopsy or resection is recommended in almost all cases where further treatment is contemplated to rule out non-neoplastic lesions and to provide histological identification and genotyping. There are also limitations to histological diagnosis, e.g. sampling errors in surgical biopsies due to intrinsic tissue heterogeneity where tumour undergrading can occur and also due to biopsying the edge of a lesion where it can be difficult to distinguish between a LGG and reactive gliosis.
Figure 1.
Contrast-enhancing malignant brain lesions—axial T1 weighted gadolinium-enhanced sequences. (a) Malignant glioma (right frontal lobe); (b) primary central nervous system lymphoma (left temporal lobe); (c) metastatic melanoma (right frontal lobe).
Figure 2.
Contrast enhancing benign brain lesions – axial T1 weighted gadolinium enhanced sequences. (a) Meningioma (intraventricular); (b) pilocytic astrocytoma (right occipital); (c) tumefactive multiple sclerosis (right parietal); (d) pyogenic abscess (left frontal lobe).
Advanced imaging techniques provide complementary information about brain tumours. Proton magnetic resonance spectroscopy (1H-MRS) allows non-invasive measurement of metabolites in brain tumours and can be used to guide biopsies, define radiotherapy targets and to monitor patients after treatment [5]. This technique is hampered by methodological issues, e.g. standardisation of data acquisition approaches across different scanning systems, the effect of field strength variations resulting from positioning of the measurement voxel in highly spatially heterogeneous tumours. Although it has been used in a research setting for the last three decades, it has only recently been added to routine clinical sequences to complement the information available from standard MRI sequences. Its use in brain tumours is limited by technical factors that render it unreliable for lesions less than 2 cm in diameter or for lesions close to bone, cerebrospinal fluid or fat because of signal contamination, e.g. base of skull, retro-orbital region. Furthermore, single-voxel spectroscopy cannot sample the whole tumour volume and therefore the information yielded may not be relevant to other parts of the tumour. This consideration is particularly important in HGGs where there are areas of tissue heterogeneity due to cystic degeneration, necrosis and variable cellular density. In contrast, MR spectroscopy (MRS) imaging, also known as chemical shift imaging (CSI), can show the whole tumour, but the signal to noise ratio and therefore the quality of the resulting spectra is significantly worse than single voxel spectroscopy and the measurement area is limited to one slice with a maximum matrix of 10 × 10 spectra. It is technically demanding to carry out CSI at long echo times (TE) and so is less useful for the determination of mobile lipids, which are best seen at short TE.
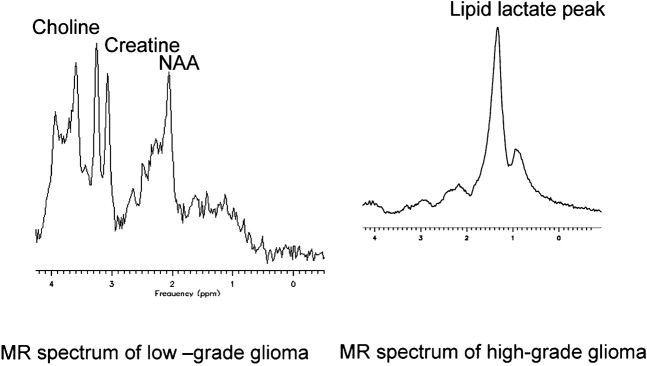
The main metabolites of interest in brain tumours are choline (Cho), creatine (Cr), N-acetyl-aspartate (NAA), myoinositol (mI), lipids and lactate (Lac). Choline metabolites consist of a number of choline-containing phospholipids and are found in greater concentrations in areas of active membrane turnover, such as in WHO grade II and III gliomas but are highly variable in glioblastomas [6]. Creatine is a combination of phosphocreatine and creatine and levels remains relatively constant in tumours, allowing measurement of ratios with other metabolites. Myoinositol is more frequently seen at high concentrations in LGGs and at very low concentrations in meningiomas. The presence of lipid/lactate usually indicates necrosis, a feature of higher-grade tumours. Typical spectra of LGG and HGG are shown in Figure 3. Different tumour types and grades contain characteristic patterns of chemicals but previous attempts to correlate MRS spectra with histological appearances have yielded inconclusive results usually because of small numbers of patients and uncertainties about the reliability of spectral changes.
Figure 3.
Typical MR spectra of gliomas taken at short echo time using point resolved spectroscopy (PRESS) sequence.
Perfusion weighted MRI (PWI) or dynamic susceptibility contrast MRI (DSC-MRI) provides information about tumour tissue perfusion and is useful in the pre-operative classification and grading of gliomas, particularly when used in combination with MRS [7]. The most common measure is regional cerebral blood volume (CBV), either as an absolute measure or relative to contralateral "normal" white matter. These measures have been shown to correlate with microvascular density (MVD) and vary with tumour grade in that maximum CBV values of LGG are significantly lower than those of HGG. However, this technique is not sensitive enough to differentiate between grade I and grade II tumours or between grade II and grade III tumours or between grade III and grade IV tumours. Recent data suggest rCBV is helpful in predicting progression in gliomas, both LGG treated conservatively [8] and LGG/HGG prior to surgery [9].
Principles of oncological treatment
There have been many therapeutic advances in recent years, yet the prognosis of gliomas has changed little and remains grim (median survival 14.6 months for patients with newly diagnosed glioblastoma multiforme and 2–3 years for patients with anaplastic astrocytomas). Therefore treatment of malignant gliomas is almost by definition palliative, and clinicians must take into account quality of life issues when making treatment decisions.
Oncological treatment is most effective for patients in the best prognostic categories as determined by a combination of patient factors (age, performance status) and tumour factors (location, histology, grade, resectability and, in certain tumours, genotype).
Surgery
Surgery for brain tumours may be for diagnostic purposes only (biopsy) or as a curative or palliative therapeutic option. Recent advances in computer-aided neuronavigation, pre-operative functional imaging (e.g. fMRI and diffusion tractography), intra-operative tumour delineation with cortical mapping (for eloquent regions) and phototherapy using 5-aminolaevulinic acid and ultraviolet light (for malignant gliomas) [10] have enabled neurosurgeons to take a more aggressive approach to tumour resection, and reduce the tumour burden for the oncologist. Curative resection is indicated for benign extra-axial tumours, e.g. meningiomas and pituitary tumours, and for some intra-axial tumours, e.g. juvenile pilocytic astrocytomas. However, the majority of adult intrinsic brain tumours are not surgically curable, and the potential benefits of prolonging survival must be weighed up carefully against the risks of causing a permanent neurological deficit. This is particularly pertinent to the field of LGG surgery where the natural history of the tumour extends for many years and the patients are usually neurologically intact and high functioning despite the presence of tumour infiltration in eloquent regions of their brain.
The preferred surgical management for HGG is maximal resection, which allows for accurate tissue diagnosis and "cytoreduction". Subgroup analysis of prognostic factors in glioma treatment trials suggest that patients who have had a gross total resection have a better response to subsequent adjuvant treatments than those who have had a partial resection or biopsy only. Other surgical options include shunting for tumour-associated hydrocephalus and aspiration of tumour-associated cysts, both of which may reduce raised pressure and neurological deficit without having any impact on the tumour itself. The approach to the surgical management of LGGs is a controversial area as there is a lack of prospective randomised evidence to show a relationship between extent of resection and survival. Most surgeons believe that a gross total resection offers a better chance of long-term survival than biopsy or partial resection. Some operate with the patient awake where the tumour is close to speech and/or motor areas. The use of cortical and subcortical electrical stimulation to map out motor pathways has enabled more radical resections with lower morbidities in LGG [11].
Radiotherapy
This is used as an adjunct to surgery in malignant tumours where there is a high chance of recurrence or as primary treatment for unresectable tumours. This is the only modality that has been shown in randomised studies to prolong survival in malignant gliomas but it rarely cures. Advances in focused radiotherapy techniques, e.g. stereotactic radiotherapy and radiosurgery, have allowed more accurate targeting of tumours close to vital structures and have significantly improved the treatment toxicity of benign extra-axial tumours, but have not impacted on the survival of HGG.
Radiotherapy is associated with both early and late brain toxicity, and this is an important consideration particularly in patients with tumours associated with a good prognosis. Examples of early toxicity include somnolence, worsening cerebral oedema and focal deficit. Late delayed toxicity includes leucoencephalopathy and cognitive decline, parkinsonism, radiation necrosis (see below) and an increased incidence of secondary tumours, specifically meningiomas and gliomas, 10 years or more after primary treatment.
Chemotherapy
As a general rule, most brain tumours are not chemosensitive, with less than 30% of recurrent HGGs showing any radiographic response to chemotherapy. A small subset of gliomas, known as anaplastic oligodendrogliomas, are highly chemosensitive when they have chromosomal losses of 1p and 19q (Figure 1). However, two recent trials addressing the role of neoadjuvant or adjuvant PCV treatment for these tumours, showed chemotherapy prolonged progression-free survival but not overall survival, but at the expense of excess toxicity in the chemotherapy arms [12,13]. These were surprising results in a tumour that was highly chemosensitive. In contrast, a landmark study in glioblastoma multiforme, a typically chemoresistant tumour, has shown a significant survival benefit in patients treated with concomitant chemoradiotherapy followed by adjuvant chemotherapy using temozolomide, an oral alkylating agent, over radiotherapy alone [14]. Median survival increased from 12.1 months to 14.6 months and 2 year survival rates increased from 10% to 26.5%. This trial has generated a resurgence of interest in the role of chemotherapy in brain tumours, having previously been reserved for treatment of glioma progression after surgery and radiotherapy.
Targeted agents
Increasing understanding of the molecular pathogenesis of malignant tumours has led to the emergence of novel therapeutic targets, principally growth factor receptors and cell cycle control enzymes and their downstream pathways. Over the last decade there has been an explosion of interest in targeted agents, i.e. monoclonal antibodies and small molecule inhibitors, which block or downregulate critical pathways in cell proliferation and angiogenesis. These are predominantly cytostatic agents and need to be given in combination with conventional cytotoxic chemotherapy and radiotherapy. However, results of Phase II studies mainly in recurrent gliomas, have been largely disappointing. The main exception to this has been with bevacizumab (Avastin), a humanised monoclonal antibody against vascular endothelial growth factor, which has shown promising activity against recurrent malignant gliomas in Phase II studies when combined with irinotecan [15]. This combination is associated with response rates, determined by conventional parameters, of more than 60%, and 6 month progression-free survival rates of 30% in GBM and 56% in AA (compared with 15% and 31% in previous negative studies). Interestingly, the pattern of progression in some patients treated with bevacizumab is different from those seen in patients treated with conventional cytotoxic agents. More patients developed diffusely infiltrating tumour progression rather than enhancing solid disease, best seen on fluid-attenuated inversion-recovery (FLAIR) sequences, suggesting that local growth is controlled through inhibition of angiogenesis but distant spread is not [16].
From an imaging perspective, anti-angiogenic agents cause rapid normalisation of tumour vasculature and reduction of oedema, which, although prolonged, eventually reverses on discontinuation of the drug [17]. On T1 weighted gadolinium images, this is seen as disappearance of contrast-enhancing tumour tissue and surrounding vasogenic oedema, which may be regarded as a marker of tumour response although does not necessarily reflect tumour cell death.
Assessment of tumour response
In clinical trials, the choice of primary and secondary endpoints is crucial in determining not just whether a new treatment is efficacious but also if it provides a clinically meaningful response. In Phase III trials, the primary endpoint is usually overall survival, which has the advantage of being "incontrovertible". In Phase II trials, however, progression-free survival at 6 months is usually taken as a marker of response, particularly in recurrent disease. The definition of progression is somewhat arbitrary. Clinical deterioration is a relatively non-specific sign of progression and may occur some months after radiological progression. Typically the appearance of new contrast-enhancing tumour tissue or the growth of previously enhancing tissue is regarded as the most reliable radiological sign of tumour progression in HGG—it is now realised this is somewhat simplistic and in some cases positively misleading. The phenomenon of "pseudoprogression" whereby new contrast-enhancing lesions are seen immediately after radiotherapy and more commonly after chemoradiotherapy (the standard treatment for glioblastoma multiforme) and then subsequently disappear without any specific treatment. This has made it more difficult to determine what constitutes true tumour progression. Conversely, treatment with anti-angiogenesis agents, which close down the blood–brain barrier and resolve oedema but only for as long as the drug is given, has led to the proposal that future studies report both radiographic and clinical response rates and incorporate findings from blood biomarker studies and physiological imaging techniques more frequently [18].
Despite the interest in quantitative imaging techniques as outlined above, there are no internationally standardised radiological methods to assess the efficacy of a certain treatment. Current assessment of tumour response in clinical practice usually relies on visual imaging, comparison of the "amount" of enhancing tumour tissue and high signal change in brain parenchyma between pre- and post-treatment images. Even in clinical trials, the assessments are basic: changes in the maximal cross-sectional area of the tumour or the product of the maximal perpendicular diameters taking into account patient condition and corticosteroid usage after the completion of treatment allows determination of treatment response according to MacDonald criteria [19]. These categorise responses into complete response, partial response, stable disease and progressive disease. More recently, a fifth category, minimal response, has been added for LGG trials as radiological changes following treatments to LGG are less obvious despite clinical improvement.
Early assessment of treatment response
Other MRI techniques, e.g. diffusion-weighted imaging (DWI), are being evaluated for the assessment of early response. These techniques measure non-volume-based tumour parameters; in the case of DWI, signal change is proportional to the random Brownian motion of water molecules, which theoretically should increase as tumour cells undergo apoptosis and necrosis following treatment due to changes in cell density. In addition, there may be reduction in the volume of regions containing high extracellular water, e.g. necrosis and cysts. These observations, first noted in rodents, have been extended into humans by measurements of tumour water diffusion in brain tumour patients treated with chemotherapy and radiotherapy using early therapeutic functional diffusion maps (fDMs) (3 weeks after the start of treatment) coregistered to pre-treatment studies [20]. These fDMs were able to predict subsequent volumetric tumour response, as measured with standard radiographic criteria, suggesting early changes in tumour diffusion could be used as a prognostic marker. Subsequent work has shown that fDM when combined with conventional radiological response can provide a more accurate prediction of patient survival than either measure alone [21]. 1H-MRS has also been shown to be a useful predictor of early treatment outcome following radiotherapy: the lactate/NAA ratio was a stronger prognostic factor for survival in malignant gliomas even more than patient age and tumour grade [22].
Even the measurement of tumour volume poses considerable technical difficulties. The conventional volume is taken from the border of contrast-enhancing tumour, but this almost certainly underestimates the true volume, as tumour cells infiltrate beyond this zone, when visualised on T2 weighted and FLAIR sequences. Because of the irregular nature of tumour enhancement, the definition of a tumour boundary is highly operator dependent, leading to considerable variability in outlining tumour volumes. This variability can be reduced with the assistance of computerised perimetry methods [23].
Distinguishing tumour recurrence from treatment effects
Radiation to the brain can cause desirable effects on brain tumour tissue and tumour vasculature leading to apoptosis and necrosis but also undesirable effects on healthy brain parenchyma, white matter pathways and vasculature. In clinical practice, radionecrosis is the most dreaded complication; this is a severe local tissue reaction with signs of a disrupted blood–brain barrier, oedema and mass effect, typically indistinguishable from recurrent tumour. Histopathological features of radionecrosis include vascular thickening, fibrinoid necrosis, thrombosis and occlusion and gliosis usually 6–12 months after radiotherapy but occasionally years and even decades later. The occurrence of radionecrosis is directed related to the dose of radiation delivered and the irradiated field volume, with a steep increase from about 5% when doses in excess of 60 Gy in 1.8–2.0 Gy fractions are given. Therefore, the standard radical dose for treatment of malignant glioma is 60 Gy in 30 fractions. With the advent of chemoradiation protocols for malignant glioma, radionecrosis is being seen earlier than with traditional radiotherapy schedules: 14% of patients had histological evidence of radionecrosis when operated within 6 months of temozolomide chemoradiotherapy [24].
Another considerable "biological" difficulty is the emergence of "pseudoprogression", which is seen in approximately 20% of patients with GBM within 1–2 months after combined chemoradiation treatment, i.e. earlier than the time period for classical radionecrosis, and is symptomatic in about one-third of cases. This takes the form of progressive enhancing lesions, which decrease in size or stabilise without any additional treatment and are frequently asymptomatic [25]. This radiological phenomenon probably represents a combination of treatment-induced necrosis and a secondary inflammatory response leading to oedema, abnormal vessel permeability causing new or increased contrast enhancement. Interestingly, it is more common in patients with methylated O6-methyl guanine-DNA methyltransferase (MGMT), whose tumours are more responsive to temozolomide treatment [26].
A number of complementary techniques, e.g. spectroscopy and perfusion, are helpful in distinguishing radiation necrosis from progressive tumour, but these techniques are rarely used in clinical trials because of the high level of specialist radiology and radiographer support required and the time-consuming post-processing analysis.
Conclusions
Imaging has an invaluable role to play in the diagnosis, treatment and monitoring of brain tumour patients but is beset by considerable difficulties relating to lack of specificity, lack of histological correlation and variability in sequence acquisition. Because of its non-invasive nature, it will always be the most useful surrogate marker of treatment, but modern therapies are producing imaging changes which are not always clinically helpful and in some cases, may give cause for heightened anxiety (pseudoprogression) or false hope (vascular shutdown with anti-angiogenesis therapy). With the increasing importance of molecular diagnosis in the stratification of patients for clinical trials, and the predictive value in relation to treatment response, it seems less and less likely that imaging will ever replace histological sampling.
References
- 1.Counsell CE, Collie DA, Grant R. Incidence of intracranial tumours in the Lothian region of Scotland, 1989–90. J Neurol Neurosurg Psychiatry 1996;61:143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO Classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannini C, Burger PC, Berkey BA, Cairncross JG, Jenkins RB, Mehta M, et al. Anaplastic oligodendroglial tumors: refining the correlation among histopathology, 1p 19q deletion and clinical outcome in Intergroup Radiation Therapy Oncology Group Trial 9402. Brain Pathol 2008;18:360–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant R. Overview: brain tumour diagnosis and management. Royal College of Physicians Guidelines. J Neurol Neurosurg Psychiatry 2004;75 Suppl 2:ii18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclerc X, Huisman AGM, Sorensen AG. The potential of proton magnetic resonance spectroscopy (1H-MRS) in the diagnosis and management of patients with brain tumors. Curr Opinion Oncol 2002;14:292–8 [DOI] [PubMed] [Google Scholar]
- 6.Howe FA, Barton SJ, Cudlip SA, Stubbs M, Saunders DE, Murphy M, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Res Med 2003;49:223–32 [DOI] [PubMed] [Google Scholar]
- 7.Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003;24:1989–98 [PMC free article] [PubMed] [Google Scholar]
- 8.Danchaivijitr N, Waldman AD, Tozer DJ, Benton CE, Brasil Caseiras G, Tofts PS, et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology 2008;247:170–8 [DOI] [PubMed] [Google Scholar]
- 9.Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2008;247:490–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, ALA-Glioma StudyGroup Fluorescence-guided surgery with 5-aminolaevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III study. Lancet Oncol 2006;7:392–401 [DOI] [PubMed] [Google Scholar]
- 11.Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 2005;76:845–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intergroup Radiation Therapy Oncology Group Trial 9402, Cairncross G, Berkey B, Shaw E, Jenkins R, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol 2006;24:2707–14 [DOI] [PubMed] [Google Scholar]
- 13.van denBent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, et al. Adjuvant procarbazine, lomustine and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendroglioma and oligoastrocytomas: a randomised European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol 2006;24:2715–22 [DOI] [PubMed] [Google Scholar]
- 14.Stupp R, Mason WP, van denBent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96 [DOI] [PubMed] [Google Scholar]
- 15.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007;13:1253–9 [DOI] [PubMed] [Google Scholar]
- 16.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, et al. Bevacizumab for recurrent malignant gliomas. Neurology 2008;70:779–87 [DOI] [PubMed] [Google Scholar]
- 17.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007;11:83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen AG, Batchelor TT, Wen PY, Zhang Wt, Jain RK. Response criteria for glioma. Nat Clin Pract Oncol 2008;5:634–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald DR, Cascino TL, Schold Sc, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80 [DOI] [PubMed] [Google Scholar]
- 20.Moffat BA, Chenevert Tl, Lawrence TS, Meyer CR, Johnson TD, Dong Q, et al. Functional diffusion map: A non-invasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci 2005;102:5524–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamstra DA, Galbán CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 2008;26:3387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarnawski R, Sokol M, Pieniazek P, Maciejewski B, Walecki J, Miszczyk L, et al. 1H-MRS in vivo predicts the early treatment outcome of postoperative radiotherapy for malignant gliomas. Int J Radiat Oncol Biol Phys 2002;52:1271–6 [DOI] [PubMed] [Google Scholar]
- 23.Sorensen AG, Patel S, Harmath C, Bridges S, Synnott J, Sievers A, et al. Comparision of diameter and perimeter methods for tumor volume calculation. J Clin Oncol 2001;19:551–7 [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following recurrent temodar and radiotherapy in patients with glioblastoma. J Neurooncol 2007;82:81–3 [DOI] [PubMed] [Google Scholar]
- 25.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Lancet Oncol 2008;9:453–61 [DOI] [PubMed] [Google Scholar]
- 26.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003 [DOI] [PubMed] [Google Scholar]