Abstract
Coronary artery disease has an important impact on the morbidity and mortality statistics and health economics worldwide. Diagnosis of coronary artery disease is important in risk stratification and guides further management. Invasive coronary angiography is the traditional method of imaging the coronary arteries and remains the gold standard. It detects luminal stenosis but provides little information about the vessel wall or plaques. Besides, not all anatomical lesions are functionally significant. This has lent itself to a wide variety of imaging techniques to identify and assess a flow-limiting stenosis. The approach to diagnosis of coronary artery disease is broadly based on anatomical and functional imaging. Coronary CT and MRI of coronary arteries provide an anatomical assessment of coronary stenosis. Coronary calcium score and coronary CT assess subclinical atherosclerosis by assessing the atherosclerotic plaque burden. The haemodynamic significance of a coronary artery stenosis can be assessed by stress radioisotope studies, stress echocardiography and stress MRI. The more recent literature also focuses on plaque assessment and identification of plaques that are likely to give rise to an acute coronary syndrome. There is an explosion of literature on the merits and limitations of the different imaging modalities. This review article will provide an overview of all the imaging modalities in the diagnosis of coronary artery disease.
Chest pain is a significant healthcare burden, accounting for 1.5% of visits to the general practitioner [1], 6% of visits to the emergency department and 27% of emergency hospital admissions [2]. Chest pain may be cardiac related or may be due to non-cardiac causes such as musculoskeletal or gastrointestinal pain. It is important to identify the subgroup of patients with ischaemic heart disease presenting with chest pain. This is because coronary artery disease (CAD) is the biggest killer in the UK, accounting for the death of one in five men and one in six women [3]. It incurs an annual cost of 9 billion pounds, 36% of which is attributed to direct healthcare costs, 43% to production losses and 21% to informal care of people with CAD [3]. The problem is exacerbated by the growing prevalence of Type 2 diabetes, obesity and an ageing population.
Clinical assessment is important in the preliminary assessment of chest pain and in determining whether it is due to CAD [4]. Further assessment with imaging is important in the:
diagnosis of CAD
assessment of the functional significance of a coronary stenosis
assessment of the viability of the territory subtended by the stenotic artery
assessment of global and regional ventricular function.
The diagnosis of CAD is not restricted to the diagnosis of luminal stenosis. It also includes a study of plaques, including plaque volume and plaque characteristics. The functional significance of a stenosis refers to whether the lesion is significant enough to cause ischaemia. Viability refers to live myocardium. Assessment of viability is important in predicting functional recovery following revascularisation. Left ventricular function (LVF) is an important prognostic consideration in the assessment of ischaemic heart disease.
The various imaging modalities available for investigation of chest pain due to suspected CAD can be broadly divided into the categories below.
-
Invasive techniques:
invasive coronary angiography, which is the traditional gold standard
fractional flow reserve (FFR)
intravascular ultrasound and optical coherence tomography.
-
Non-invasive imaging techniques
-
direct visualisation of the coronary arteries:
coronary calcium score (CAC)
coronary CT using electron beam CT (EBCT) or multidetector CT (MDCT)
magnetic resonance angiography of the coronary arteries.
-
Assessment of the functional significance of coronary stenosis:
myocardial perfusion scintigraphy, which includes single photon emission CT (SPECT) and positron emission tomography (PET)
stress echocardiography (SE)
cardiac MRI (CMR) including stress CMR and delayed enhancement sequences.
-
The article provides an overview of the various imaging techniques, including:
the pathophysiology of angina and the ischaemic cascade
the rationale for combining anatomical and functional evaluation
the integration of these techniques within the framework of the recent National Institute for Health and Clinical Excellence (NICE) guideline [4]
the diagnostic and prognostic accuracy of various imaging modalities
issues such as radiation consideration and newer developments.
The article does not discuss the imaging techniques and the interpretation of findings in detail. It also does not discuss the role of various imaging modalities in the context of an acute coronary syndrome. These are described elsewhere in this issue.
Pathophysiology
Physiological determinants of myocardial oxygenation
Myocardial oxygen consumption and oxygen delivery determine the myocardial tissue oxygenation as outlined in Table 1 [5,6].
Table 1. Factors determining myocardial oxygenation.
| Determinants of myocardial oxygen consumption |
| 1. Ventricular myocardial mass |
| 2. Myocardial work load, which is influenced by the heart rate and the systemic blood pressure |
| 3. Myocardial wall tension, which is determined by the ventricular pre-contraction volume |
| 4. Contractility |
| Determinants of oxygen delivery to the myocardium |
| 1. The arterial oxygen concentration which is determined by haemoglobin concentration and haematocrit |
| 2. Coronary blood flow which is determined by the perfusion pressure and the resistance of the coronary blood vessels in the distal microcirculation |
Pathophysiology of angina
Angina is the result of an imbalance between myocardial oxygen consumption and delivery of oxygen to the myocardium (Figure 1) [6].
Figure 1.
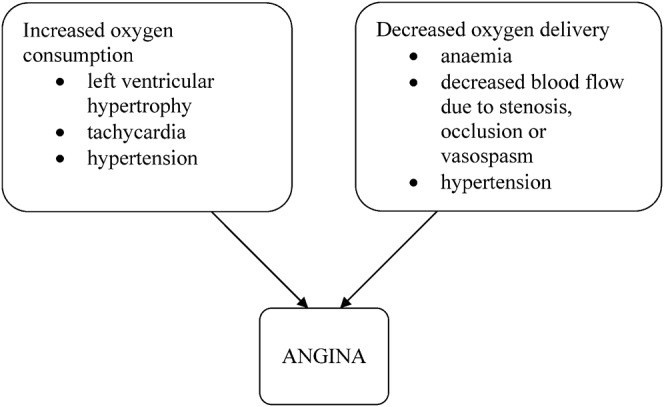
Factors leading to angina.
The ischaemic cascade
Development of angina is the end result of a sequence of events resulting from an imbalance between myocardial oxygen consumption and myocardial oxygen delivery called the ischaemic cascade. It refers to the temporal sequence of pathophysiological events that occurs within seconds of occlusion or chronic stenosis of a coronary artery (Figure 2) [7].
Figure 2.
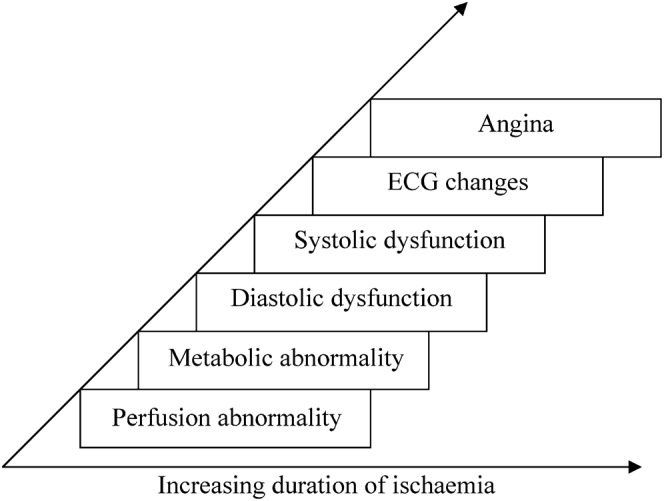
Ischaemic cascade. ECG, electrocardiogram.
The ischaemic changes become irreversible after 30 min, when myocardial necrosis sets in. It starts in the subendocardium and moves towards the epicardium as a wave front phenomenon. This is reflected in the pattern of delayed enhancement seen on MRI. Enhancement due to an infarct is initially typically subendocardial in location before progressing towards the epicardium.
Physiological basis of functional assessment
The ischaemic cascade forms the basis of functional imaging. This can be assessed by demonstrating perfusion or wall motion abnormalities on stress MRI, SE, SPECT and PET imaging brought on by “stressing the heart” with adenosine, dipyridamole, exercise or dobutamine.
Stress is achieved either in the form of:
Vasodilatory stress. This mechanism is based on the variable ability of coronary arteries to vasodilate, resulting in maldistribution of blood flow between regions supplied by normal and stenotic arteries. During stress, the non-stenotic coronary artery dilates, resulting in a fourfold increase in blood flow downstream. The stenotic vessel, on the other hand, is already maximally dilated at rest in order to maintain myocardial oxygenation. It is unable to dilate any further. This loss of vasodilatory reserve, also called the coronary flow reserve, results in a reverse coronary steal. The blood flow is diverted from areas supplied by a stenotic vessel to those subtended by non-stenotic vessels with consequent hypoperfusion in the region supplied by the stenotic artery [8]. Ischaemia is diagnosed primarily by the demonstration of a hypoperfusion defect on the post-stress images which is not present on the non-stress (i.e. rest) images. Stress-induced wall motion abnormalities may also occur, particularly when the ischaemia is extensive and associated with severe stenosis greater than 70% [9].
Ionotropic stress. This mechanism relies on an increased oxygen demand as a consequence of the ionotropic and chronotropic actions of the stressor. Vasodilatation is largely secondary to the increased oxygen demand and is not as profound as that achieved with adenosine or dipyridamole [10]. Wall motion abnormalities occur in the territory supplied by the stenotic artery due to a mismatch between oxygen demand and supply. Ischaemia is diagnosed primarily by the presence of inducible wall motion abnormality. Perfusion defects, although less specific, may also be demonstrated and form the basis of dobutamine SPECT imaging [11].
Adenosine and dipyridamole are predominantly vasodilatory agents. Exercise and dobutamine are predominantly ionotropic stress agents.
Perfusion abnormalities occur earlier than wall motion abnormalities in the ischaemic cascade. This understanding of the ischaemic cascade explains the better sensitivity of techniques that identify perfusion abnormalities and better specificity of techniques that identify wall motion abnormalities in the diagnosis of ischaemia [12].
The functional significance of a lesion can also be determined invasively by measuring the fractional flow reserve (FFR) at the time of a coronary angiogram. FFR is expressed as a ratio of the coronary pressure distal to a stenosis to simultaneous aortic pressure measurement during maximal hyperaemia. An FFR less than 0.75 is considered to be functionally significant [13].
Rationale for integrated anatomical and functional imaging
In guiding treatment options
The debate regarding percutaneous intervention (PCI) vs optimum medical therapy (OMT) continues. The COURAGE (Clinical Outcomes Utilizing Revascularization and AGgressive drug Evaluation) trial showed that in stable CAD with more than 70% stenosis, the adverse event rate (defined as all-cause mortality, non-fatal myocardial infarction, stroke and hospitalisation for acute coronary syndrome) was similar in patients randomised to an initial strategy of OMT alone and those treated with PCI and OMT [14].
Recent publications such as the nuclear substudy of the COURAGE trial [15], the 1 and 2 year follow-up of the FAME study (Fractional flow reserve versus Angiography for Multivessel Evaluation) [16,17] and the 2 and 5 year follow-up of the DEFER study [18,19] highlight the importance of looking for objective evidence of ischaemia prior to PCI. These studies highlight several important clinically relevant points.
PCI in FFR-guided functionally significant coronary artery stenosis may improve patient outcome by reducing adverse cardiac events. These studies confirm the findings from earlier large observational myocardial perfusion studies, which suggested that revascularisation in patients with more than 10% ischaemic myocardium reduced adverse events [20].
Intervention in functionally significant stenosis results in a higher percentage of patients experiencing relief from angina with consequent improvement in their quality of life.
On the other hand, revascularisation of functionally non-significant lesions actually increases the risk of cardiac event rates. It also does not relieve anginal symptoms.
These conclusions have important clinical ramifications. The decision to revascularise has traditionally been based on visual anatomical assessment of stenosis on invasive coronary angiogram. However, there is a lack of correlation between anatomical severity and functional significance. The classic study of Gould and Lipscomb [21] showed that a stenosis of less than 50% is unlikely to be functionally significant. Based on FFR measurement, stenoses of severity between 50% and 90% and, in particular, between 50% and 70% show a wide variability in their functional significance (Table 2) [22].
Table 2. Functional severity of stenoses as determined by fractional flow reserve [22].
| Stenosis severity(%) | Functionally significant (%) | Functionally non-significant (%) |
| 50–70 | 35 | 65 |
| 71–90 | 80 | 20 |
| 91–99 | 96 | 4 |
Factors that may make a 50–70% lesion functionally significant include:
the length of stenosis and the number of stenoses in the vessel [23]
the location of the lesion: a proximally located vessel narrowing results in a larger area of ischaemic myocardium than a distally located stenosis [4]
factors leading to increased oxygen demand and consumption such as left ventricular hypertrophy or tachycardia (Table 1) [5,6]
factors leading to decreased arterial oxygen concentration, e.g. anaemia (Table 1) [5,6].
On the other hand, the presence of collateral vessels may ensure enough blood flow to a region supplied by a severely stenotic artery so as to not show objective evidence of ischaemia [24].
An individualised approach to multivessel CAD based on intervention targeted to culprit functionally significant lesions is likely to improve clinical outcome.
Role in refining risk stratification
The different imaging modalities provide complementary information about different aspects of CAD. An integrated approach refines risk stratification by providing incremental information over any single technique. In a study involving 541 patients who underwent both SPECT imaging and coronary CT, both modalities provided independent prognostic information. The annualised hard event rate (defined as an all-cause mortality or non-fatal myocardial infarction) was 1.8% for none/mild CAD and 4.8% for significant CAD on CT. The annualised hard event rate was 1.1% for a normal SPECT and 3.8% for an abnormal SPECT study. The two techniques, when combined, provided additional prognostic information (Table 3) [25].
Table 3. Annualised cardiac event rate on combining coronary CT and gated single photon emission CT (SPECT) imaging [25].
| Normal SPECT imaging (%) | Abnormal SPECT imaging (%) | |
| Normal/mild coronary artery disease on CT | 1 | 3.7 |
| Significant coronary artery disease on CT | 3.8 | 9 |
Addition of plaque analysis further enhanced the risk prediction to 10.8%. The plaque composition also influenced prognostication. The risk increased with two or more segments with non-calcified plaques, three or more segments with mixed plaques and four or more segments with calcified plaques [25].
In identifying subclinical atherosclerosis
Coronary calcium and coronary CT diagnose the presence of plaques. In an asymptomatic patient or in a patient with no objective ischaemia on functional imaging, their presence is an indicator of subclinical atherosclerosis. Those with subclinical atherosclerosis may benefit from lifestyle modifications and aggressive medical therapy as part of secondary prevention strategies [26].
Guidelines for an integrated approach to chest pain based on the NICE recommendations
NICE is an independent UK-based organisation. It provides evidence-based national guidelines on various matters relating to health. The NICE guideline, issued in March 2010, recommends a structured, evidence-based cost-effective model incorporating anatomical and functional assessment in a patient with stable chest pain of recent onset [4].
The salient features of the NICE guideline are:
A structured systematic “back to basics” approach to a patient presenting with chest pain based primarily on clinical history and assessment. The pre-test probability of CAD is determined by the age and gender of patient and the nature of the chest pain, presence of cardiovascular risk factors such as diabetes, smoking, hypercholesterolaemia, history of known CAD and resting 12 lead electrocardiogram (ECG) and biochemical markers.
Non-anginal chest pain need not be investigated further for CAD.
A patient with typical or atypical stable chest pain with a pre-test likelihood of CAD of between 10% and 90% is investigated further (see Table 4).
The use of exercise ECG for a patient with no known history of CAD is discouraged owing to its low sensitivity and specificity.
In patients with known CAD, functional imaging or exercise ECG is recommended for patients in whom the diagnosis of angina is not possible on clinical assessment.
Table 4. Suggested first-line diagnostic investigation for chest pain [4].
| Pre-test probability | Suggested first-line investigation |
| <10% | Consider other causes of chest pain |
| Between 10% and 29% | Consider coronary calcium |
| If coronary calcium score is zero consider other causes of chest pain | |
| If coronary calcium score is between 1 and 400 consider functional imaging for further assessment | |
| If coronary calcium score is >400 consider coronary angiography | |
| Between 30% and 60% | Consider functional imaging |
| Between 61% and 90% | Consider coronary angiography if revascularisations is a consideration or functional imaging if revascularisation is not a consideration |
| More than 90% | Manage as angina |
Implications of the NICE guideline
It is anticipated that there will be a substantial increase in the number of patients undergoing non-invasive imaging and invasive angiography [27]. This necessitates a considerable improvement in the infrastructure of imaging facilities in terms of the equipment, manpower and technical expertise. Local guidelines, although broadly based on the NICE guideline, need to take into account the availability of local resources and expertise in implementing the NICE recommendations.
Diagnostic and prognostic accuracy of different imaging modalities
There is a wide variation in literature on the subject owing to different methodologies involved, different prevalence of disease in the populations studied and different definitions of significant CAD. Most studies define significant stenosis as 50% or more based on the original study by Gould [21]. However, 70% or more stenosis is considered severe stenosis in day-to-day practice [28] and this cut-off has been used in some studies to define significant stenosis. Invasive coronary angiogram is still considered to be a gold standard. This, in itself, is a flaw as an anatomical yardstick is used to validate the diagnostic accuracy of functional assessment. The rest of the article looks at the role of different imaging modalities, with particular reference to their accuracy in the diagnosis of CAD, prognostication and assessment of viability.
Single photon emission CT
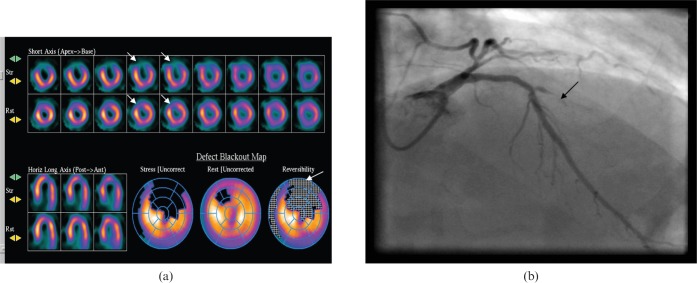
SPECT imaging has been available since the 1970s and has given us a large body of evidence confirming its diagnostic and prognostic value. The commonly used radio-isotopes are thallium-201 and technetium-based agents such as 99Tcm sestamibi and 99Tcm tetrofosmin. Ischaemia is suspected when there is reduced tracer uptake on the stress acquisition which is reversible on the rest acquisition (Figure 3). A fixed defect, i.e. a defect present on both stress and rest acquisitions, is suggestive of an infarct provided attenuation artefacts are ruled out (Figure 4).
Figure 3.
(a) Reversible ischaemia on myocardial scintigraphy scan in the anterior wall (arrows). (b) An occluded diagonal artery (arrow) on invasive coronary angiogram.
Figure 4.
Infarct in the inferior wall identified by the fixed defect on stress and rest acquisitions on myocardial perfusion scintigraphy scan (arrows). Courtesy of Dr A Kelion, Consultant Cardiologist, Harefield Hospital, UK.
Diagnostic accuracy
The sensitivity and specificity for the diagnosis of significant coronary stenosis (defined as ≥50% stenosis) were 86% and 74%, respectively [29].
A false negative study may be a feature of three-vessel and left main stem disease because SPECT assesses relative perfusion. Normal perfusion is seen in up to 13–15% of patients with left main stem disease on account of balanced ischaemia in multivessel disease [30,31].
False positive tests due to attenuation artefacts lower the specificity. For example, an elevated diaphragm results in an apparent fixed defect in the inferior wall in men, and breast artefact gives rise to an apparent defect in the anterior wall in women. Implementing gated studies [32], attenuation correction algorithm [33] and prone imaging [34] help improve the specificity by reducing the number of equivocal scans in such cases.
Referral bias, introduced by the fact that only patients with a positive test will undergo an invasive angiogram, also falsely lowers the specificity.
Prognostic considerations
A negative study confers an annualised risk of less than 1% of adverse cardiac events [35].
An abnormal study is associated with an annual event rate of 6.7–7% [29,35]. The annual event rate increases with increasing severity of the perfusion defect.
The warranty period refers to the frequency of follow-up testing following a negative study. The warranty period is approximately 5 years in a clinically stable patient with no new symptoms or signs. The individual risk and the warranty period are dependent on the age and sex of the patient, stress-induced ECG changes and associated comorbidities such as diabetes and renal dysfunction. The annual risk varies from 1.4% to 1.8%. The risk is highest for an 80-year-old female diabetic patient, in whom the warranty period is only 1–2 years [20].
Assessment of viability
Viability assessment on SPECT is based on demonstration of the integrity of the cell membrane following an injection of a perfusion tracer. Pooled meta-analysis of thallium and tetrofosmin studies suggests good sensitivity of 83–88% and a modest specificity of 49–69% for prediction of regional functional recovery after revascularisation [36]. This suggests that it has a good negative predictive value. The poor positive predictive value is due to the poor spatial resolution of the technique. Subendocardial infarcts are beyond the spatial resolution of SPECT and are likely to be missed leading to overestimation of viability [37].
Assessment of cardiac function
Analysis of LVF on gated studies correlated well with MRI in a meta-analysis of nine studies. However, the margin of error was higher in women with smaller left ventricular volumes, with dilated cardiomyopathy and in the presence of global subendocardial perfusion defects [38].
Role of perfusion scintigraphy scan
The European and American guidelines recommend the stress electrocardiography test as a first-line of investigation in patients with an intermediate pre-test probability owing to its wide availability [39,40]. A perfusion scintigraphy scan is recommended for the diagnosis of CAD in the following conditions:
contra-indications to performing stress ECG, e.g. severe arterial hypertension, left ventricular hypertrophy
inability to perform stress ECG
equivocal stress ECG
abnormal resting ECG which would make interpretation of stress ECG difficult.
In patients with known CAD, it assesses the haemodynamic significance of a coronary lesion and is involved in risk stratification and prognosis.
Positron emission tomography
PET consists of perfusion imaging with a perfusion tracer (rubidium-82, nitrogen-13 ammonia or oxygen-15 water) and functional metabolic imaging with 18F-fluorodeoxyglucose (FDG).
Mismatch between flow and metabolism, i.e. reduced flow with normal or increased FDG uptake, suggests reversible ischaemia. Matched reduction in blood flow and metabolism suggests an infarct.
Diagnostic accuracy
In a meta-analysis of 19 studies, PET had a sensitivity of 92% and a specificity of 85% for diagnosing significant CAD (defined as ≥50%) [41].
Prognostic value
A study of 1441 patients who underwent rubidium PET study confirmed that the all-cause mortality increased with increasing severity of perfusion defect and with decreasing left ventricular ejection fraction (LVEF) over a follow-up period of 2.7 years [42]. The annualised cardiac event rate was 0.4% in patients with normal studies. It increased to 2.3% with mild perfusion abnormalities and 7% with moderate to severe perfusion defects [43].
Assessment of viability
As with SPECT, PET has good sensitivity and moderate specificity for predicting functional recovery post-revascularisation [44].
Comparison with SPECT
In a study looking at age-, gender- and body mass-matched patients who had PET (n = 112) or SPECT (n = 112), PET was superior to SPECT in many aspects [45]. PET had higher diagnostic accuracy than SPECT (87% vs 71%) for stenosis of more than 50%. It correctly diagnosed multivessel disease in more patients. There was less gut interference and fewer attenuation artefacts, resulting in superior quality images. It was quicker than SPECT because of the short half-life of the perfusion tracers. Rubidium-82 has a half life of 72 s, nitrogen-13 ammonia has 10 min and oxygen-15 water has 2 min.
Unlike SPECT, PET has the advantage of being able to measure myocardial blood flow in absolute units, which is important in the assessment of the distal coronary microcirculation [46].
Despite its superiority over SPECT imaging, the widespread use of PET is hampered by the requirement for expensive PET cameras and for cyclotron or rubidium generators.
Stress echocardiography
SE is a low-cost, widely available procedure which is based on assessment of regional wall motion abnormality induced by exercise or increasing doses of dobutamine. Stress-induced new or worsening regional or global wall motion abnormality is a reliable predictor of ischaemia.
Diagnostic accuracy
The sensitivity and specificity of SE in the diagnosis of CAD varies with the technique used. The sensitivity is 80%, 85% and 78% and specificity is 86%, 76% and 91% for dobutamine, exercise and dipyridamole, respectively [47,48]. The sensitivity and specificity are reduced in patients with severe left ventricular dysfunction.
Prognostic information
A negative SE confers a 0.5–0.8% risk of cardiac death or non-fatal myocardial infarction [49,50]. An abnormal SE is associated with an increased risk of adverse cardiac events. The risk is increased with resting left ventricular dysfunction, extensive ischaemia and extensive wall motion abnormality [48,51].
Viability
Viability imaging is based on demonstration of contractile reserve, i.e. the ability of dysfunctional myocardium to contract with low doses of an ionotropic agent. SE had a sensitivity of 84% and a specificity of 81% for prediction of functional recovery. False negatives may be due to the presence of fibrosis or the disruption of the contractile apparatus in viable tissue. Contractile response of the myocardium adjacent to an infarct can give rise to a false positive result [36].
Assessment of cardiac function
LVF is mainly assessed by M mode and two-dimensional echocardiography [52]. It is widely available, inexpensive and does not involve the use of radiation. However, it is unreliable in progressive ventricular dilatation as it relies on geometric assumptions regarding the left ventricular cavity [53].
Comparison with SPECT imaging
Pooled meta-analyses show that SPECT is more sensitive and SE is more specific, both in the diagnosis of significant CAD and in the assessment of viability [54].
Role of stress echocardiography
Indications for SE include:
diagnosis of CAD
risk stratification in patients with known CAD
pre-operative risk assessment in patients undergoing non-cardiac surgery
assessment of valvular dysfunction [51].
It is most useful in patients in whom stress ECG is contraindicated, not feasible, equivocal or submaximal [51].
Cardiac MRI
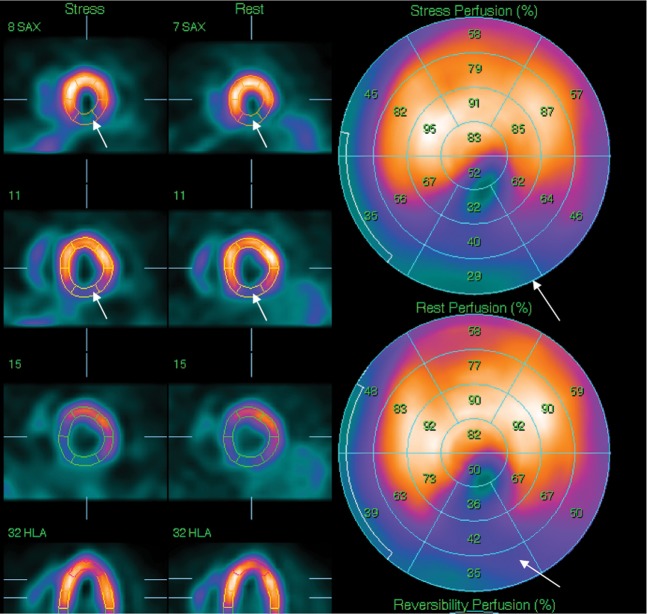
Stress is achieved by the same mechanisms as for other techniques, i.e. exercise or pharmacological. The myocardium is imaged during the first pass of a bolus of gadolinium during stress. The normally perfused myocardium enhances with contrast.
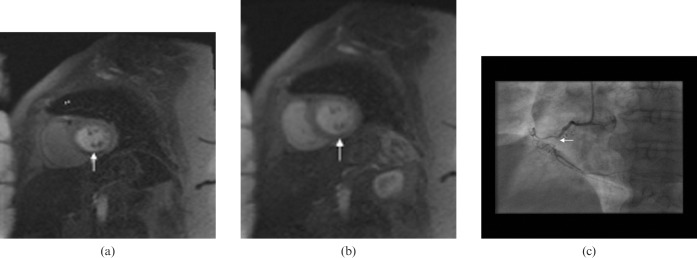
Reversible ischaemia is visually assessed as a reversible low-signal defect in the absence of delayed enhancement (Figure 5). An infarct shows up as an enhancing area as opposed to normal myocardium, which is black or “nulled” on the delayed enhancement sequences with gadolinium (Figure 6).
Figure 5.
(a) A hypoperfusion defect in the inferior wall on stress MRI (arrow). (b) No hyperfusion defect in the inferior wall on rest images (arrow). (c) Occlusion in the mid-right coronary artery (arrows) on coronary angiogram.
Figure 6.
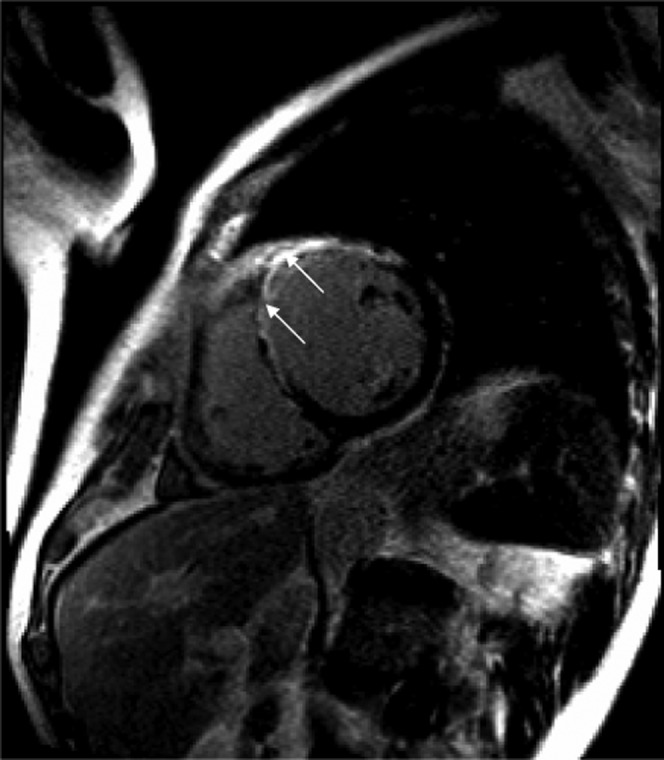
Enhancement in an infarct involving the anterior and anteroseptal walls of the left ventricle on delayed enhancement images on MRI (arrows).
Diagnostic accuracy
A recent meta-analysis confirmed a high sensitivity of 89% and a moderate specificity of 80% for the diagnosis of significant CAD in a population with a high prevalence of CAD of 57% [55]. The value of stress CMR in low-prevalence populations is not clear. False positive tests can be attributed to the presence of artefacts due to susceptibility (called dark rim artefacts), poor gating and motion artefacts [56].
Prognostic evaluation
A negative adenosine stress perfusion CMR conferred a low cardiac event rate of 1%, both in the low to intermediate risk population and in patients with known CAD [57–59]. An abnormal adenosine stress CMR was associated with a 12-fold increased risk of a cardiac event, and an abnormal dobutamine stress perfusion was associated with a 5-fold risk of a cardiac event over a follow up period of 2.3 years [60].
Assessment of viability
Viability imaging on CMR relies on the demonstration of scar tissue 10–20 min after the administration of gadolinium on the delayed enhancement inversion gradient echo sequence. In a study involving 50 consecutive patients who were imaged before and after revascularisation, Kim et al [61] showed that the extent of the infarct on delayed enhancement sequences predicted functional recovery after revascularisation (Table 5). The extent of the infarct was expressed as a percentage of the myocardium that enhanced with contrast.
Table 5. Extent of delayed enhancement and functional recovery after revascularisation [61].
| Extent of delayed enhancement | Segments which show functional recovery (%) |
| 0 | 78 |
| 1–25 | 60 |
| 26–50 | 42 |
| 51–75 | 10 |
| >75 | 2 |
Thus, absence of enhancement and enhancement of more than 75% of myocardium were the best predictors of functional recovery 79±36 days after revascularisation.
Assessment of cardiac function
This is considered to be the gold standard for global and regional left ventricular functional analysis. It is superior to echocardiography for the following reasons:
the newer sequence, called the steady-state free-precession sequence, allows good demarcation of the endocardial border and blood pool contrast; and
unlike echo, there is no geometric assumption and the LVEF can be calculated with reasonable accuracy even in distorted ventricles [62].
Comparison with SPECT
A comparison of stress CMR and SPECT in 234 patients in 18 centres worldwide was the basis of the MR-IMPACT (magnetic resonance imaging for myocardial perfusion assessment in coronary artery disease) trial. It showed that stress CMR using 0.1 mmol kg–1 gadolinium performed better than SPECT (area under the curve of 0.86 vs 0.67) and that stress MR performed better than SPECT in the diagnosis of multivessel disease [63].
CMR also consistently detects more subendocardial defects than SPECT or PET imaging. Nearly half of the segments with subendocardial infarcts are missed on SPECT [64] and PET [65].
Comparison with stress echocardiography
Delayed enhancement CMR has a better negative predictive value than SE, particularly in segments with severe dysfunction [61]. Dobutamine echocardiography has a low sensitivity of 26% in severe left ventricular dysfunction [66], the very segments whose viability assessment needs to be accurate. This is because contractility depends on the delivery of adequate amount of oxygen to an intact contractile apparatus. In severely dysfunctional segments, ionotropic reserve is hampered owing to an exhausted coronary flow reserve to a possibly disrupted contractile apparatus.
Coronary artery calcium
This is a marker of subclinical atherosclerosis and reflects the total atherosclerotic burden including calcified and non-calcified plaques. The most widely used method for quantifying coronary calcium is the Agatston score [67]. It is based on identifying calcium on the basis of its density (>130 Hounsfield units) (Figure 7). It assigns a CT factor to the coronary calcium based on the Hounsfield unit in all the coronary arteries and compiles a total score based on age and gender [68].
Figure 7.
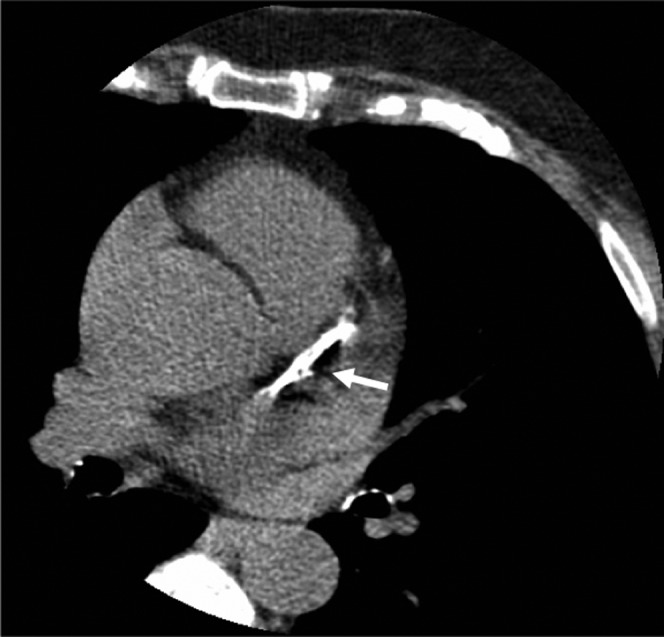
Calcification in the left anterior descending artery on a coronary calcium score study (arrow).
Prognostic role of coronary calcium in asymptomatic patients
Coronary calcium is an independent risk factor superior to and additive to traditional risk factors in patients without known CAD, irrespective of their ethnicity [69-72].
Absent coronary calcium was associated with an event rate of less than 1.01% over 50 months. A coronary calcium score of zero was associated with a low incidence of significant coronary artery stenosis on invasive angiogram, a low likelihood of acute coronary syndrome and a low incidence of an abnormal myocardial perfusion scintigraphy scan [72].
Prognostic role of coronary calcium in symptomatic patients
Although most of the prognostic data are derived from studies involving asymptomatic patients, the prognostic role also extends to the symptomatic population. In a meta-analysis of 7 studies involving 3924 patients, 1.8% of patients with no coronary calcium had a cardiovascular event compared with 8.99% of symptomatic patients with coronary calcium followed over 42 months [72].
Role of coronary calcium in the diagnosis of obstructive CAD in patients presenting with stable chest pain
Coronary calcium has an important role to play in patients presenting with stable chest pain, with a pre-test probability of 10–29% as suggested by the NICE guideline (Table 5) [4]. The presence of coronary calcium is sensitive but not specific for the diagnosis of significant coronary artery stenosis [72]. A clear relationship exists between increasing coronary calcium scores and the severity of coronary stenosis and the number of stenotic vessels [73]. Absent coronary calcium is indicative of the absence of significant stenosis. However, this has to be interpreted with caution. Both the Multi-Ethnic Study of Atherosclerosis [73] and a recent meta-analysis [72] showed that absent coronary calcium may be associated with significant coronary artery stenosis in 2–4% of patients. These patients were younger, and hence absent coronary calcium should be interpreted with caution in patients under 50 years.
Role of coronary calcium in patients presenting with acute chest pain to the emergency department
A limited number of studies of patients presenting to the emergency department with acute chest pain show that in patients with negative cardiac enzymes and no electrocardiographic changes, absent coronary calcium excludes acute coronary syndrome with a high sensitivity of 99% and a negative predictive value of 99% [72]. These patients can be discharged from the unit promptly and safely with a low risk of a cardiac event [74].
Role of coronary calcium in renal disease
Coronary calcium is deposited in a non-atherosclerotic process in the tunica media as a consequence of altered calcium metabolism [75]. It is high and progressive in renal patients, particularly in patients on dialysis [76]. It is associated with a significantly increased cardiovascular risk [77]. The role of coronary calcium is not clear because of limited studies on the subject. More prospective studies are required to clearly ascertain the relationship between increased cardiovascular risk and coronary calcium.
Role of coronary calcium in diabetic subjects
Several studies demonstrate that:
Coronary calcium is an independent prognostic indicator in diabetic patients.
Increasing coronary calcium is associated with an increased risk of cardiovascular events. For every increase in coronary calcium the risk is higher in diabetics than non-diabetic patients.
Absent coronary calcium is associated with a lower risk of events, a lower incidence of myocardial perfusion abnormalities and a short-term survival rate similar to that of non-diabetic patients [78-80].
Comparison with other imaging modalities
Absent coronary calcium is associated with a low incidence of ischaemia on SPECT/PET. Only 6% of patients with absent coronary calcium had ischaemia compared with 20% of patients with a high coronary calcium score [81,82]. Increasing coronary calcium is associated with an increased risk of an adverse cardiac event, both in patients with normal perfusion scans and in those with perfusion abnormalities on PET imaging [83].
Coronary CT
Technological advancements in hardware and software of newer generation CT scanners have improved spatial and temporal resolution and z-axis coverage while reducing the radiation dose. Faster gantry rotation times, wide detector designs, dual-source technology and newer reconstruction methods have resulted in improvements in image acquisition, reconstruction, work flow and analysis.
Diagnostic value
Diagnosis of CAD involves detection of stenosis and plaque analysis (Figure 8).
Figure 8.
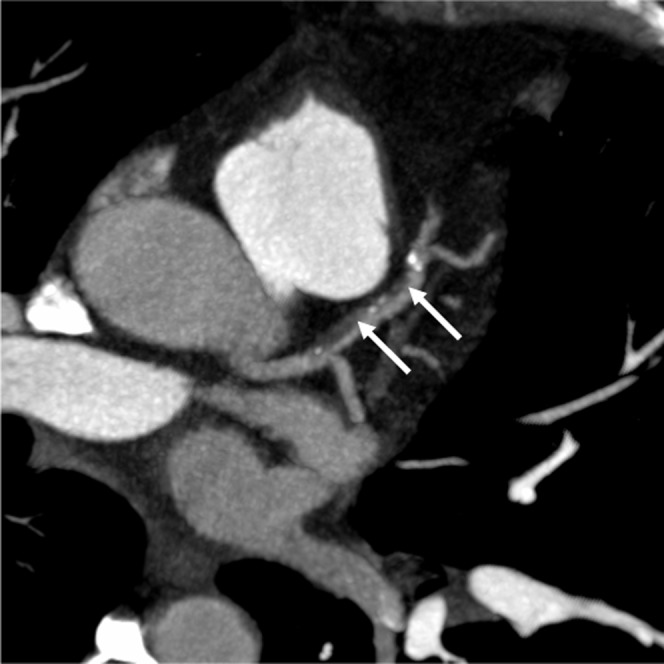
CT appearances of mixed plaque (arrows) in the left anterior descending artery.
In the diagnosis of coronary stenosis
Multicentre prospective studies [28,84,85] and a recent meta-analysis [86] confirm an excellent sensitivity of 99% for 64 slice coronary CT. Coronary CT rules out coronary stenosis with a high degree of confidence in low-, intermediate- and high-risk populations [28,84,85].
However, its specificity varies between 64% and 89% [84,86-88]. The relatively modest specificity, particularly in high-risk patients, is due to a number of factors, including heavily calcified vessels [28], smaller vessels [89] and the presence of stents [90]. Artefacts introduced by poor image quality due to high heart rates, arrhythmias and motion may be falsely interpreted as stenoses [86]. Thus, there is a tendency to overestimate stenoses, particularly in high-risk patients.
In plaque assessment
Assessment of the atherosclerotic burden by CT is based on plaque volume assessment. There is a good correlation between plaque volume estimation on intravascular ultrasound and on CT [91]. Plaque volume estimation has the potential for monitoring response to lipid-lowering therapy [92]. However, it is affected by several variables including the image quality and interobserver variability [93].
Plaques may also be implicated in the development of acute coronary syndrome. Low attenuation plaques (i.e. non-calcified plaques), plaques with spotty calcification (mixed plaques) and those associated with constrictive remodelling are more likely to result in an acute coronary syndrome [94].
Prognostic value
There are few data regarding long-term prognostic information, particularly with the newer generation of CT scanners. In a recent meta-analysis, the annualised cardiac event rate was 0.17% for a normal coronary CT over a median follow-up of 20 months. The adverse annual event rate was 8.8% for an abnormal coronary CT and the risk rose with increasing severity of coronary stenosis [95].
Role of coronary CT in a stable patient with suspected CAD
Most of the data regarding the diagnostic accuracy of coronary CT, as described above, relate to stable patients with suspected CAD. The high negative predictive value in intermediate-risk patients safely reduces unnecessary referrals for invasive angiograms [96]. Its modest positive predictive value limits its diagnostic accuracy, particularly in high-risk patients [84]. Thus, coronary CT is of most value in the intermediate-risk population.
Role of coronary CT in acute chest pain
Patients presenting to the emergency department with acute chest pain can be stratified on the basis of coronary CT provided they have no ECG changes and have normal cardiac enzymes [97,98]. Small studies show that up to 50% of such patients do not have significant coronary stenosis [97] and can be safely discharged with a low 1 year event rate [98]. Coronary CT also has a role to play in a “triple rule-out” test, which aims to rule out important non-cardiac causes of chest pain such as an acute aortic syndrome and pulmonary embolism. There are issues such as increased radiation dose and difficulty in ensuring adequate contrast opacification in three vascular territories [90]. More data, particularly from large randomised controlled trials, are needed to evaluate the clinical utility of the triple rule out test in day-to-day practice.
MRI of coronary arteries
This involves free breathing three-dimensional acquisition of the coronary artery with real-time assessment of the diaphragm using a navigator (Figure 9). It has a sensitivity of 72% and a specificity of 87% for diagnosing coronary artery stenosis of 50% or more [99]. Its predictive value is best for proximal and mid-segments [100]. Its clinical utility is limited by the fact that it is time-consuming and 30–50% of segments cannot be evaluated [100].
Figure 9.
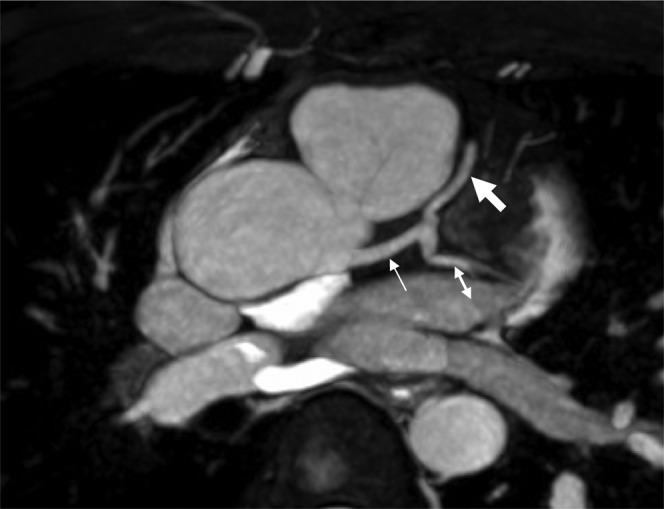
Three-dimensional navigator image of the left main stem (thin arrow), left anterior descending artery (thick arrow) and circumflex artery (double-headed arrow) on MRI.
Radiation considerations
The background risk of radiation is 1–3 mSv. Radiological procedures may be associated with an exposure to radiation over and above the background risk of radiation. The radiation dose is expressed as an effective dose in millisieverts (mSv) [101]. The average risk of dying of malignancy due to exposure to radiation is estimated to be 5–7.9 per 100 individuals in the general population per sievert [102]. The effective radiation dose for cardiac imaging procedures is summarised in Table 6.
Table 6. Effective radiation doses for various imaging modalities.
| Effective radiation dose (mSv) | |
| Tetrofosmin stress rest [101] | 9–13 |
| Thallium stress rest [101,103] | 22–40 |
| FDG-PET [104] | 14 |
| Rubidium 82 [104] | 5 |
| Coronary calcium [104,105] | 1–3 |
| Coronary CT [106,107] | 1–12 |
FDG PET, 18F-fluorodeoxyglucose positron emission tomography.
Future direction
Technological innovations in myocardial perfusion and plaque imaging have pushed the boundaries of cardiac imaging.
Perfusion imaging
Echocardiography
Myocardial contrast echocardiography assesses myocardial perfusion, viability and myocardial blood flow by using intravenous microbubbles of contrast [108,109]. The microbubbles are coated with lipid, albumin or polymers. They generate an ultrasound signal as they transit through the myocardial capillary circulation. This helps visualise the myocardial capillary bed during a continuous infusion of microbubbles of contrast during vasodilatory or ionotropic stress [110]. The use of intravenous contrast agent combined with three-dimensional echocardiography has made real-time three-dimensional myocardial contrast echocardiography an emerging technique for assessing tissue perfusion. It has the advantage of volumetric data acquisition and delineation of perfusion defects [111].
SPECT imaging
A shift from sodium iodide crystals to newer generation crystals such as cadmium zinc telluride and caesium iodide has led to improved detector efficiency. Improved detector configurations have resulted in ultra-fast SPECT cameras. Iterative reconstruction methods have led to quicker acquisition with better contrast resolution and reduced radiation dose. The introduction of the new selective vasodilatory agent, regadenoson, will result in fewer side-effects.
Innovations in the detector crystals, hardware design including detector configuration, reconstruction methods and vasodilator stress agents have led to faster acquisition times with better spatial and contrast resolution.
CT perfusion
CT perfusion studies with adenosine on 64 slice, 256 slice and dual-source scanners are associated with sensitivity and specificity comparable to those obtained with SPECT imaging with acceptable radiation doses [112,113].
Initial studies with CT adenosine stress suggest a potential for demonstrating coronary anatomy, ischaemia and infarct in a single procedure. However, more studies are needed before it can be considered for widespread clinical use.
Plaque analysis
Rupture or fissuring of plaques leads to an acute coronary syndrome. Plaque composition has an important role to play in plaque rupture. Lipid-rich plaques with a thin fibrous cap containing macrophages are more likely to rupture [114]. Identification of plaque composition is the basis of CMR and CT imaging. CMR imaging focuses on identification of the lipid core, the fibrous cap and haemorrhage within the plaque. The use of plaque-avid contrast agents is an important area of research in MRI [115]. PET concentrates on demonstration of metabolic activity in a vulnerable plaque [116]. Invasive methods such as intravascular ultrasound and optical coherence tomography are catheter-based techniques used at the time of coronary angiography to assess plaque composition. Intravascular ultrasound is based on a greyscale image generated by the reflection of ultrasound [117]. Optical coherence tomography is based on reflection of light resulting in high-resolution tomographic images [118]. Both techniques assess plaque composition and identify rupture or fissuring of the fibrous cap.
Conclusion
The different cardiac imaging modalities provide complementary information about various aspects of CAD. Coronary CT and MRI of the coronary arteries provide anatomical information. Functional significance of a coronary stenosis is assessed by stress CMR, radio-isotope studies and SE. Subclinical atherosclerosis is assessed by coronary calcium score and coronary CT. The NICE guideline provides a framework which incorporates anatomical and functional imaging in the setting of stable chest pain of recent onset. An integrated approach combining anatomical and functional imaging is important in guiding treatment options and in risk stratification.
References
- 1.Nilsson S, Scheike M, Engblom D, Karlsson LG, Mölstad S, Akerlind I, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract 2003;53:378–82 [PMC free article] [PubMed] [Google Scholar]
- 2.Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005;91:229–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British Heart Foundation UK coronary heart disease statistics 2009–10. London, UK: BHF; 2009 [Google Scholar]
- 4.National Institute for Health and Clinical Excellence. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin (clinical guideline 95), 2010. Available at: www.nice.org.uk/guidance/CG95. [Google Scholar]
- 5.Braunwald E. Control of myocardial oxygen consumption: physiologic and clinical considerations. Am J Cardiol 1971;27:416–32 [DOI] [PubMed] [Google Scholar]
- 6.Crossman DC. The pathophysiology of myocardial ischaemia. Heart 2004;90:576–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol 1987;59:23C–30C [DOI] [PubMed] [Google Scholar]
- 8.Verani MS. Pharmacological stress with adenosine for myocardial perfusion imaging. Semin Nucl Med 1991;21:266–72 [DOI] [PubMed] [Google Scholar]
- 9.Paetsch I, Jahnke C, Wahl A, Gebker R, Neuss M, Fleck E, et al. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation 2004;110:835–42 [DOI] [PubMed] [Google Scholar]
- 10.Fung A, Gallagher K, Buda A. The physiologic basis of dobutamine as compared with dipyridamole stress interventions in the assessment of critical coronary stenosis. Circulation 1987;76:943–51 [DOI] [PubMed] [Google Scholar]
- 11.Elhendy A, Bax JJ, Poldermans D. Dobutamine stress myocardial perfusion imaging in coronary artery disease. J Nucl Med 2002;43:1634–46 [PubMed] [Google Scholar]
- 12.Leong-Poi H, Rim S-J, Le DE, Fisher NG, Wei K, Kaul S. Perfusion versus function: the ischemic cascade in demand ischemia: implications of single-vessel versus multivessel stenosis. Circulation 2002;105:987–92 [DOI] [PubMed] [Google Scholar]
- 13.Pijls NH, De Bruyne B, Peels K, Van DerVoort PH, Bonnier HJ, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703–8 [DOI] [PubMed] [Google Scholar]
- 14.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16 [DOI] [PubMed] [Google Scholar]
- 15.Shaw LJ, Berman DS, Maron DJ, Mancini GBJ, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–91 [DOI] [PubMed] [Google Scholar]
- 16.Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van't Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24 [DOI] [PubMed] [Google Scholar]
- 17.Pijls NHJ, Fearon WF, Tonino PAL, Siebert U, Ikeno F, Bornschein B, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 2010;56:177–84 [DOI] [PubMed] [Google Scholar]
- 18.Bech GJW, De Bruyne B, Pijls NHJ, de Muinck ED, Hoorntje JCA, Escaned J, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 2001;103:2928–34 [DOI] [PubMed] [Google Scholar]
- 19.Pijls NHJ, van Schaardenburgh P, Manoharan G, Boersma E, Bech J-W , van't Veer M, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER study. J Am Coll Cardiol 2007;49:2105–11 [DOI] [PubMed] [Google Scholar]
- 20.Hachamovitch R, Hayes S, Friedman JD, Cohen I, Shaw LJ, Germano G, et al. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: What is the warranty period of a normal scan? J Am Coll Cardiol 2003;41:1329–40 [DOI] [PubMed] [Google Scholar]
- 21.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 1974;34:48–55 [DOI] [PubMed] [Google Scholar]
- 22.Tonino PAL, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–21 [DOI] [PubMed] [Google Scholar]
- 23.Feldman RL, Nichols WW, Pepine CJ, Conetta DA, Conti CR. The coronary hemodynamics of left main and branch coronary stenoses. The effects of reduction in stenosis diameter, stenosis length, and number of stenoses. J Thorac Cardiovasc Surg 1979;77:377–88 [PubMed] [Google Scholar]
- 24.Tayebjee MH, Lip GYH, MacFadyen RJ. Collateralization and the response to obstruction of epicardial coronary arteries. QJM 2004;97:259–72 [DOI] [PubMed] [Google Scholar]
- 25.van Werkhoven JM, Schuijf JD, Gaemperli O, Jukema JW, Boersma E, Wijns W, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol 2009;53:623–32 [DOI] [PubMed] [Google Scholar]
- 26.Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, et al. From vulnerable plaque to vulnerable patient—Part III: executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) task force report. Am J Cardiol 2006;98:2H–15H [DOI] [PubMed] [Google Scholar]
- 27.Fox KAA, McLean S. Nice guidance on the investigation of chest pain. Heart 2010;96:903–6 [DOI] [PubMed] [Google Scholar]
- 28.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32 [DOI] [PubMed] [Google Scholar]
- 29.Underwood SR, Anagnostopoulos C, Cerqueira M, Ell PJ, Flint EJ, Harbinson M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging 2004;31:261–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melikian N, De Bondt P, Tonino P, De Winter O, Wyffels E, Bartunek J, et al. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. J Am Coll Cardiol Intv 2010;3:307–14 [DOI] [PubMed] [Google Scholar]
- 31.Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol 2007;14:521–8 [DOI] [PubMed] [Google Scholar]
- 32.Smanio P, Watson D, Segalla D, Vinson E, Smith W, Beller G. Value of gating of technetium-99m sestamibi single-photon emission computed tomographic imaging. J Am Coll Cardiol 1997;30:1687–92 [DOI] [PubMed] [Google Scholar]
- 33.Dondi M, Fagioli G, Salgarello M, Zoboli S, Nanni C, Cidda C. Myocardial SPECT: what do we gain from attenuation correction (and when)? Q J Nucl Med Mol Imaging 2004;48:181–7 [PubMed] [Google Scholar]
- 34.Berman DS, Kang X, Nishina H, Slomka PJ, Shaw LJ, Hayes SW, et al. Diagnostic accuracy of gated Tc-99m sestamibi stress myocardial perfusion SPECT with combined supine and prone acquisitions to detect coronary artery disease in obese and nonobese patients. J Nucl Cardiol 2006;13:191–201 [DOI] [PubMed] [Google Scholar]
- 35.Iskander S, Iskandrian AE. Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol 1998;32:57–62 [DOI] [PubMed] [Google Scholar]
- 36.Bax JJ, Wijns W, Cornel JH, Visser FC, Boersma E, Fioretti PM. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J Am Coll Cardiol 1997;30:1451–60 [DOI] [PubMed] [Google Scholar]
- 37.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374–9 [DOI] [PubMed] [Google Scholar]
- 38.Ioannidis JPA, Trikalinos TA, Danias PG. Electrocardiogram-gated single-photonemission computed tomography versus cardiacmagnetic resonance imaging for the assessmentof left ventricular volumes and ejection fraction: A meta-analysis. J Am Coll Cardiol 2002;39:2059–68 [DOI] [PubMed] [Google Scholar]
- 39.Fox K, Garcia MAA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary. Eur Heart J 2006;27:1341–81 [DOI] [PubMed] [Google Scholar]
- 40.Antman EM, Smith SC, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, et al. , with Task Force Members. ACC/AHA/ASNC Guidelines for the Clinical Use of Cardiac Radionuclide Imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol 2003;42:1318–33 [DOI] [PubMed] [Google Scholar]
- 41.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur SR, Reddy P, Carlos RC. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol 2008;15:444–51 [DOI] [PubMed] [Google Scholar]
- 42.Lertsburapa K, Ahlberg AW, Bateman TM, Katten D, Volker L, Cullom SJ, et al. Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol 2008;15:745–53 [DOI] [PubMed] [Google Scholar]
- 43.Yoshinaga K, Chow BJW, Williams K, Chen L, deKemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol 2006;48:1029–39 [DOI] [PubMed] [Google Scholar]
- 44.Gerber BL, Ordoubadi FF, Wijns W, Vanoverschelde J-LJ , Knuuti MJ, Janier M, et al. Positron emission tomography using18F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction. Results from the European Community concerted action multicenter study on use of18F-fluoro-deoxyglucose positron emission tomography for the detection of myocardial viability. Eur Heart J 2001;22:1691–701 [DOI] [PubMed] [Google Scholar]
- 45.Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 2006;13:24–33 [DOI] [PubMed] [Google Scholar]
- 46.Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med 2009;50:1076–87 [DOI] [PubMed] [Google Scholar]
- 47.Beleslin BD, Ostojic M, Djordjevic-Dikic A, Babic R, Nedeljkovic M, Stankovic G, et al. Integrated evaluation of relation between coronary lesion features and stress echocardiography results: the importance of coronary lesion morphology. J Am Coll Cardiol 1999;33:717–26 [DOI] [PubMed] [Google Scholar]
- 48.Marwick TH, Case C, Sawada S, Rimmerman C, Brenneman P, Kovacs R, et al. Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol 2001;37:754–60 [DOI] [PubMed] [Google Scholar]
- 49.McCully RB, Roger VL, Mahoney DW, Karon BL, Oh JK, Miller FA, et al. Outcome after normal exercise echocardiography and predictors of subsequent cardiac events: follow-up of 1,325 patients. J Am Coll Cardiol 1998;31:144–9 [DOI] [PubMed] [Google Scholar]
- 50.Chung G, Krishnamani R, Senior R. Prognostic value of normal stress echocardiogram in patients with suspected coronary artery disease—a British general hospital experience. Int J Cardiol 2004;94:181–6 [DOI] [PubMed] [Google Scholar]
- 51.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, et al. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 2008;9:415–37 [DOI] [PubMed] [Google Scholar]
- 52.Marwick TH. Techniques for comprehensive two dimensional echocardiographic assessment of left ventricular systolic function. Heart 2003;89:2iii–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boudoulas H, Ruff PD, Fulkerson PK, Lewis RP. Relationship of angiographic and echographic dimensions in chronic left ventricular dilatation. Am Heart J 1983;106:356–62 [DOI] [PubMed] [Google Scholar]
- 54.Schinkel AFL, Bax JJ, Geleijnse ML, Boersma E, Elhendy A, Roelandt JRTC, et al. Noninvasive evaluation of ischaemic heart disease: myocardial perfusion imaging or stress echocardiography? Eur Heart J 2003;24:789–800 [DOI] [PubMed] [Google Scholar]
- 55.Hamon M, Fau G, Nee G, Ehtisham J, Morello R, Hamon M. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson 2010;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerber BL, Raman SV, Nayak K, Epstein FH, Ferreira P, Axel L, et al. Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson 2008;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lerakis S, McLean D, Anadiotis A, Janik M, Oshinski J, Alexopoulos N, et al. Prognostic value of adenosine stress cardiovascular magnetic resonance in patients with low-risk chest pain. J Cardiovasc Magn Reson 2009;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, et al. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol 2006;47:1427–32 [DOI] [PubMed] [Google Scholar]
- 59.Pilz G, Jeske A, Klos M, Ali E, Hoefling B, Scheck R, et al. Prognostic value of normal adenosine-stress cardiac magnetic resonance imaging. Am J Cardiol 2008;101:1408–12 [DOI] [PubMed] [Google Scholar]
- 60.Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, et al. Prognostic value of cardiac magnetic resonance stress tests. adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation 2007;115:1769–76 [DOI] [PubMed] [Google Scholar]
- 61.Kim RJ, Wu E, Rafael A, Chen E-L , Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53 [DOI] [PubMed] [Google Scholar]
- 62.Natale L, Meduri A, Caltavuturo C, Palladino F, Marano P. MRI assessment of ventricular function. Rays 2001;26:35–44 [PubMed] [Google Scholar]
- 63.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 2008;29:480–9 [DOI] [PubMed] [Google Scholar]
- 64.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374–9 [DOI] [PubMed] [Google Scholar]
- 65.Klein C, Nekolla SG, Bengel FM, Momose M, Sammer A, Haas F, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 2002;105:162–7 [DOI] [PubMed] [Google Scholar]
- 66.Bonow RO. Identification of viable myocardium. Circulation 1996;94:2674–80 [DOI] [PubMed] [Google Scholar]
- 67.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32 [DOI] [PubMed] [Google Scholar]
- 68.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc 1999;74:243–52 [DOI] [PubMed] [Google Scholar]
- 69.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003;228:826–33 [DOI] [PubMed] [Google Scholar]
- 70.Bellasi A, Lacey C, Taylor AJ, Raggi P, Wilson PWF, Budoff MJ, et al. Comparison of prognostic usefulness of coronary artery calcium in men versus women (results from a meta- and pooled analysis estimating all-cause mortality and coronary heart disease death or myocardial infarction). Am J Cardiol 2007;100:409–14 [DOI] [PubMed] [Google Scholar]
- 71.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–45 [DOI] [PubMed] [Google Scholar]
- 72.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Hoffman U, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2:675–88 [DOI] [PubMed] [Google Scholar]
- 73.Rosen BD, Fernandes V, McClelland RL, Carr JJ, Detrano R, Bluemke DA, et al. Relationship between baseline coronary calcium score and demonstration of coronary artery stenoses during follow-Up: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol Img 2009;2:1175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Georgiou D, Budoff MJ, Kaufer E, Kennedy JM, Lu B, Brundage BH. Screening patients with chest pain in the emergency department using electron beam tomography: a follow-up study. J Am Coll Cardiol 2001;38:105–10 [DOI] [PubMed] [Google Scholar]
- 75.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 2002;61:638–47 [DOI] [PubMed] [Google Scholar]
- 76.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342:1478–83 [DOI] [PubMed] [Google Scholar]
- 77.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002;39:695–701 [DOI] [PubMed] [Google Scholar]
- 78.Elkeles RS, Godsland IF, Feher MD, Rubens MB, Roughton M, Nugara F, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J 2008;29:2244–51 [DOI] [PubMed] [Google Scholar]
- 79.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43:1663–9 [DOI] [PubMed] [Google Scholar]
- 80.Anand DV, Lim E, Hopkins D, Corder R, Shaw LJ, Sharp P, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J 2006;27:713–21 [DOI] [PubMed] [Google Scholar]
- 81.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Hoffman U, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2:675–88 [DOI] [PubMed] [Google Scholar]
- 82.Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol 2004;44:923–30 [DOI] [PubMed] [Google Scholar]
- 83.Schenker MP, Dorbala S, Hong ECT, Rybicki FJ, Hachamovitch R, Kwong RY, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation 2008;117:1693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meijboom WB, Meijs MFL, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CAG, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44 [DOI] [PubMed] [Google Scholar]
- 85.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36 [DOI] [PubMed] [Google Scholar]
- 86.Mowatt G, Cook JA, Hillis GS, Walker S, Fraser C, Jia X, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart 2008;94:1386–93 [DOI] [PubMed] [Google Scholar]
- 87.Stein PD, Yaekoub AY, Matta F, Sostman HD. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med 2008;121:715–25 [DOI] [PubMed] [Google Scholar]
- 88.Sun Z, Lin C, Davidson R, Dong C, Liao Y. Diagnostic value of 64-slice CT angiography in coronary artery disease: a systematic review. Eur J Radiol 2008;67:78–84 [DOI] [PubMed] [Google Scholar]
- 89.Dodd JD, Rieber J, Pomerantsev E, Chaithiraphan V, Achenbach S, Moreiras JM, et al. Quantification of nonculprit coronary lesions: comparison of cardiac 64-MDCT and invasive coronary angiography. AJR Am J Roentgenol 2008;191:432–8 [DOI] [PubMed] [Google Scholar]
- 90.Sheth T, Dodd JD, Hoffmann U, Abbara S, Finn A, Gold HK, et al. Coronary stent assessability by 64 slice multi-detector computed tomography. Catheter Cardiovasc Interv 2007;69:933–8 [DOI] [PubMed] [Google Scholar]
- 91.Springer I, Dewey M. Comparison of multislice computed tomography with intravascular ultrasound for detection and characterization of coronary artery plaques: a systematic review. Eur J Radiol 2009;71:275–82 [DOI] [PubMed] [Google Scholar]
- 92.Burgstahler C, Reimann A, Beck T, Kuettner A, Baumann D, Heuschmid M, et al. Influence of a lipid-lowering therapy on calcified and noncalcified coronary plaques monitored by multislice detector computed tomography: results of the New Age II Pilot Study. Invest Radiol 2007;42:189–95 [DOI] [PubMed] [Google Scholar]
- 93.Ferencik M. Assessment of coronary plaque burden by computed tomography: getting closer—step by step. Heart 2010;96:575–6 [DOI] [PubMed] [Google Scholar]
- 94.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319–26 [DOI] [PubMed] [Google Scholar]
- 95.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 2011;57:1237–47 [DOI] [PubMed] [Google Scholar]
- 96.Danciu SC, Herrera CJ, Stecy PJ, Carell E, Saltiel F, Hines JL. Usefulness of multislice computed tomographic coronary angiography to identify patients with abnormal myocardial perfusion stress in whom diagnostic catheterization may be safely avoided. Am J Cardiol 2007;100:1605–8 [DOI] [PubMed] [Google Scholar]
- 97.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) Trial. J Am Coll Cardiol 2009;53:1642–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med 2009;53:295–304 [DOI] [PubMed] [Google Scholar]
- 99.Schuijf JD, Bax JJ, Shaw LJ, de Roos A, Lamb HJ, van derWall EE, et al. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J 2006;151:404–11 [DOI] [PubMed] [Google Scholar]
- 100.Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation 2008;118:586–606 [DOI] [PubMed] [Google Scholar]
- 101.Mettler FA, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008;248:254–63 [DOI] [PubMed] [Google Scholar]
- 102.Committee to Assess Health Risks from Exposureto Low Levels of Ionizing Radiation Board on Radiation Effects Research Division on Earth and Life Studies National Research Council of the National Academies Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII-Phase 2. Washington, DC: National Academies Press; 2006 [Google Scholar]
- 103.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290–305 [DOI] [PubMed] [Google Scholar]
- 104.Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009;119:1056–65 [DOI] [PubMed] [Google Scholar]
- 105.Hunold P, Vogt FM, Schmermund A, Debatin JF, Kerkhoff G, Budde T, et al. Radiation exposure during cardiac CT: effective doses at multi-detector row CT and electron-beam CT. Radiology 2003;226:145–52 [DOI] [PubMed] [Google Scholar]
- 106.Alkadhi H, Stolzmann P, Scheffel H, Desbiolles L, Baumüller S, Plass A, et al. Radiation dose of cardiac dual-source CT: the effect of tailoring the protocol to patient-specific parameters. Eur J Radiol 2008;68:385–91 [DOI] [PubMed] [Google Scholar]
- 107.Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500–7 [DOI] [PubMed] [Google Scholar]
- 108.Carr CL, Lindner JR. Myocardial perfusion imaging with contrast echocardiography. Curr Cardiol Rep 2008;10:233–9 [DOI] [PubMed] [Google Scholar]
- 109.Dijkmans PA, Senior R, Becher H, Porter TR, Wei K, Visser CA, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol 2006;48:2168–77 [DOI] [PubMed] [Google Scholar]
- 110.Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging 2009;2:412–24 [DOI] [PubMed] [Google Scholar]
- 111.Bhan A, Kapetanakis S, Rana BS, Ho E, Wilson K, Pearson P, et al. Real-time three-dimensional myocardial contrast echocardiography: is it clinically feasible? Eur J Echocardiogr 2008;9:761–5 [DOI] [PubMed] [Google Scholar]
- 112.Blankstein R, Shturman LD, Rogers IS, Rocha-Filho JA, Okada DR, Sarwar A, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol 2009;54:1072–84 [DOI] [PubMed] [Google Scholar]
- 113.George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang H-J , Bluemke DA, et al. Adenosine Stress 64- and 256-row detector computed tomography angiography and perfusion imaging: clinical perspective. Circ Cardiovasc Imaging 2009;2:174–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664–72 [DOI] [PubMed] [Google Scholar]
- 115.Kramer CM, Narula J. Atherosclerotic plaque imaging: the last frontier for cardiac magnetic resonance. J Am Coll Cardiol Img 2009;2:916–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825–31 [DOI] [PubMed] [Google Scholar]
- 117.Garcia-Garcia HM, Costa MA, Serruys PW. Imaging of coronary atherosclerosis: intravascular ultrasound. Eur Heart J 2010;31:2456–69 [DOI] [PubMed] [Google Scholar]
- 118.Stamper D, Weissman NJ, Brezinski M. Plaque characterization with optical coherence tomography. J Am Coll Cardiol 2006;47(Suppl. 8):C69–79 [DOI] [PubMed] [Google Scholar]