Abstract
Cardiomyopathies (CMPs) are a group of often inherited diseases characterised by abnormalities and associated dysfunction of heart muscle. In the past decade, cardiovascular magnetic resonance (CMR) has emerged as a powerful tool in their assessment, providing data that are complementary to other aspects of clinical evaluation. Key advantages of CMR are three-dimensional visualisation of the heart and its relationship to thoracic structures; gold-standard quantification of cardiac volumes and function, which can safely be repeated over time (no ionising radiation is involved); and tissue characterisation to detect focal scar and fatty infiltration. This paper reviews the role of CMR in the clinical assessment of patients with CMPs.
Cardiomyopathies (CMPs) are a group of often inherited diseases characterised by abnormalities and associated dysfunction of the heart muscle [1,2]. The classification and diagnosis of CMPs are based on morphological and functional cardiac assessment. The main forms of CMPs are hypertrophic, dilated, restrictive and right ventricular (RV) CMP.
Historically, echocardiography has played a key role in the diagnosis and assessment of CMPs. In recent decades, cardiovascular magnetic resonance (CMR) has emerged as a powerful tool in CMP assessment, providing data which complement those obtained by other aspects of clinical evaluation (including history taking, examination, electrocardiography (ECG), echocardiography, Holter monitoring and exercise testing). CMR has several advantages: three-dimensional visualisation of the heart and its relationship to thoracic structures; gold-standard quantification of cardiac volumes and function with standardised protocols [3,4]; excellent spatial and temporal resolution; tissue characterisation using either contrast or non-contrast techniques to detect focal scar and fatty infiltration; and no ionising radiation, allowing scans to be repeated safely as often as is necessary. CMR also has disadvantages, including greater cost, relatively poor availability and the fact that it cannot be performed at the bedside or in patients with pacemakers or implantable cardioverter defibrillators (ICDs). CMR is also inferior to echocardiography in the assessment of diastolic function and in measuring flow velocity in dynamic outflow tract obstruction.
Echocardiography and CMR are complementary imaging modalities. CMR is particularly useful in cases where the diagnosis is clear, for family evaluation where ECG is abnormal but the echocardiogram is normal and for the exclusion of mimics (phenocopies); it may also have a role in predicting prognosis, particularly in hypertrophic CMP (HCM). This paper reviews the role of CMR in the assessment of CMPs.
Hypertrophic cardiomyopathy
HCM is the most common genetic cardiac disease. It is defined morphologically by left ventricular (LV) enlargement in the absence of cardiac or systemic diseases that are capable of producing such hypertrophy [5].
Focal hypertrophy, anatomy and early disease expression
In HCM, the cardiac hypertrophy may be focal, and this can be challenging to diagnose using echocardiography. For example, the LV apex is susceptible to near-field artefact on echocardiography, and thus subtle early hypertrophy of the basal anterior wall is often difficult to assess. CMR is particularly valuable in patients who have a high pre-test probability of having early disease expression (i.e. in patients with a family history of HCM and abnormal ECG), especially when echocardiogram fails to show any evidence of LV hypertrophy. In these cases, CMR detects missed hypertrophy in up to 6% of cases in some series [6]. The extent of hypertrophy required for a diagnosis of HCM depends on patient factors (e.g. it will be different in children) and how wall thickness is measured. By echocardiography, maximal LV wall thickness should be ≥15 mm. When CMR is used, however, cine images are able to distinguish compacted from non-compacted myocardium more clearly. When compacted myocardium alone is measured, a lower threshold is usually appropriate: borderline hypertrophy may be 12 mm, established hypertrophy 14 mm. CMR can also provide additional morphological and functional information in HCM; for example, it can be used to confirm the presence of RV hypertrophy, apical micro-aneurysms and subtle ventricular architectural abnormalities, such as multiple clefts and papillary muscle abnormalities. In some circumstances, distinguishing early HCM from, say, hypertensive heart disease may be difficult. The use of CMR may reduce the “grey zone” of uncertainty. Nevertheless, the scan results, as ever, require interpretation within the clinical context, particularly with regard to family history and ECG results.
Outflow tract obstruction
Echocardiography is the gold-standard investigation for non-invasive quantification of LV outflow tract (LVOT) obstruction, particularly for beat-to-beat variation after a Valsalva manoeuvre (this will exacerbate any resting outflow tract gradient) or during exercise. CMR is helpful in complex cases, such as those involving multilevel obstruction (intracavity, subvalvular membrane or valvular obstruction) or RV outflow tract obstruction, or where invasive gradient reduction therapy is planned or has previously taken place. Complete or incomplete systolic anterior motion (SAM) of the mitral valve, the extent and localisation of the septal contact and, sometimes, an impact septal lesion can be visualised. SAM is associated with posteriorly directed mitral regurgitation; if such regurgitation is central, it could imply additional mitral valve pathology.
Tissue characterisation
Tissue characterisation using the late gadolinium enhancement (LGE) technique is a unique property of CMR and has an important role in the differential diagnosis and risk stratification of patients with HCM (Figure 1). LGE is performed after a bolus of contrast (gadolinium-DPTA), an extracellular agent that passively accumulates in areas of interstitial expansion where kinetics are slower and there is increased extracellular space. Following administration, after a delay of typically 5–10 min, an inversion recovery sequence is performed with the inversion time set to null normal myocardial tissue. LGE allows for the in vivo imaging of focal expansion of the extracellular space in the myocardium, which might be caused by focal scarring or an infiltrative process. The localisation and extent of the scarring, together with other morphological and functional abnormalities, can help in the differential diagnosis of HCM, and can predict the risk of heart failure and sudden death [7,8].
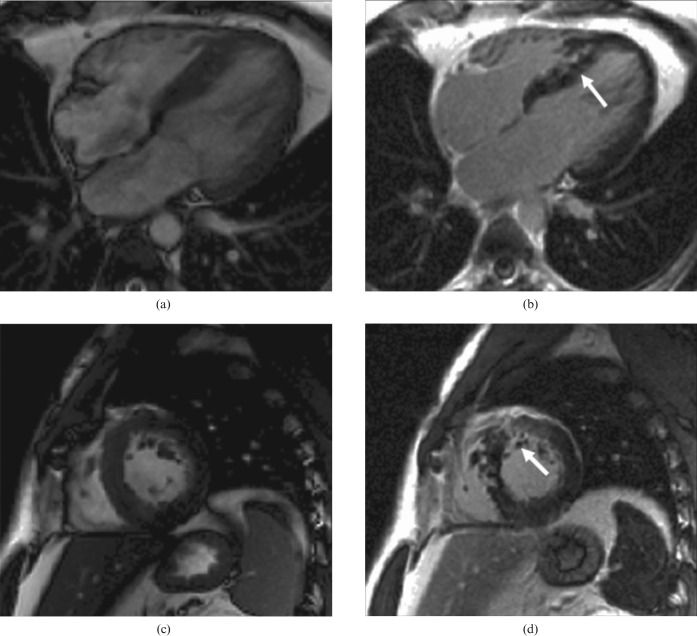
Figure 1.
Hypertrophic cardiomyopathy. Note left ventricular hypertrophy and, after contrast, late gadolinium enhancement of the hypertrophied areas and right ventricular insertion points (arrows). (a, c) Steady-state free precession cine in diastole [(a) four-chamber view; (c) short-axis view]. (b, d) Inversion recovery after gadolinium bolus [(b): four-chamber view; (d) short-axis view].
Phenocopies
Several conditions can mimic HCM. Cardiac amyloidosis is typically characterised on CMR by cardiac decompensation (pleural and/or pericardial effusions or ascites), concentric biventricular hypertrophy, reduced long-axis function and valvular thickening. On contrast imaging, typical, often subendocardial, LGE (resulting from the expansion of extracellular space by amyloid fibril deposition) and unusual gadolinium kinetics may be seen. In particular, gadolinium wash-out from blood can be fast (probably secondary to gadolinium distribution into the total body amyloid load) and this, in combination with increased volume of distribution in myocardium, can make the T1 values of blood and myocardium very similar. Indeed, myocardium may have shorter T1 values and more contrast than blood, i.e. the “myocrit” is lower than the haematocrit [9]. Sometimes, LGE can be diffuse (Figure 2). Cardiac sarcoidosis may present with parenchymal lung changes and mediastinal lymphadenopathy, together with LV hypertrophy (LVH), regional wall motion abnormalities, aneurysms and focal scarring on LGE (Figure 3). Anderson–Fabry disease, a rare X-linked lysosomal storage disease, is characterised by concentric LV hypertrophy, valvular thickening and characteristic basal inferolateral LGE in up to 50% of patients (Figure 4), and is often not detectable by any other test [10]. Hypereosinophilic syndrome [11] manifests with characteristic biventricular subendocardial LGE with apical thrombosis (with preserved underlying wall motion) (Figure 5). Other features include valvular leaflet retraction abnormalities with regurgitation and biatrial enlargement. Occasionally HCM can be mimicked by intracardiac masses, such as fibromas, lipomas or malignant primary and secondary tumours, which are usually readily distinguished by tissue characterising sequences.
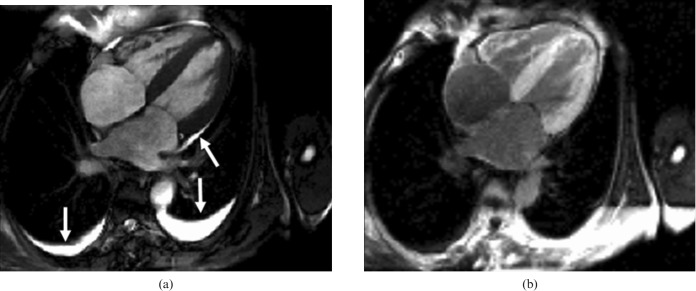
Figure 2.
Cardiac amyloidosis. Note the bilateral pleural effusions and small pericardial effusion (arrows). The patient has mildly impaired radial systolic left ventricular function but severely impaired long-axis function with concentric left ventricular hypertrophy and dilated atria. (a) Steady-state free precession cine image in diastole, four-chamber view. (b) Inversion recovery after gadolinium bolus, four-chamber view; note the dark blood pool and bright myocardium.
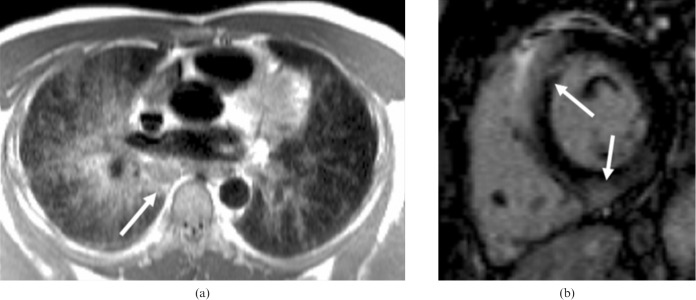
Figure 3.
A patient with sarcoidosis and paroxysmal complete heart block on 24 h electrocardiography. (a) Transverse multislice half-Fourier acquisition single-shot turbo spin echo images, showing extensive lung parenchymal abnormalities (right>left) and lymphadenopathy (arrow). (b) Short axis inversion recovery late gadolinium enhancement image shows patchy fibrosis (arrows).
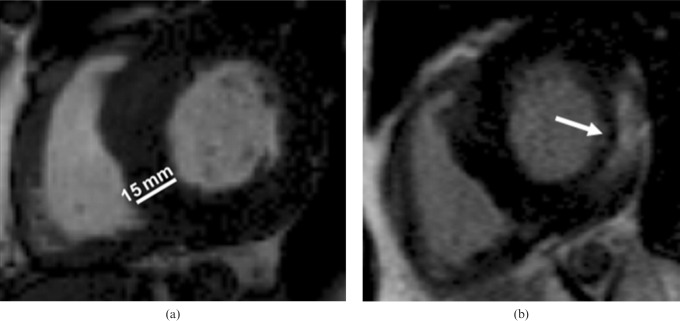
Figure 4.
Short-axis still steady-state free precession cine in diastole of a patient with Anderson–Fabry disease, showing (a) concentric left ventricular hypertrophy (15 mm, white line) and right ventricular hypertrophy. (b) Typical subepicardial late gadolinium enhancement in the inferolateral wall (arrow).
Figure 5.
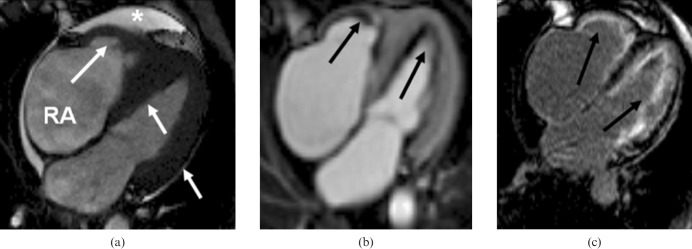
Hypereosinophilic syndrome. (a) Still four-chamber steady-state free precession cine in diastole shows increased left and right ventricular wall thickness (particularly at the apex) (white arrows), moderate left atrium dilatation, severe tricuspid regurgitation (not shown), right atrium (RA) dilatation and pericardial effusion (white star). (b) Inversion recovery early gadolinium image shows diffuse left and right subendocardial thrombi. These were confirmed histologically. (c) Inversion recovery late gadolinium image shows diffuse right and left subendocardial fibrosis.
Dilated cardiomyopathy
Dilated CMP (DCM) is characterised by cardiac enlargement and systolic dysfunction of the left or both ventricles. CMR can directly measure cardiac volumes, without the need for geometrical assumptions, providing high accuracy and reproducibility. Several conditions can result in LV dilatation and dysfunction, the most common being myocardial infarction (ischaemia), genetic conditions, myocarditis and cardiac toxins (e.g. alcohol, chemotherapy). Other rarer aetiologies include tachycardiomyopathy, global hibernation and iron overload.
Ischaemic cardiomyopathy
CMR can differentiate ischaemic CMP from non-ischaemic CMPs through the use of LGE imaging, even when the heart is globally dilated and dysfunctional. This is important as the treatments for these two groups are very different. Infarction is characteristic in that it always causes subendocardial LGE, which extends variably transmurally to the epicardium. It also follows a coronary territory distribution (Figure 6). The absence of LGE in a dysfunctional segment of myocardium implies the potential for recovery with time (stunning), medical treatment or revascularisation (hibernation), biventricular pacing (dyssynchrony) or removal of a toxin (e.g. alcohol) [12]. Conversely, CMR could rule out expensive, invasive and potentially dangerous revascularisation or resynchronisation of transmural scar, which would achieve little [13]. Rarely, extensive triple vessel disease may cause global hibernation with little or no infarction. Similarly, dual pathology may exist: DCM with triple vessel disease. Under such circumstances, additional definitive coronary imaging or stress testing may be advisable.
Figure 6.
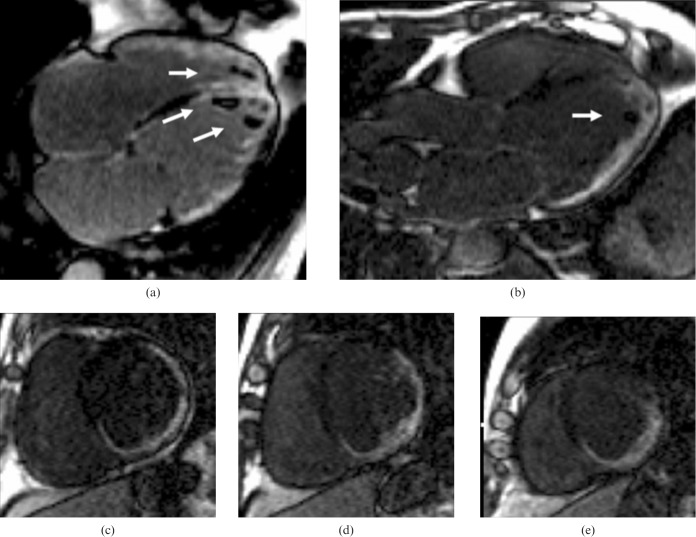
Ischaemic cardiomyopathy in a patient with triple vessel disease. Inversion recovery images after gadolinium bolus show extensive transmural late gadolinium enhancement and apical thrombi in the left and right ventricles (arrows). (a, b) Four-chamber (left) and left ventricular outflow tract (right) views. (c–e) Short axis views.
Non-ischaemic dilated cardiomyopathy
Non-ischaemic DCM may demonstrate either no LGE or mid-wall LGE in areas not corresponding to a coronary territory (Figure 7). The aetiology of this disease is often unknown, but may be the result of a previous myocarditis or genetic myopathic process, and it is associated with an adverse prognosis [14]. Additional features that can be detected using CMR include valvular regurgitation, apical thrombus, dyssynchrony with or without posterior scar, signs of decompensation, cardiac iron, LVH, RV involvement and atrial size.
Figure 7.
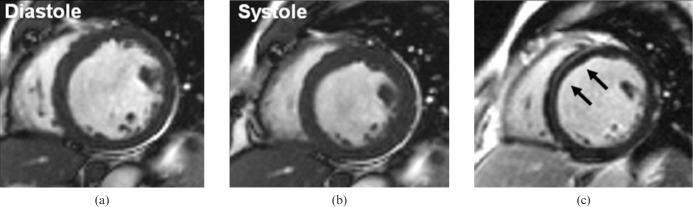
Dilated cardiomyopathy. Note the dilated left ventricular cavity and poor systolic function. Late gadolinium enhancement reveals mid-wall hyperenhancement (arrows). The images are steady-state free precession cine in diastole (a) and systole (b) and inversion recovery after gadolinium bolus (c).
Arrhythmogenic right ventricular cardiomyopathy
Arrhythmogenic RV CMP (ARVC) is an uncommon genetic heart muscle disease. It is characterised clinically by ventricular arrhythmias, sudden cardiac death and heart failure, and histologically by myocyte loss and fatty or fibro-fatty replacement, particularly in the RV [15]. LV involvement is increasingly recognised. The diagnosis is complex and relies on several major and minor diagnostic criteria, which have been modified recently [16]. The diagnostic criteria take into account family history, arrhythmias, ECG abnormalities (i.e. repolarisation and depolarisation), genetic analysis, tissue characterisation (i.e. endomyocardial biopsy), and functional and/or structural abnormalities on imaging (i.e. echocardiography or CMR). These criteria emphasise the importance of formal RV volume quantification, with major and minor criteria based on specific cut-offs for indexed diastolic RV volumes and RV ejection fraction. The presence of minor regional wall motion abnormalities (i.e. hypokinesia) is no longer a diagnostic criterion.
CMR has an important role in the diagnosis of ARVC. It allows for three-dimensional visualisation of the LV or RV pathologies that may mimic ARVC, such as thoracic abnormalities (i.e. pectus excavatum, carinatus or rib-cage abnormalities), abnormal heart positions that can result in distortion of RV shape (e.g. the position resulting from a partially absent pericardium), false regional wall motion and ECG abnormalities. Common other ARVC mimics detected by CMR are sarcoidosis, RV infarction and left-to-right shunt leading to RV dilatation (i.e. atrial septal defects or anomalous pulmonary venous drainage).
A comprehensive CMR evaluation of patients with possible ARVC [3] should include the study of functional and morphological abnormalities using cine imaging in long axis, short axis and axial views to analyse global RV and LV function and regional wall-motion abnormalities.
Minor regional wall-motion abnormalities in the proximity of the moderator band insertion point should be interpreted with caution because they could be part of the normal spectrum [16]. Focal RV wall thinning (RV wall thickness <2 mm) has particular significance if associated with regional function abnormalities. There may be localised dilatation, usually in the right ventricular outflow tract, and/or trabecular disarray (i.e. increased and prominent RV trabeculations), but these are difficult to quantify.
Tissue characterisation is an important part of CMR assessment. CMR can detect fatty infiltration and fibrosis, which may be useful for the diagnosis [17]. Tissue characterisation, however, is not included in the current diagnostic criteria for ARVC. Intramyocardial fat can be detected by T1 weighted turbo spin echo imaging with and without fat suppression. Myocardial fat infiltration on CMR is suspicious, but care should be taken in its interpretation because it is non-specific and can occur in scar tissue from any cause. Furthermore, pericardial fat can mimic intramyocardial fat, especially in obese and elderly patients or in those with a history of corticosteroid use, in whom fat infiltration may be present. Fibrosis is assessed by LGE (Figure 8), but it is not easily visualised in the thin RV free wall where partial voluming may cause an artefact. CMR can also evaluate the presence of LGE in the left ventricle—a more robust finding.
Figure 8.
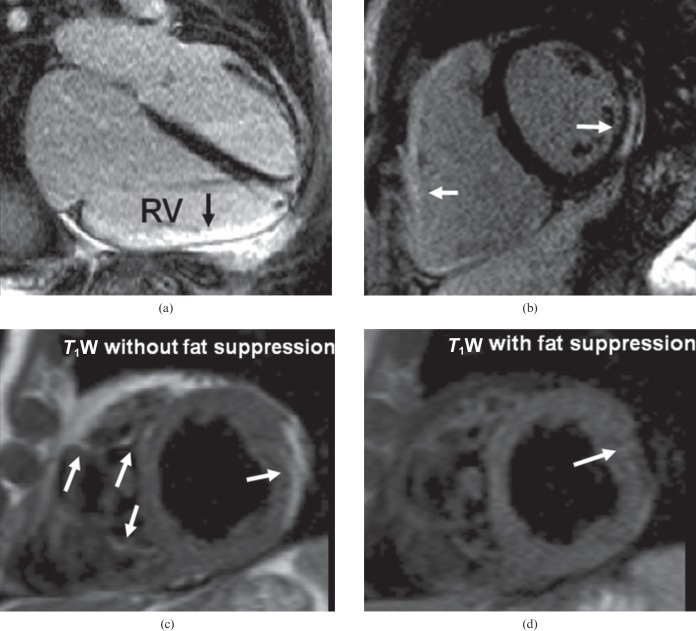
Arrhythmogenic right ventricular (RV) cardiomyopathy: note the dilated right ventricle (larger than the left ventricle) which had impaired systolic function on cine imaging. (a) Inversion recovery after gadolinium, four-chamber view, shows enhancement of the RV free wall. (b) Inversion recovery after gadolinium image confirms the RV free wall late gadolinium enhancement and shows left ventricular involvement with mid-ventricular inferolateral subepicardial enhancement. (c, d) T1 weighted (T1W) images without (c) and with (d) fat suppression from another patient with arrhythmogenic right ventricular cardiomyopathy shows fat infiltration in the right and left ventricles (arrows).
Restrictive cardiomyopathy
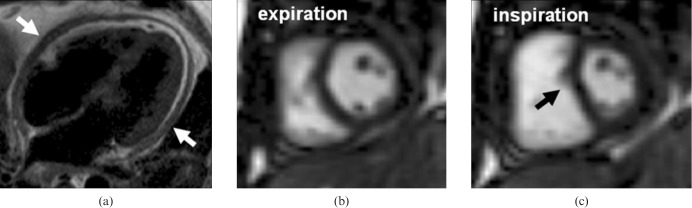
Restrictive CMP (RCM) is the least common genetic heart muscle disease and is characterised by diastolic dysfunction, restrictive cardiac physiology, normal LV volumes and near-normal mass, and radial systolic function. Bi-atrial enlargement is characteristic. One of the clinical challenges in patients with suspected RCM is its differentiation from pericardial constriction, which shares a similar clinical presentation but is treatable with pericardectomy. CMR is helpful because it can detect the anatomy of any pericardial thickening, the haemodynamics of constriction and any abnormality of the underlying myocardium (Figure 9). The pericardium can be delineated on black-blood imaging and inflammation can be imaged using LGE (owing to the increased extracellular space associated with oedema and breakdown of cell walls). Tethering to underlying myocardium and lack of slippage can be seen using tagging [18], and real-time cine-CMR [19] may show ventricular–ventricular interaction (compression of the left ventricle by the right ventricle during deep inspiration), which is a hallmark of constriction. Multimodality imaging is often warranted and typically includes echocardiography, invasive haemodynamic assessment and CT, the last offering excellent anatomic delineation of pericardium and calcification.
Figure 9.
Pericardial constriction. (a) T1 weighted four-chamber view showing marked concentric pericardial thickening. Middle and right panels: still diastolic frames from real-time sequence during expiration (b) and deep inspiration (c). With inspiration, there is preferential right ventricular filling and consequent underfilling of the coupled left ventricle, resulting in flattening of the ventricular septum.
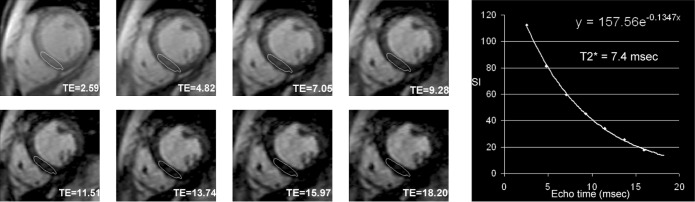
Several restrictive CMPs are associated with characteristic CMR findings, such as endomyocardial fibrosis, HCM and amyloidosis. CMR can reliably quantify myocardial iron loading in patients with haemochromatosis or iatrogenic overload (i.e. transfusion-dependent conditions, particularly thalassaemia) through the T2* mapping technique (Figure 10). Cardiac iron accumulation increases local magnetic field inhomogeneity and shortens T2* relaxation time. Iron overload is associated with an adverse prognosis, and CMR accurately, identifies patients who are at risk of heart failure and arrhythmias [20], allowing targeted intensification of chelation therapy.
Figure 10.
Single breath-hold spoiled gradient multi-echo T2 weighted sequence in a patient with thalassaemia major and severe cardiac iron loading. The left panel shows signal decay in the myocardium at different echo times (TE, in ms) that is due to iron overload. A region of interest is traced in the mid-septum and plotting of a curve of the signal intensity vs TE (right panel) allows calculation of cardiac T2* (in this case 7.4 ms) from the equation of the curve (the reciprocal of the exponent).
Other cardiomyopathies
Left ventricular non-compaction
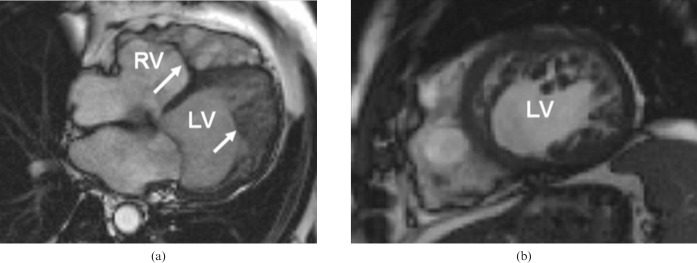
Left ventricular non-compaction (LVNC) is a developmental disorder [21] that is characterised by increased trabeculations and intratrabecular recesses in the heart. In some circumstances, this can be associated with CMP (DCM or HCM) [22] and related clinical features such as heart failure, thrombo-embolism and ventricular arrhythmias. CMR can accurately identify the non-compacted and compacted tissue layers [23] (Figure 11). A non-compacted to compacted layer ratio, measured at end-diastole, of 2.3 or more has been suggested to yield a high diagnostic accuracy, but there are some concerns about this simplistic approach to the diagnosis of non-compaction, particularly as ever increasing image quality allows the detection of trabeculae that were previously occult [23]. Trabeculations are mainly located at the apex and in the mid-inferior and lateral walls.
Figure 11.
Left ventricular non-compaction. Steady-state free precession cine images in diastole. Note the dilated left ventricle (LV), which has impaired function, and the marked hypertrabeculation of both ventricles (arrowed). (a) Four-chamber view, (b) short axis view. RV, right ventricle.
Tako-tsubo cardiomyopathy
Tako-tsubo CMP, or transient LV apical ballooning, is a cause of reversible LV systolic dysfunction. It presents with a myocardial infarct-like clinical syndrome and it is often preceded by emotional stress or exacerbation of an existing medical condition [24]. It results in dyskinetic mid to apical segments, hyperdynamic basal segments (which can result in systolic anterior motion of the mitral valve and LVOT obstruction) and angiographically normal coronary arteries. On CMR, there is typically no or minimal LGE and LV systolic function tends to normalise.
Conclusion
CMR is increasingly recognised as an important tool in the investigation of CMPs and, where available, forms part of routine clinical work-up for patients with these conditions. It is now considered the gold-standard investigation for cardiac morphology and function and offers unique insights into pathophysiology using tissue characterisation with emerging roles in research, diagnostics, prognostics and disease monitoring.
References
- 1.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270–6 [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–16 [DOI] [PubMed] [Google Scholar]
- 3.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 2008;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hundley WG, Bluemke D, Bogaert JG, Friedrich MG, Higgins CB, Lawson MA, et al. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson 2009;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687–713 [DOI] [PubMed] [Google Scholar]
- 6.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005;112:855–61 [DOI] [PubMed] [Google Scholar]
- 7.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003;41:1561–7 [DOI] [PubMed] [Google Scholar]
- 8.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;56:867–74 [DOI] [PubMed] [Google Scholar]
- 9.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005;111:186–93 [DOI] [PubMed] [Google Scholar]
- 10.Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, et al. Gadolinium enhanced cardiovascular magnetic resonance in Anderson–Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J 2003;24:2151–5 [DOI] [PubMed] [Google Scholar]
- 11.Chang SA, Kim HK, Park EA, Kim YJ, Sohn DW. Images in cardiovascular medicine. Loeffler endocarditis mimicking apical hypertrophic cardiomyopathy. Circulation 2009;120:82–5 [DOI] [PubMed] [Google Scholar]
- 12.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53 [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal NR, Martinez MW, Gersh BJ, Chareonthaitawee P. Role of cardiac MRI and nuclear imaging in cardiac resynchronization therapy. Nat Rev Cardiol 2009;6:759–70 [DOI] [PubMed] [Google Scholar]
- 14.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1977–85 [DOI] [PubMed] [Google Scholar]
- 15.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009;373:1289–300 [DOI] [PubMed] [Google Scholar]
- 16.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain A, Tandri H, Calkins H, Bluemke DA. Role of cardiovascular magnetic resonance imaging in arrhythmogenic right ventricular dysplasia. J Cardiovasc Magn Reson 2008;10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima S, Yamada N, Goto Y. Diagnosis of constrictive pericarditis by tagged cine magnetic resonance imaging. N Engl J Med 1999;341:373–4 [DOI] [PubMed] [Google Scholar]
- 19.Francone M, Dymarkowski S, Kalantzi M, Rademakers FE, Bogaert J. Assessment of ventricular coupling with real-time cine MRI and its value to differentiate constrictive pericarditis from restrictive cardiomyopathy. Eur Radiol 2006;16:944–51 [DOI] [PubMed] [Google Scholar]
- 20.Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, et al. Cardiac T2 magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009;120:1961–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantazis AA, Elliott PM. Left ventricular noncompaction. Curr Opin Cardiol 2009;24:209–13 [DOI] [PubMed] [Google Scholar]
- 22.Biagini E, Ragni L, Ferlito M, Pasquale F, Lofiego C, Leone O, et al. Different types of cardiomyopathy associated with isolated ventricular noncompaction. Am J Cardiol 2006;98:821–4 [DOI] [PubMed] [Google Scholar]
- 23.Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46:101–5 [DOI] [PubMed] [Google Scholar]
- 24.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–41 [DOI] [PubMed] [Google Scholar]