Abstract
This article gives an overview of the role of imaging in the diagnosis and management of acute coronary syndrome.
Coronary heart disease (CHD) is a major cause of morbidity and mortality. CHD is the most common cause of death in the UK. 94 000 deaths were attributed to CHD from 2006 to 2007. In addition, it is estimated that, annually, 275 000 people have a myocardial infarction [1]. However, the prevalence of chest pain is even higher (20–40% of the population) [2,3]. Chest pain is therefore a major problem for casualty departments, and accounts for approximately 5% of visits. These patients have a spectrum of cardiac risk from those with typical symptoms and abnormal electrocardiography (ECG) who require immediate catheter angiography with a view to intervention; at the other end of the spectrum are those of low risk with atypical symptoms and a normal ECG who can be discharged without investigation. Unfortunately, between these two groups are a large number of patients with diagnostic uncertainty. Most of these patients, because of diagnostic uncertainty, are admitted for observation and diagnostic testing. Therefore, chest pain accounts for 40% of emergency hospital admissions [4,5]. However, fewer than 20% of hospitalised patients have subsequent confirmation of acute coronary syndrome (ACS) [6], and 2–10% of patients are mistakenly discharged [7,8]. Such discharged patients have a high mortality.
Patients presenting to emergency departments therefore represent a major problem to the healthcare system, in terms of both volumes of patients requiring hospital admission and cost.
Diagnosis of patients with acute chest pain
Clinical evaluation is fundamental to diagnosis, risk stratification and decision-making in patients with suspected ACS. A detailed history remains the cornerstone of the evaluation of patients with suspected ischaemic coronary syndromes. A diagnosis of ACS can be made based solely on history if there is a compelling clinical scenario in a patient with a moderate or high probability of ACS. However, the majority of patients require further evaluation. History is also important for the assessment of prognosis and risk stratification. Clinical risk scores identify patients who benefit from more aggressive treatment and there are a number used (e.g. TIMI, GRACE and PURSUIT). Discussion of these is outside the realms of this article.
The criteria for diagnosis of acute myocardial infarction (AMI) depend on finding evidence of myocardial necrosis in the appropriate clinical setting. The criteria have been set out in an expert consensus document [9] and are as follows.
The detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit together with evidence of myocardial ischaemia with at least one of the following:
symptoms of ischaemia
ECG changes indicative of new ischaemia (new ST–T changes or new left bundle branch block)
development of pathological Q waves in the ECG
imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.
Although biomarkers (usually troponin) are central in the diagnosis of myocardial infarction, they are reliant on myocardial cell death (which is clearly what we wish to avoid) of sufficient degree to elevate plasma levels. Values that are above the 95th percentile should be considered an indication of myocardial necrosis. Cardiac troponins are extremely specific for cardiac damage; however, myocardial damage is not specific to ACS. An elevated troponin in a patient with, for example, sepsis or pulmonary embolism indicates that there has been myocardial damage but does not mean they have had a coronary event. Over-reliance on troponins can lead to misdiagnosis and inappropriate treatment and must always be taken as supportive evidence in the appropriate clinical situation.
Troponin elevation takes time to develop. Studies have demonstrated that in more than 90% of acute myocardial infarctions it takes 6–12 h from symptom onset for the cardiac troponin I and T immunoassays to exceed the threshold considered normal [10,11]. This means that, for rapid diagnosis, the findings on ECG and imaging are of vital importance.
The diagnostic strategy for patients with ST segment elevation is clear (with urgent coronary revascularisation with primary percutaneous coronary angioplasty or thrombolysis) [12]. However, there is still a lack of such a service in many centres owing to the lack of a critical number of interventional cardiologists. This will probably result in a greater reliance on non-invasive imaging even in this group.
In patients without ST segment elevation diagnosis is more challenging. Most patients in the emergency department (55–60% [13]) have no worrisome ECG abnormalities and no history of coronary artery disease (CAD). Typically, such patients are admitted and are further risk stratified (usually by the TIMI score [14]), with those of intermediate to low risk undergoing a period of observation with serial enzymes with or without some form of stress testing, stress echocardiography or resting nuclear scans [15]. Such an approach is detrimental to patients (delayed diagnosis results in greater myocardial cell death) and is expensive, in terms of both resources and hospital beds.
In the face of such clinical uncertainty, there is a great opportunity for non-invasive imaging to provide a cost-effective solution in terms of both the exclusion of coronary disease in patients presenting with chest pain in the casualty department and the management of patients with coronary disease by means of diagnosis and risk stratification. The aim of this article is to outline the evidence for the use of imaging with regards to the diagnosis of ACS.
Understanding the problem: pathophysiology of coronary artery disease
CAD is the development of cholesterol-rich plaques within the walls of coronary arteries (atherosclerosis). However, the clinical manifestations of this process are varied. The atherosclerotic process is typically a chronic inflammatory process that, like any chronic inflammatory process, causes fibrosis and calcification. This fibrosis can advance insidiously to narrow the lumen of the coronary artery. This in turn can compromise the blood supply to the myocardium, and the affected individual will often develop predictable exertional chest discomfort, or “stable” angina. However, not all patients, even with critical stenoses, will have microvascular myocardial ischaemia because of the ability of the heart to adapt with collateralisation from other vessels and conditioning of the myocardium to ischaemia. Typically, myocardial blood flow does not fall below normal resting levels until a coronary artery obstruction of 85–90% of the luminal area [16].
At any stage in the development of atherosclerosis, and often when the coronary artery lumen is narrowed only slightly or not at all, an unstable plaque may develop a tear of its inner lining cell layer (intima), exposing the underlying cholesterol-rich atheroma to the vessel lumen. This atheroma is extremely thrombogenic and initiates platelet aggregation and thrombus formation. If the volume of thrombus is sufficient to occlude the lumen, then ST elevation myocardial infarction (STEMI) ensues. Time to cell death is variable, but has been shown to develop in less than 20 min in some animal models. However, complete necrosis requires at least 2–4 h. This is the rationale for early revascularisation strategies.
If the thrombus is only partially occlusive, or is temporary, then myocardial ischaemia is less severe. This may result in myocardial ischaemia with cell death (with elevation of cardiac-specific biomarkers such as troponin), when it is described as a non-STEMI (NSTEMI). If myocardial ischaemia is present, but without evidence of cell death (normal serum troponin levels), this is known as unstable angina (UA). These two conditions are collectively known as non-ST elevation acute coronary syndrome (NSTEACS). Such cases will be associated with severe vessel stenoses. However, such a thrombus may be transient because of either spontaneous or therapeutic thrombolytic therapy and, if imaging is delayed, it follows that stenoses in this situation may be moderate or mild and the diagnosis may be missed.
Another important consideration is the presence of coincidental CAD. The prevalence of asymptomatic non-obstructive CAD is high, especially in the elderly population. This disease may not be the cause of the patient's pain and it is important not to ascribe the pain to being cardiac just because of the presence of CAD. Confirmatory evidence must be sought so as to not “convert” an individual with non-cardiac chest pain and coincidental CAD into a cardiac patient.
Anatomical or functional imaging: which way to go?
Investigation of chest pain with anatomical imaging
One rationale for the imaging of patients with acute chest pain is to directly image the coronary arteries to look for the severe stenosis or occlusion that is causing the myocardial ischaemia. Catheter-based angiography is highly accurate in this regard. Its role in high-risk patients and typical symptoms is well established and without question. In patients with atypical symptoms but worrisome ST segment elevation, urgent angiography will also identify potential lesions that require intervention and allow treatment at the same time.
However, for intermediate- and low-risk patients, the majority of whom do not have an ACS, its use is questionable and will not result in early discharge even in those with normal coronary arteries (because of the bed stay required after the procedure). In this group, non-invasive coronary artery imaging with CT angiography holds great promise. It is highly accurate at exclusion of CAD. It is also highly sensitive in the majority of patients for the evaluation of stenotic disease. It not only aids in diagnosing luminal narrowings, as does catheter angiography, but it also demonstrates the atheroma in the wall and allows diagnosis of important non-coronary causes of chest pain (e.g. acute aortic syndromes) as well as providing potential other causes (e.g. hiatus hernia).
The problem with an anatomy-based algorithm, however, is that it is difficult to determine solely based on anatomy whether a particular lesion is the cause of the presenting symptoms. Patients may have both chest discomfort from a non-cardiac source and a lesion on anatomical imaging that is not the cause of their symptoms. There is also a small but important group of patients who have cardiac chest pain that is not secondary to CAD (e.g. myocarditis). Although CT may give some clues to these (either with pericardial effusion or with reduced myocardial contractility), the diagnosis requires additional investigation.
Investigation of chest pain with functional imaging
The other approach is to look at the effects of ischaemia on the myocardium. This can be done by examining perfusion of the myocardium by radionuclide myocardial perfusion imaging, magnetic resonance myocardial imaging or echocardiography. Alternatively, one of the consequences of ischaemia is reduced myocardial contractility, which can be examined with echocardiography or MRI. Although these approaches are looking for the pathophysiology of the chest pain, this may be absent if the chest pain has resolved. This is a shortcoming as, in clinical practice, at the time of the examination the majority of patients are pain free [17]. In the absence of pain, the demonstration of an abnormality (either perfusion or motion) is unreliable [17].
Another problem is that myocardial ischaemia may be limited to only part of the ventricular wall (usually the subendocardium). Contractility or perfusion abnormalities in this context may be absent or not detected.
In patients who are pain free, functional testing has to be directed at detecting haemodynamically significant disease. As stated in the anatomical imaging section, not all patients who have had an acute myocardial event will have a residual significant stenosis.
The reader should be aware that the separation between anatomical and functional imaging is becoming blurred. While CT is at present an anatomical test, there are emerging preliminary data of its use in the evaluation of perfusion (with multi-energy techniques). In addition, positron emission tomography/CT scanners are now frequently equipped with cardiac-enabled 64-slice systems that allow both CT angiography and exquisite perfusion data.
However, at present, it can be seen from the above discussion that both anatomical and functional approaches have their merits and drawbacks and that the approach taken at any institution will depend on the availability and expertise. Whatever approach is taken for the imaging strategy, to be effective it must:
be available 24 h a day, 7 days a week (as patients present at any time)
be rapidly available (so patients with myocardial infarction receive appropriate rapid treatment)
be rapid to perform and interpret.
In addition, for any investigation to be cost-effective, it must have a high negative predictive value and demonstrate other important causes for chest pain to allow safe discharge of patients.
Imaging techniques
Myocardial perfusion imaging: rest imaging
The use of rest myocardial perfusion imaging (MPI), with either 99mTc–sestamibi or 99mTc–tetrofosmin, in patients with acute chest pain for detecting ACS has been well validated [18-20], with perfusion abnormalities correlating well with both the anatomical size of infarction and the risk stratification (Figure 1). In the context of acute chest pain (<6 h), its sensitivity for detecting myocardial infarction is high (approximately 92%; range, 90–100%) [21,22]. However, the specificity of rest imaging is suboptimal (67–78% in the above recent papers) and has positive predictive values of 43–45%. One of the main reasons for this is that hypoperfusion can be due to chronic ischaemia, artefacts or unstable angina without myocardial necrosis. Thus, abnormal MPI is not specific for ACS. Among a trial of 2475 patients who presented with chest pain, randomisation to a strategy with acute rest MPI did not affect triage decisions in those patients in whom the eventual diagnosis was myocardial infarction or unstable angina; however, among those patients without acute coronary ischaemia (85% of the patients), MPI did reduce the rate of admission [23].
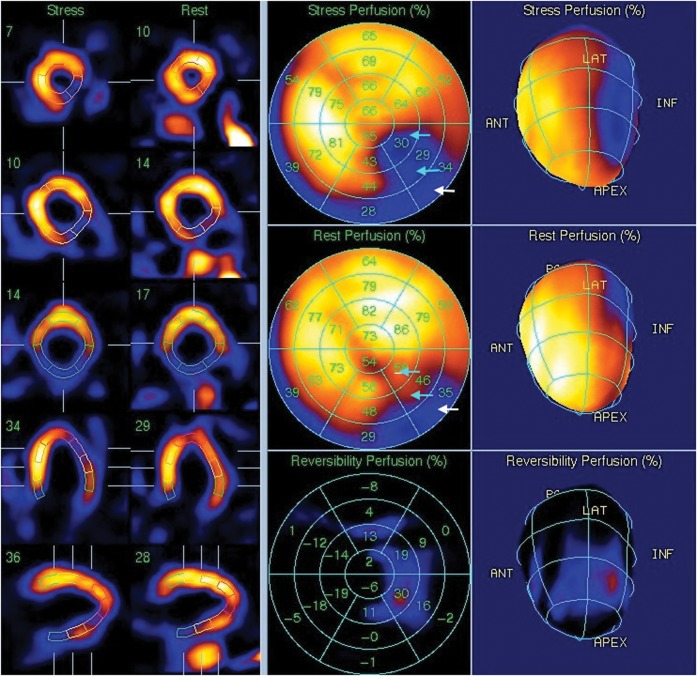
Figure 1.
Rest and stress images from a cardiac nuclear medicine myocardial imaging study. The images demonstrate a severe perfusion defect in the inferolateral wall. This is partly fixed (in the basal segment; white arrows) and partly reversible (in the distal and mid-inferolateral wall; cyan arrows) and is typical for an area of ischaemia.
The strength of resting MPI lies with its high negative predictive value (approaching 100%) and its value in short-term risk stratification. Patients with a normal rest MPI have a very low (<1%) 30 day cardiac event rate, whereas patients with abnormal rest MPI may have a 10–20% 30 day cardiac event rate. However, the timing of rest MPI is crucial. Optimal timing is during pain, and certainly no longer than 6 h following relief of pain. Partly for this reason, chest pain centres have been developed within the USA, which usually have good access to MPI. Despite this, the majority of patients present when pain free [17]. This problem is likely to be worse in the UK because of the general lack of a round-the-clock MPI service in the UK.
Echocardiography
Echocardiography has the advantage of being real time, free of ionising radiation and relatively cheap. In the acute setting, echocardiography is useful in that it can identify wall-motion abnormalities that may be the consequence of acute myocardial ischaemia in patients with non-diagnostic ECG changes and ongoing chest pain. In this context, echocardiography has a high sensitivity (>90%) and high negative predictive value (>95%) for myocardial infarction and ischaemia [24-26]. However, ACS may occur without regional wall-motion abnormality, and the specificity of echocardiography may be as low as 53% [25].
Microbubble contrast agents have been introduced to enhance the delineation of left ventricular endocardial borders [27], and myocardial contrast echocardiography can be used to assess microvascular perfusion. Microbubbles are entirely intravascular and, at steady state during a continuous intravenous infusion of microbubbles, the number of microbubbles entering or leaving any microcirculatory unit is constant, and will depend on the flow rate. Destroying microbubbles with an ultrasound pulse, and then determining the rate of replenishment of microbubbles into tissue, can allow an estimate of perfusion. As expected, such perfusion is reduced in ACS. Comparative trials between MPI and contrast echocardiography have shown similar results [28].
As with MPI, the assessment of patients needs to occur in a relatively short time-frame relative to the patient's chest pain. While the time to perform echocardiography is considerably less than for MPI, it has its own problems with poor acoustic windows in some patients and the need for considerable operator expertise in the interpretation of the images as wall-motion abnormalities are subjective, as are perfusion defects. However, in expert hands, microbubble contrast-enhanced echocardiography is an effective tool that could reduce unnecessary admission with associated cost savings [29].
Cardiovascular MRI
The role of cardiovascular magnetic resonance (CMR) imaging continues to expand. However, its role in the context of ACS is less well established. Access to the MRI scanner is typically limited and round-the-clock services are unusual in the UK. In addition, comprehensive CMR imaging typically takes between 30 min and 1 h, and patients with pacemakers, defibrillators or implanted pumps cannot undergo MRI. Therefore, it is unlikely that CMR imaging will develop into a front-line investigation for patients presenting to casualty. However, CMR imaging can provide unique information in chest pain syndromes that can aid in diagnosis and improve risk stratification after an event.
CMR protocols and technique
Images are usually obtained with breath-hold, but patients in New York Heart Association Class III or IV dyspnoea can be imaged using free-breathing sequences. Data acquisition is usually synchronised to the patient's ECG and acquired throughout the cardiac cycle. The individual images or movies (cine loops) that are acquired over several cardiac cycles are then gated using the patient's ECG. If the patient's rhythm is irregular or there are frequent ectopics, real-time acquisition can be used; however, the spatial resolution of these images is lower.
The balanced steady-state free precession (b-SSFP) sequence is the mainstay of cardiac functional assessment. Images are reconstructed as cine loops and displayed as movie clips and can be used for evaluation of global functioning parameters, ventricular end-systolic and end-diastolic volumes, stroke volume, ejection fraction and myocardial mass. The images can also be used for evaluation of regional wall motion. Although echocardiography still remains the gold standard for assessment of valve function and morphology, morphology can be assessed on cine CMR images, and phase-contrast velocity mapping can be used for calculation of the peak velocity and regurgitant fractions. Cardiac morphology can be assessed using spin echo sequences. These typically produce black blood images, and the images can be acquired with T1 or T2 weighting with or without fat suppression. T1 weighting is used to assess cardiac anatomy. T2 weighted images can be used to assess myocardial oedema.
Myocardial perfusion can be assessed using contrast-enhanced CMR (DE-CMR) imaging. First-pass imaging after the injection of a bolus of gadolinium can be used for quantitative or qualitative assessment. Late gadolinium enhancement (LGE or CE-CMR) is the key strength of CMR imaging, and is considered the gold standard for the assessment of myocardial infarction and scarring [1]. LGE images are obtained between 7 and 15 min after gadolinium injection. Initially, a multiphase inversion recovery SSFP scan is acquired through the ventricular mid-short axis (varied T1 scout). The optimal inversion time (TI) for nulling the myocardium is determined. Subsequent inversion recovery images are obtained using the TI interval to assess LGE. The images are acquired as a short-axis stack in the same position as those for functional assessment, single slices in the four chamber, ventricular long axis and left ventricular outflow tract views. The TI is increased as required for the subsequent images to allow for the delay in myocardial nulling due to gadolinium wash-out.
Pharmacological stress (adenosine or dobutamine) is used to unmask latent myocardial ischaemia. Physical exercise within the magnet leads to degradation of image quality. Following the standard sequences, first-pass perfusion is performed at stress, followed by rest using saturation-prepared turbo flash acquisition. Three slices orientated in the short axis (base, mid and apex) are acquired using a breath-hold command for up to 1 min. The stress and rest images are reviewed as cine loops for comparison of ventricular wall motion and perfusion defects.
CMR can be used in the following situations.
Detection of acute coronary syndrome
Resting CMR imaging assessment can detect ACS with a high sensitivity and specificity and is a stronger predictor of CAD than ECG and serum marker (troponin), especially in patients with unstable angina and NSTEMI. Regional wall motion may remain abnormal for several hours after transient ischaemia, owing to myocardial stunning, and is the most sensitive element of CMR assessment [30]. A standard CMR imaging protocol includes first-pass perfusion at rest with assessment of regional wall motion, assessment of ventricular function and volumes followed by an LGE sequence (Figure 2). Additional assessment with adenosine stress (AS-CMR) or dobutamine stress (DS-CMR) may be required, and is proven to be safe in high-risk patients with NSTEMI or unstable angina (Figure 3) [31]. In low-risk patients presenting with symptoms of ACS, AS-CMR or DS-CMR has a high sensitivity for detection of CAD and a high negative predictive value in defining the population at low risk of future cardiac events [32,33].
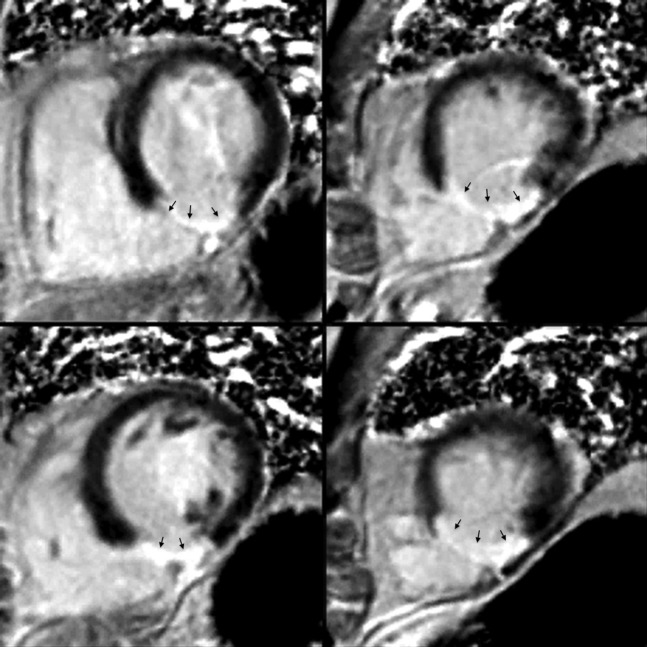
Figure 2.
Delayed enhancement coronary MRI. A stack of short-axis phase-segmented inversion recovery images in a patient with a transmural inferior infarction, demonstrate full-thickness delayed enhancement in the inferior wall (black arrows).
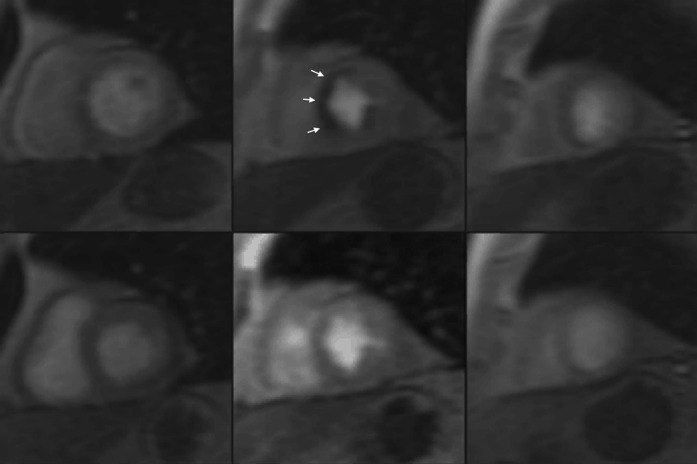
Figure 3.
Adenosine stress coronary MRI. A stack of short-axis images (basal, mid-cavity and apical) of the left ventricle at peak stress (top) and rest (bottom) demonstrate a reversible perfusion defect in the mid-cavity septal segments (white arrows).
Compared with radionuclide imaging, CMR imaging has the advantage of higher spatial resolution, sensitivity and freedom from radiation exposure. Lengthy examination times and lack of availability, however, mean its use in ACS is limited.
Management of patients with known acute coronary syndrome
It is safe for patients to undergo CMR within 24 h of an AMI. Coronary stents are not a contra-indication to CMR assessment because the artefact related to the stents is insignificant and does not interfere with image analysis [34]. The basic protocol in these patients (following AMI) includes cine imaging and delayed contrast enhancement imaging, with additional T2 weighted spin echo imaging or first-pass contrast-enhanced imaging to further characterise the infarcted territory.
CMR is the gold standard for the assessment of left and right ventricular ejection fraction and volumes [35]. Cine CMR images used in the quantitative assessment of left and right ventricular indices are highly reproducible and prone to very little interobserver variability [36]. Left ventricular end-systolic volume and ejection fraction are strong predictors of long-term prognosis in patients following an AMI [37]. These measurements also provide the accurate and reproducible indices for follow-up assessment of functional recovery in patients undergoing early reperfusion therapy. In patients who receive delayed or no reperfusion therapy, CMR imaging can be used for accurate assessment of ventricular remodelling.
LGE or DE-CMR is considered the most sensitive technique for the detection of infarcted myocardium. In studies performed following septal ablation for hypertrophic cardiomyopathy, LGE has been shown to demonstrate myocardial injury as early as 1 h after injury following the procedure [38]. There is evidence to suggest that LGE is useful in differentiating acute ischaemia from chronic irreversible myocardial injury and influencing further management of patients with ACS [39]. The extent of coronary obstruction leading to infarction can be predicted by assessing the transmurality and distribution of delayed enhancement. Infarction caused by occlusion of small or distal coronary branches not amenable to revascularisation will cause small, well-circumscribed but transmural enhancement (infarction). Proximal coronary occlusion that has been successfully revascularised will produce a large area of subendocardial enhancement corresponding to the arterial territory. Right ventricular and inferior wall involvement can be detected with higher sensitivity than with ECG and echocardiography. Prognosis after AMI has been shown to be closely related to infarction size. In a recent study, several factors, including ejection fraction and ventricular volumes, were shown to be associated with overall outcome; however, the size of the infarction, as demonstrated by LGE, was shown to be the strongest predictor of future adverse events and to have an incremental prognostic value [40]. LGE can be used to accurately quantify the infarction size in terms of absolute mass or to express it as a percentage of ventricular mass. LGE, along with T2 weighted imaging, can help to quantify areas of reversible and irreversible injury and be used to follow up the efficacy of revascularisation procedures [41].
Following AMI, there is an increase in the water content of the affected myocardium. This myocardial oedema is a transient phenomenon and usually resolves at between 3 and 12 weeks. T2 weighted imaging may help to visualise infract-related oedema without the use of contrast agent [38]. Since myocardial oedema occurs before irreversible damage, a single study using T2 weighted imaging could be used to assess the area at risk and the eventual infarction size. Currently, this technique has little use in the clinical setting; however, it may be useful in the comparison and evaluation of revascularisation strategies in the future. T2 weighted imaging, however, is prone to artefacts, and further research is required to establish the reproducibility and value of this technique in the clinical setting.
Microvascular obstruction (MVO) is another major prognostic factor after reperfusion therapy for AMI. MVO is defined as damage to the myocardial microcirculation within an infarcted area following restoration of epicardial coronary flow. The extent of MVO usually stabilises between 2 and 9 days following AMI, and optimal assessment of MVO can be performed during this period [42]. Contrast-enhanced CMR imaging is very sensitive to the detection of MVO. Both first-pass imaging and LGE can be used to detect MVO; however, first-pass imaging has a higher sensitivity. On first-pass imaging, MVO can be detected as an area of hypoenhancement of varying transmurality. On LGE, MVO can be detected as an area of non-enhancement within the area of late enhancement. It is essential to maintain a strict image acquisition protocol for the accurate detection and quantification of MVO and for assessment of progression on subsequent follow-up studies.
CMR imaging can be used to assess infarction-related complications such as papillary muscle dysfunction/infarction leading to mitral regurgitation, ventricular pseudo-aneurysm formation and pericardial effusion. LGE can be used to detect areas of infarction that may be at a high risk of developing these complications. First-pass imaging can be used to detect left ventricular thrombus following an infarction. Thrombi are avascular structures that produce a low signal on the background of contrast-enhanced blood in the ventricular cavity.
Patients presenting with ACS symptoms but unobstructed coronaries
In a small but significant number of patients presenting with chest pain, the laboratory tests reveal raised troponin, but coronary artery angiography reveals normal or non-flow-limiting CAD. There are a number of potential causes of this presentation, including acute myocarditis, cardiac infarction with coronary artery recanalisation due to thrombolytic therapy, coronary artery embolism and non-cardiac causes of raised troponin [43]. A number of studies have revealed that these patients have a poorer prognosis than patients with ACS receiving revascularisation therapies. This, in part, can be explained by the lack of accurate diagnosis and appropriate treatment in this difficult group of patients [44]. CMR imaging has the ability to identify areas of inflammation and myocardial damage and can be used to differentiate conditions that closely mimic ACS, such as myocarditis or tako-tsubo cardiomyopathy. Standard CMR sequences can provide quantitative information about ventricular function and aid in identifying the myocardial areas affected. Additional sequences using T2 weighted imaging and LGE are used to delineate the underlying aetiology.
CMR is now the imaging technique of choice for the diagnosis and follow-up of myocarditis [45]. In patients with suspected myocarditis, CMR imaging must be used for assessment in the acute phase, usually within 7 days of presentation [46]. T2 weighted imaging can delineate areas of myocardial oedema and increased extracellular water, and LGE sequences are used to detect areas of enhancement in the early phase. The pattern of LGE is usually patchy and multifocal, often extending from the subepicardial region to the mid-myocardium with subendocardial sparing [47]. Studies have demonstrated that using LGE corresponds to areas of active myocarditis and enhances the diagnostic yield of endomyocardial biopsies by directing the site of tissue sampling [48,49].
Multidetector CT angiography
Multidetector CT angiography (MDCTA) is a variation on standard CT angiography requiring ECG gating to allow appropriate timing of scanning relative to the patient's heart rhythm. The technique has seen massive technological advances over the past 5 years, which show no signs of slowing. Temporal and spatial resolution have continued to improve, and radiation doses have fallen dramatically with new methods of scanning. A recent meta-analysis of MDCTA in elective patients showed a pooled sensitivity of 90%, specificity of 97%, a negative predictive value of 99% and a positive predictive value of 76% for the detection of significant stenoses compared with invasive angiography [50].
MDCTA has the unique ability to non-invasively detect both significant coronary artery stenoses and coronary atherosclerotic plaque [51,52], as well as allow assessment of the aorta, pulmonary arteries and the adjacent structures, all within a single breath-hold examination (Figure 4). This makes it ideally suited to the investigation of patients with low to moderate probability of coronary disease presenting with acute chest pain, and its use in this indication been supported in the recent American College of Cardiology/American Heart Association guidelines and National Institute for Health and Clinical Excellence (NICE) documentation [1,53].
Figure 4.
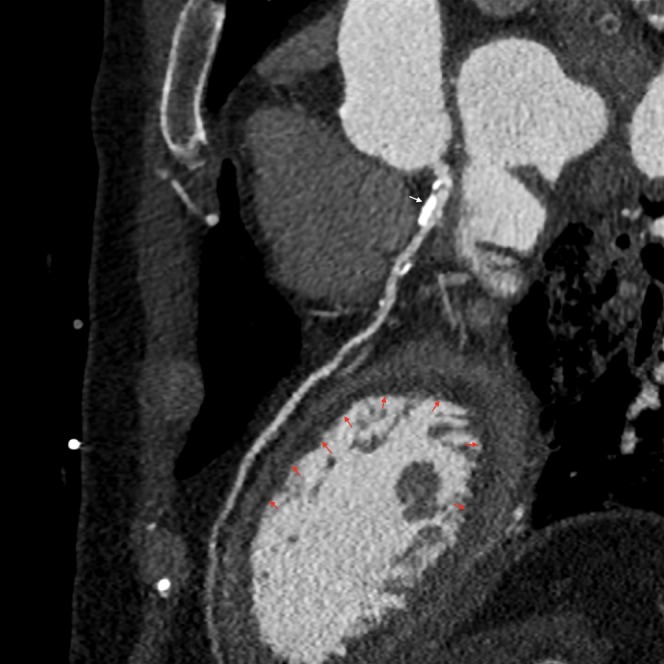
64-slice contrast-enhanced multidetector CT in a patient after cardiac arrest demonstrating large areas of subendocardial hypoenhancement (red arrows) indicating myocardial ischaemia. This is secondary to a severe band-like stenosis in the left main stem coronary artery (white arrow).
Equipment required
Cardiac CT can reliably be performed on any 64-slice CT with the appropriate hardware and software. Retrospective upgrades are possible on existing equipment. Prospective gating is considered essential because of the dramatic dose saving that can be achieved with its use. Contrast injection rates of 5–6.5 ml s–1 are routinely used and a dual-headed pump is recommended (to allow the use of a saline chaser).
Patient considerations
Image quality in MDCTA is improved dramatically in patients with a slow regular heart rate who can comply with breath-hold and who have relatively low levels of coronary artery calcification. These are evident on clinical assessment and initial calcium scoring. Patients with rapid rhythms that cannot be controlled with β-blockade benefit from the increased temporal resolution of dual-source CT systems. Patients with irregular rhythms can be scanned, but at higher radiation doses (as most, if not all, of the cardiac cycle needs evaluation) and with lower diagnostic rates than would be normally expected. Patients who are unable to comply with breath-hold are best investigated with another modality.
Coronary artery calcium can be assessed by performing low-dose calcium scoring prior to MDCTA (110 kV, 100 mA); this has little effect on the estimated Agatston score [54]. There is no consensus on what levels of calcium will prohibit CTA; however, high calcium levels undoubtedly reduced the positive predictive value of CTA. New technologies, for example high-definition CT, are likely to reduce its importance. However, in the context of exclusion of disease, approximately half the patients have no evidence of any disease (including coronary calcium).
Use of intravenous β-blockade, provided the usual contra-indications are observed, is safe in patients with acute chest pain and should be considered in patients with heart rates above 65 bpm [55]. Sublingual nitroglycerin is sometimes used to invoke coronary vasodilatation; there is, however, no evidence to show that it increases accuracy and it may cause overestimation of stenoses and induce headache.
Scanning technique
Detailed discussion about technique is inappropriate here; in brief, there are three methods of scanning:
retrospective ECG-gated helical (retrospective helical)
prospective ECG-triggered axial (prospective axial)
prospective ECG-triggered high-pitch helical (prospective helical–high pitch).
The last is only possible on dual-source systems and will not be considered further. Retrospective scanning allows multiphase data acquisition owing to continuous radiation throughout the cardiac cycle. This is sometimes useful, in terms of both better coronary visualisation and allowing evaluation of myocardial contractility and ejection fraction. This, however, comes at the expense of high radiation dose [the median effective dose reported recently from a large international observational study was 12 mSv (interquartile range 8–18 mSv)] [56]. Prospective scanning has limited phase data but allows a significant reduction in radiation to approximately 20% of that of retrospective scanning [57]. The latter should therefore be the default technique in patients with heart rates below 65 bpm and a regular rhythm.
Radiation doses can be reduced significantly by patient-specific scanning parameters (kilovolts and milliamperes) linked to the patient's body mass index. The success of such strategies is linked to operator experience [56], and all centres considering the introduction of the service should ensure full training of both radiographic and medical staff.
Advantages of multidetector CT angiography in the evaluation of acute chest pain
While MDCTA at present is technically challenging compared with other types of CT examination, it does have a number of definite advantages in the assessment of patients presenting with acute chest pain in the UK:
64-slice CT is widely available to most casualty departments
most CT services are operated around the clock because of the demands for other investigation (e.g. the evaluation of stroke)
it can be performed rapidly, so patients with positive examinations can receive appropriate rapid treatment
it can demonstrate alternative causes for the patient's chest pain (which can occur in over 20% of patients) [58]
it has a reported negative predictive value approaching 100%.
The last fact is extremely important in the context of patients presenting to casualty. As stated above, 40% of hospital admissions are patients with acute chest pain, yet only 20% of these will have an ACS (and only half have any evidence of CAD; see below). Therefore, a rapid, widely available test that can exclude disease would be not only useful but also cost-effective.
MDCTA is a new technique and, as such, prognostic data are inevitably lacking. Emerging data suggest that, in stable patients with chest pain, those with no detectable plaque have a very low (all cause) mortality rate of 0.3% [59], and that MDCTA adds significant prognostic data to myocardial perfusion imaging [60]. There are some single-centre data regarding its efficacy in acute chest pain in the low- to intermediate-risk category [61-63]. A recent meta-analysis of these data, looking at 16 studies in 1119 patients, demonstrated pooled sensitivity of 96% and specificity of 92% for diagnosis of significant coronary artery stenosis, and concluded that “MDCTA accurately detects and noninvasively establishes or excludes obstructive CAD as the cause of chest pain. MDCTA has the potential to substantially alter the algorithms, used for chest pain assessment in the ED” [64].
One of these, the Rule Out Myocardial Infarction Using Computer Assisted Tomography study, demonstrates a number of important points. This study prospectively enrolled 368 acute chest pain patients with inconclusive initial emergency department evaluation in a protocol to receive a 64-slice coronary CTA scan [65]. This was reported within an average time of 25 min. Among the study patients, 31 (8.4%) were judged to have had ACS. Over a 6 month follow-up, none of the patients without ACS had a clinical outcome event. By coronary CTA, 50% of patients were completely free of CAD, 31% had non-obstructive plaque and 19% had significant obstructive CAD. Comparing the presence of any coronary plaque with the consensus diagnosis of ACS, coronary CTA had a sensitivity of 100% and a specificity of 54%. The presence of obstructive CAD had a sensitivity of 77% and a specificity of 87% for the consensus diagnosis of ACS. Of the 34 patients with significant obstructive CAD by CT, 14 were not diagnosed as having ACS, and none of these patients had a follow-up event out to 6 months.
What the study illustrated is that a significant number of patients with entirely normal coronaries undergo admission and further investigation, often with invasive angiography and that, in the USA at least, MDCTA can be a rapid and effective triage tool for those patients with no CAD. Clearly, not all patients with significant CAD have an acute ACS, and it is in this group of patients where the diagnostic algorithm is less clear and there is risk of overinvestigation once the presence of atheroma has been demonstrated.
Not only will MDCTA prevent overinvestigation, but it is also likely to result in fewer admissions. In one of the largest studies to date, 476 (84%) of 568 patients with suspected ACS who were at low risk as indicated by their TIMI score were discharged from the emergency department after cardiac CTA, and none had adverse cardiac events at 30 days [66]. A recent large study of 785 consecutive patients compared MDCTA with stress MPI for the investigation of acute chest pain. At 3 months' follow-up, 0.3% of the negative MDCTA patients and 3% of the negative MPI patients developed ACS or died [58]. Further small studies also show similar results. As acknowledged in the recent NICE document on the management of acute chest pain [1], MDCTA is likely to be important in low- to intermediate-risk patients but requires further randomised controlled trials with adequate sample sizes to detect important differences in hard outcomes.
Conclusions
Acute chest pain is an important clinical problem. The use of “front door” imaging is likely to be an effective strategy to prevent unnecessary admission in terms of not only patient benefit but also cost-effectiveness. All the imaging modalities here can be effective with the appropriate expertise. MPI has been used for the longest period of time and has proven prognostic data. However, despite considerable investment in the USA in its use in acute chest pain units, it has limitations and has failed to be developed as a service in the UK. CMR imaging has the best tissue characterisation of all the investigations and can demonstrate tissue oedema, wall-motion abnormalities and perfusion defects. However, at present it is a lengthy examination best suited to problem-solving rather than “front door” evaluation. Echocardiography is fast, cheap and widely available, but requires good acoustic windows and high operator expertise.
Of all the imaging modalities, MDCTA is most likely to emerge as the primary “front door” investigation of patients with acute chest pain but with no history of CAD or worrying ECG changes. It is widely available, quick and, with good technique, highly accurate. Its ability to detect both occlusive and non-occlusive coronary atheroma gives it an extremely high negative predictive value for the exclusion of CAD. It also has the ability to demonstrate alternative aetiologies for the patient's chest pain and exclude the other potentially life-threatening conditions of acute aortic syndromes. Its widespread use should result in the safe discharge of large numbers of patients (approximately half) who would normally have undergone hospital admission. However, in those patients in whom it demonstrates coronary atheroma, the management decisions are less clear. Large multicentre trials have yet to be performed to clearly define the role of CT and are eagerly awaited. Owing to the high incidence of coincidental coronary atheroma, it will be important to demonstrate that, in those patients with significant disease, the cause of chest pain is cardiac. In this group, functional imaging and the use of cardiac enzymes will remain central in patient management.
References
- 1.National Institute of Healthand Clinical Excellence Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. London, UK: NICE, 2010 [Google Scholar]
- 2.Ruigomez A, Rodrigeuz LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract 2006;23:167–74 [DOI] [PubMed] [Google Scholar]
- 3.Goodcare S, Cross E, Arnold J, et al. The health care burden of acute chest pain. Heart 2005;91:229–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy NF, MacIntyre K, Capewell S, et al. Hospital discharge rates for suspected acute coronary syndromes between 1990 and 2000: population based analysis. BMJ 2004;328:1413–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodacre S, Cross E, Arnold J, et al. The health care burden of acute chest pain. Heart 2005;91:229–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope JH, Aufderheide TP, Selker HP, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–70 [DOI] [PubMed] [Google Scholar]
- 7.Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol 1987;60:219–24 [DOI] [PubMed] [Google Scholar]
- 8.Rusnak RA, Stair TO, Hansen K, et al. Litigation against the emergency physician: common features in cases of missed myocardial infarction. Ann Emerg Med 1989;18:1029–34 [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD, et al;onbehalfofthejointESC/ACCF/AHA/WHFTaskForcefortheRedefinitionofMyocardialInfarction Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173–95 [DOI] [PubMed] [Google Scholar]
- 10.Fesmire FM, Fesmire CE. Improved identification of acute coronary syndromes with second generation cardiac troponin I assay: utility of 2-hour delta cTnI ≥ + 0.02 ng/mL. J Emerg Med 2002;22:147–52 [DOI] [PubMed] [Google Scholar]
- 11.Bakker AJ, Koelemay MJW, Gorgels JPMC, et al. Failure of new biochemical markers to exclude acute myocardial infarction at admission. Lancet 1993;342:1220–2 [DOI] [PubMed] [Google Scholar]
- 12.Antman EM, Anbe DT, Armstrong PW, et al. doi: 10.1016/j.jacc.2004.07.014. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction); 2004. Available at: www.acc.org/clinical/guidelines/stemi/index.pdf. [DOI] [PubMed] [Google Scholar]
- 13.Gerber TC. Emergency department assessment of acute-onset chest pain: contemporary approaches and their consequences. Mayo Clin Proc 2010;85:309–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antman EM, Cohen M, Bernink PJLM, et al. The time risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284:835–42 [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol 2007;50:1–15717601538 [Google Scholar]
- 16.Gould KL, Lipscomb K. Effects on coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 1974;34:48–55 [DOI] [PubMed] [Google Scholar]
- 17.Wackers FJT. Chest pain in the emergency department: role of cardiac imaging. Heart 2009;95:1023–30 [DOI] [PubMed] [Google Scholar]
- 18.Heller GV, Stowers SA, Hendel RC, et al. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and non-diagnostic electrocardiograms. J Am Coll Cardiol 1998;31:1011–17 [DOI] [PubMed] [Google Scholar]
- 19.Kontos MC, Jesse RL, Schmidt KL, et al. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol 1997;30:976–82 [DOI] [PubMed] [Google Scholar]
- 20.Gomez MA, Anderson JL, Karagounis LA, et al;forthe ROMIO., Study Group An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study (ROMIO). J Am Coll Cardiol 1996;28:25–33 [DOI] [PubMed] [Google Scholar]
- 21.Kontos MC, Jesse RL, Anderson FP, et al. Comparison of myocardial perfusion imaging and cardiac troponin I in patients admitted to the emergency department with chest pain. Circulation 1999;99:2073–8 [DOI] [PubMed] [Google Scholar]
- 22.Heller J, Kontos MC, Tatum JL, et al. Comprehensive strategy for the evaluation and triage of the chest pain patient. Ann Emerg Med 1997;29:116–25 [DOI] [PubMed] [Google Scholar]
- 23.Udelson JE, Beshansky JR, Ballin DS, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. JAMA 2002;288:2693–700 [DOI] [PubMed] [Google Scholar]
- 24.Peels CH, Visser CA, Funke Kupper AJ, et al. Usefulness of two dimensional echocardiography for immediate detection of myocardial ischemia in the emergency room. Am J Cardiol 1990;65:687–91 [DOI] [PubMed] [Google Scholar]
- 25.Sabia P, Afrookteh A, Touchstone DA, et al. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction. A prospective study using two-dimensional echocardiography. Circulation 1991;84:I85–92 [PubMed] [Google Scholar]
- 26.Kontos MC, Arrowood JA, Paulsen WHJ, et al. Early echocardiography can predict cardiac events in emergency department patients with chest pain. Ann Emerg Med 1998;31:550–7 [DOI] [PubMed] [Google Scholar]
- 27.Ward RP, Mor-Avi V, Lang RM. Assessment of left ventricular function with contrast echocardiography. Cardiol Clin 2004;22:211–19 [DOI] [PubMed] [Google Scholar]
- 28.Kaul S, Senior R, Harrel FE, et al. Incremental value of cardiac imaging in patients presenting to the emergency department with chest pain and without ST-segment elevation: a multicenter study. Am Heart J 2004;148:129–36 [DOI] [PubMed] [Google Scholar]
- 29.Wyrick JJ, Kalvaitis S, McConnell J, et al. Cost efficiency of myocardial contrast echocardiography in patients presenting to the emergency department with chest pain of suspected cardiac origin and a non-diagnostic electrocardiogram. Am J Cardiol 2008;102:649–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation 2003;107:531–7 [DOI] [PubMed] [Google Scholar]
- 31.Plein S, Greenwood JP, Ridgway JP, et al. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004;44:2173–81 [DOI] [PubMed] [Google Scholar]
- 32.Nandalur KR, Dwamena BA, Choudhri AF, et al. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol 2007;50:1343–53 [DOI] [PubMed] [Google Scholar]
- 33.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation 2007;115:1769–76 [DOI] [PubMed] [Google Scholar]
- 34.Beek AM, van Rossum AC. Cardiovascular magnetic resonance imaging in patients with acute myocardial infarction. Heart 2010;96:237–43 [DOI] [PubMed] [Google Scholar]
- 35.Bogaert JG, Bosmans HT, Rademakers FE, et al. Left ventricular quantification with breath-hold MR imaging: comparison with echocardiography. Magma 1995;3:5–12 [DOI] [PubMed] [Google Scholar]
- 36.Kondo C, Caputo GR, Semelka R, et al. Right and left ventricular stroke volume measurements with velocity-encoded cine MR imaging: in vitro and in vivo validation. AJR Am J Roentgenol 1991;157:9–16 [DOI] [PubMed] [Google Scholar]
- 37.White HD, Norris RM, Brown MA, et al. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987;76:44–51 [DOI] [PubMed] [Google Scholar]
- 38.Schulz-Menger J, Gross M, Messroghli D, et al. Cardiovascular magnetic resonance of acute myocardial infarction at a very early stage. J Am Coll Cardiol 2003;42:513–18 [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Aty H, Zagrosek A, Schulz-Menger J, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 2004;109:2411–16 [DOI] [PubMed] [Google Scholar]
- 40.Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart 2008;94:730–6 [DOI] [PubMed] [Google Scholar]
- 41.Friedrich MG, Abdel-Aty H, Taylor A, et al. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol 2008;51:1581–7 [DOI] [PubMed] [Google Scholar]
- 42.Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998;97:765–72 [DOI] [PubMed] [Google Scholar]
- 43.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005;142:786–91 [DOI] [PubMed] [Google Scholar]
- 44.Dokainish H, Pillai M, Murphy SA, et al. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS-TIMI-18 substudy. J Am Coll Cardiol 2005;45:19–24 [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Aty H, Boye P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815–22 [DOI] [PubMed] [Google Scholar]
- 46.Roditi GH, Hartnell GG, Cohen MC. MRI changes in myocarditis: evaluation with spin echo, cine MR angiography and contrast enhanced spin echo imaging. Clin Radiol 2000;55:752–8 [DOI] [PubMed] [Google Scholar]
- 47.Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006;114:1581–90 [DOI] [PubMed] [Google Scholar]
- 48.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 2004;109:1250–8 [DOI] [PubMed] [Google Scholar]
- 49.De Cobelli F, Pieroni M, Esposito A, et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 2006;47:1649–54 [DOI] [PubMed] [Google Scholar]
- 50.Mowatt G, Cummins E, Waugh N, et al. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess 2008;12(17). [DOI] [PubMed] [Google Scholar]
- 51.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment based comparison with intravascular ultrasound. Circulation 2004;109:14–17 [DOI] [PubMed] [Google Scholar]
- 52.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol 2005;46:147–54 [DOI] [PubMed] [Google Scholar]
- 53.American College of Cardiology / American Heart Association ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: executive summary. Circulation 2007;116:803–77 [Google Scholar]
- 54.Nakazato R, Dey D, Gutstein A, et al. Coronary artery calcium scoring using reduced tube voltage and radiation dose protocol with dual source computed tomography. J Cardiovasc Comput Tomogr 2009;3:394–400 [DOI] [PubMed] [Google Scholar]
- 55.Shim SS, Kim Y, Lim SM. Improvement of image quality with beta-blocker premedication on ECG-gated 16-MDCT coronary angiography. AJR Am J Roentgenol 2005;184:649–54 [DOI] [PubMed] [Google Scholar]
- 56.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500–7 [DOI] [PubMed] [Google Scholar]
- 57.Roobottom CA, Mitchell G. Morgan-Hughes G. Radiation-reduction strategies in cardiac computed tomographic angiography. Clin Radiol 2010;65:859–67 [DOI] [PubMed] [Google Scholar]
- 58.Beigel R, Oieru D, Goitein O, et al. Usefulness of routine use of multidetector coronary computed tomography in the “fast track” evaluation of patients with acute chest pain. Am J Cardiol 2009;103:1481–6 [DOI] [PubMed] [Google Scholar]
- 59.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–70 [DOI] [PubMed] [Google Scholar]
- 60.van Werkhoven JM, Schuijf JD, Gaemperli O, et al. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol 2009;53:623–32 [DOI] [PubMed] [Google Scholar]
- 61.Chang SA, Choi SI, Choi EK, et al. Usefulness of 64-slice multi-detector computed tomography as an initial diagnostic approach in patients with acute chest pain. Am Heart J 2008;156:375–83 [DOI] [PubMed] [Google Scholar]
- 62.Goldstein JA, Gallagher MJ, O'Neill WW, et al. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol 2007;49:863–71 [DOI] [PubMed] [Google Scholar]
- 63.Hoffmann U, Nagurney JT, Moselewski F, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation 2006;114:2251–60 [DOI] [PubMed] [Google Scholar]
- 64.Athappan G, Habib M, Ponniah T, et al. Multi-detector computerized tomography angiography for evaluation of acute chest pain: a meta analysis and systematic review of literature. Int J Cardiol 2010;141:132–40 [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann U, Bamberg F, Chae C, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the Rule Out Myocardial Infarction Using Computer Assisted Tomography (ROMICAT) trial. J Am Coll Cardiol 2009;53:1642–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollander JE, Chang AM, Shofer FS, et al. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med 2009;53:295–304 [DOI] [PubMed] [Google Scholar]