Abstract
During the last two decades, radionuclide myocardial perfusion scintigraphy (MPS) has become established as the main functional cardiac imaging technique for the assessment of ischaemic heart disease (IHD). Despite a growing number of alternative functional imaging techniques, MPS still remains the most widely used technique, with a wealth of literature supporting its usefulness in assessing IHD and predicting prognosis. The technique itself has evolved, making it more reliable and robust, with additional ventricular functional information that further defines the prognosis in these patients. With the advent of hybrid single photon emission with CT and positron emission tomography with CT cameras together with the development of new camera technology that enables faster images with less radiation and better resolution, MPS will remain an essential part of IHD investigation. There are new promising radiopharmacological developments and applications such as radiolabelled fatty acids and meta-iodobenzylguanidine. These will widen the scope of nuclear medicine imaging to include patients with cardiac failure and acute chest pain presenting to accident and emergency departments. Nuclear medicine cardiac investigations will continue to have an essential role in the diagnosis, stratification and prognosis of patients with cardiac disease, complementing the new developing cardiac modalities such as CT coronary angiography and MRI.
Myocardial perfusion scintigraphy (MPS) using single photon emission computed tomography (SPECT) or positron emission tomography (PET) is a well-established mode of investigation in the diagnosis of acute-onset chest pain as well as evaluation of patients with known coronary artery disease. The demand for it has increased unabated despite ever-increasing availability of new imaging modalities such as CT and MRI: 8.5 million MPS studies were performed in the USA alone in 2008 [1], a 370% increase from 2.3 million since 2004. Functional imaging has remained an important part of the investigation of ischaemic heart disease (IHD), and its role in the UK has been re-iterated by the new National Institute for Health and Clinical Excellence (NICE) guidelines for acute chest pain [2]. The biggest strengths of nuclear medicine MPS are the large, well-conducted trials and the literature for establishing the specificity, sensitivity, accuracy as well as prognostication of patients. The prognostic significance of normal and abnormal MPS with long-term follow-up and angiographic correlation has been extensively investigated and established [3,4]. It has been shown to be cost-effective when utilised for investigating and managing patients with IHD [5]. In this review, we shall look at the past and present developments in nuclear medicine cardiac imaging for both IHD and non-IHD.
Cardiac SPECT and SPECT/CT
Past developments in imaging myocardium
Hardware
SPECT imaging has superseded planar perfusion imaging. Multiple planar images of the heart using photons (γ-rays) emitted by radiopharmaceuticals are acquired by rotating detector heads around the patient, and myocardial perfusion images are reconstructed using principles close to CT imaging. It is widely available and used to assess myocardial perfusion as well as ventricular function in suspected IHD. Since its introduction in the late 1980s for routine clinical practice, the principle has remained the same; however, there have been several significant modifications for better and optimal imaging of the heart, for example 180° acquisitions have almost entirely replaced 360° acquisitions. This has resulted in faster acquisition times, in particular by using two-headed cameras at a 90° angle. Back-projection reconstruction of the images has now been mostly replaced by iterative reconstruction. This technique requires high computing power as it is calculation intensive. Although it has routinely been available in nuclear medicine since the 1990s, its use has been widespread only in the last decade. This technique is now being introduced to CT imaging owing to the even higher computing power required for higher resolution of CT images. This method of reconstruction has reduced the artefacts from adjacent hot structures such as the liver and gut. The protocols are standardised and have been well documented by professional bodies in the UK [6], Europe [7] and the USA [8] in an attempt to establish a uniform standard for imaging the heart. This should achieve consistent accuracy for this test, enabling the results of various studies to be translated into daily clinical practice. However, guidelines have a lag time, and often with cautious use of the new technology and software it is possible to achieve better results. Attenuation correction to reduce soft-tissue attenuation artefacts was introduced using external beam (transmission) images using either a γ-emitting source or, more commonly, a low-dose CT in conjunction with SPECT imaging (SPECT/CT). This has inherent problems, but it is generally accepted that it helps to improve the accuracy in reporting [9].
Radiopharmaceuticals
Thallium-201 chloride was the first pharmaceutical to be widely used clinically for imaging myocardial perfusion [10]. It is an excellent agent for imaging perfusion and is still used to image the heart in many centres. Because of its relatively long half-life and low-energy X-ray emission, it is not the ideal agent for imaging, giving a relatively large radiation dose with lower image quality than technetium agents. It behaves like the K+ ion (via Na/K-ATPase), and is redistributed fairly rapidly, starting 20 min after injection [11]. This enables imaging of both ischaemic (stress) and resting images (following redistribution of the agent) without the need for re-injection. On the other hand, rapid redistribution images have to be obtained immediately following stress and cannot be repeated for unsatisfactory images such as patient movement. When planar imaging was the standard, it was possible to obtain images within 15 min. With SPECT imaging, which takes up to 45 min, in theory the later images may represent the redistribution stage, which may affect the images adversely. Technetium-based agents for assessing myocardial perfusion are well established [12]. This has the convenience of production by local radiopharmacies from cold kits and there is no or minimal redistribution, so that images can be obtained many hours later with no loss of diagnostic accuracy. This makes the planning and timing of imaging more convenient and, if the images are unsatisfactory (movement or gut activity), rescanning can be performed. Two technetium-based agents, namely sestamibi and tetrofosmin, are now both widely used since their introduction in the early 1990s. They have similar characteristics for visualisation of myocardial perfusion. Because of the lower radiation dose to the patient, higher injection activity (5–10 times that of thallium), which more than compensates for the lower percentage uptake in the myocardium, and better photon characteristics, better images can be acquired in a shorter time. With technetium agents, 8- or even 16-bin gated studies (for left ventricular function assessment) can consistently be obtained in daily clinical practice with a relatively standard 20 min acquisition.
Software
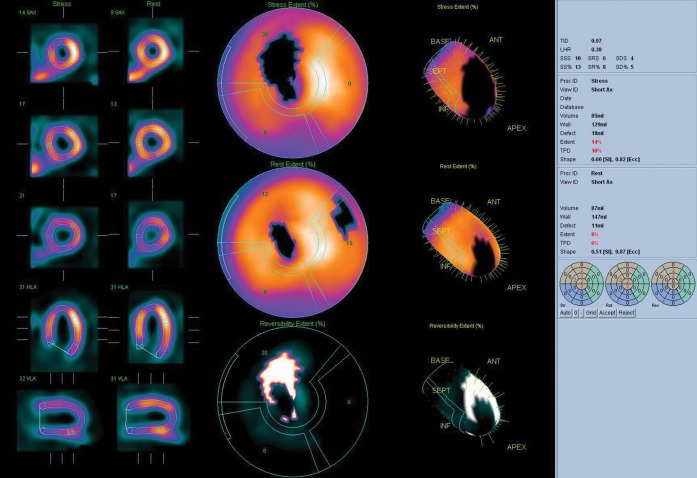
The display and presentation of perfusion images comparing two sets of images acquired during stress and rest has been challenging, in particular if there are extracardiac high-count areas such as a hot gallbladder or activity in the adjacent gastrointestinal tract. The development of cardiac packages has enabled reliable automatic normalisation of myocardial activity for rest and stress images for comparison. Such packages provide semi-quantification measurements for planar [13] and SPECT images. They also enable easily automated display of related short and long axes of SPECT images for side-by-side comparison and provide automated estimation of the defect size (Figure 1). In addition, comparison with normal libraries can help to identify artefacts due to breast or diaphragm and aid in the correct interpretation of images [14]. Quantification of the defect either by a segmental scoring system or by calculation of the percentage of fixed (infarcted) and reversible (ischaemic) defects has helped to stratify the significance of defects and patient prognosis. The algorithms used for cardiac edge detection have been refined to be more robust and reliable.
Figure 1.
Patient with a large ischaemic area in the left anterior descending artery (LAD) territory. The image shows a reversible (white out) and fixed (black out) defect in LAD territory. The extent of the lesion is calculated to be 10% and the calculated prognostic score is 14, which puts the patient in the high-risk category.
Stressing
MPS is used to look at the changes in blood flow with stress. Traditionally, physical stressing is used to increase oxygen demand in healthy arteries, leading to coronary vasodilatation and increased blood flow. In stenosed arteries, there is no increased flow despite the increased demand for oxygen. This results in ischaemia with associated symptoms (pain) and echocardiographic changes because of myocardial hypoxia. In patients unable to do physical exercise, dobutamine is used to the same effect. Dipyridamole induces coronary vasodilatation without increasing the myocardial oxygen demand by inhibiting the breakdown of adenosine, which is a potent coronary vasodilator. The coronary vasodilator effect of dipyridamole is more pronounced than that caused by stress-induced ischaemia, and thus is most suitable for MPS. Direct infusion of adenosine for coronary vasodilatation was soon introduced; this has a short half-life of about 10 s, with the effects being reversible within minutes of stopping the infusion. This makes adenosine a fast, safe and reliable drug to use for assessing the myocardial perfusion reserve in normal coronary arteries and the loss of perfusion reserve in significantly stenosed coronary arteries (commonly termed “ischaemia”). These agents are now widely used to assess myocardial perfusion with no loss of diagnostic accuracy for identifying significant stenosis [15].
New developments
Hardware developments
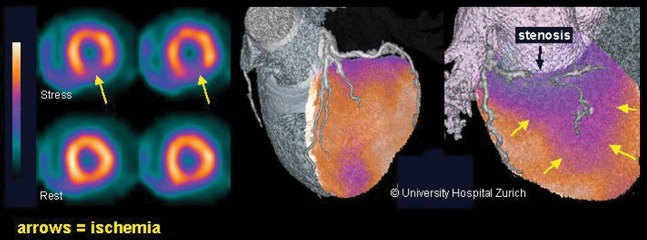
With increasing demand on MPS services, one of the major limiting factors has been the relatively slow image acquisition possible with Anger cameras. Several new cardiac camera systems have now been developed that enable faster acquisition with better resolution. Some use new, novel approaches utilising either moving sodium iodide crystals with coupled photomultipliers or clever collimator systems to increase the sensitivity by focusing on a small area (such as the heart). Another exciting development is the introduction of solid-state cameras with no moving parts for tomographic acquisitions. Some of these systems use several fixed pinhole collimators at different angles focused on a relatively small organ (heart) to reconstruct perfusion images. At the same time, the development and use of resolution recovery algorithms has enabled better images with lower counts. Combining the hardware and software developments with the new camera systems, one can now acquire a myocardial tomographic study in less than 5 min with almost twice the resolution of the standard Anger gamma cameras and lower injected activity and radiation to the patients [16,17]. In theory, even dynamic tomographic images can be performed to enable absolute blood flow measurements using technetium-based agents for MPS. The new fast cameras also enable tomographic multigated blood pool studies (SPECT/multigated acquisition scan) as a fast, plausible investigation for both left and right ventricular function, including phase and diastolic function assessment at rest and stress. Another parallel development has been the development of the combined structural and functional images. The advantages of combining the two technologies has led to the widespread use of hybrid cameras with sequential CT and SPECT or PET imaging. There are now several camera systems combining the fast-acquisition gamma cameras with high-resolution cardiac CT (hybrid cardiac cameras). Image coregistration using these hybrid systems enables more informed and better interpretation of both coronary CT findings as well as nuclear medicine perfusion imaging (Figure 2) [18,19]. This has enabled some departments to develop quick diagnostic algorithms by combining coronary anatomy with physiological information on blood flow in one patient visit. Combined images enable better diagnosis, stratification and treatment planning for patients with suspected coronary artery disease. Combining calcium scoring, coronary CT and perfusion imaging in one patient visit is possible, and there are several departments looking at the most effective use of such protocols in daily clinical practice to enable faster and better diagnosis of coronary artery disease and streamlining the most appropriate treatment for patients.
Figure 2.
Hybrid image combining a myocardial perfusion image and CT coronary angiography. It clearly demonstrates the lesion in the coronary artery (black arrow) and the associated reduction in perfusion (blue/purple area shown with yellow arrows). Hybrid imaging helps to clearly identify the functionally significant coronary stenosis (Society of Nuclear Medicine image of the year [18]).
Software developments
We have already mentioned the software development for image acquisition and reconstruction that has led to faster and better quality imaging with less radiation to the patient. In addition, there are now several new software packages available for displaying images. The new display software packages have better edge detection algorithms with better display of normalised images. The introduction of extensive normal libraries has enabled more accurate semiquantitative assessment of left ventricular perfusion. There are also expert systems for interpretation and automatic reporting of myocardial perfusion images [20]. Gating the study enables measurement of various left ventricular functional parameters such as volumes, ejection fraction and diastolic function. There is a growing literature on the clinical and prognostic significance of these various perfusion and left ventricular function parameters [21]. The reporting aid software has enabled more accurate and objective assessment of the heart. There is now a growing literature on the uses of MPS in cardiac failure. In addition to providing information on perfusion, it can give an accurate assessment of both systolic and diastolic left ventricular function, including phase and movement asynchrony which, in conjunction with CT anatomy information from hybrid imaging, can be used in planning for positioning of various intracardiac devices [22-25]. Another potential growth area in nuclear cardiac imaging is SPECT blood pool imaging. There are now software packages that help to measure left as well as right ventricular function, which potentially is more accurate than the traditional planar images, and give additional information on regional wall movement and various aspects of phase analysis (Figure 3) [26]. With the new developments in cameras and software, these studies can be performed in a few minutes without the need for the patient to lie flat for long periods, which can be difficult in cardiac failure patients.
Figure 3.
Multigated single photon emission CT (SPECT MUGA) study of a patient with heart failure. Count and volume-based cardiac function can be assessed. Right and left ventricular parameters include diastolic function and phase analysis of both the right and left ventricles.
Radiopharmaceuticals
Thallium was the first agent routinely used in assessing myocardial ischaemia. Technetium-based agents (2-methoxyisobutylisonitrile and tetrofosmin) with minimal redistribution, better imaging characteristics and less radiation to the patient are now widely used. For the foreseeable future, MPS will continue to be used for assessment of ischaemia. There is now a resurgence and development of both neutral and cationic technetium- and iodine-based agents to further improve the characteristics of perfusion radiopharmaceuticals. The new technological advances described will enable faster imaging and/or reduced dose. Faster redistributing agents such as Tc–teboroxime [12] and the developmental Tc–tricarbonyl complex (99Tcm-15C5-1-PNP, with relatively fast redistribution and low liver uptake) [27], which were impractical (because of too fast a redistribution for standard 20 min imaging using standard gamma cameras), may be re-introduced for use with the new fast systems. In theory, a complete study with calcium scoring followed by stress–rest images and, if necessary, complemented with coronary CT can be performed in 30 min using a fast redistribution agent and new fast hybrid cameras.
With the rapid growth of molecular imaging, several new metabolic markers have been developed for SPECT imaging, which promises an exciting future for the development of metabolic function imaging of the heart. These agents may potentially have a great impact on nuclear medicine departments. With the shortage of and price increases in technetium generators and greater availability of 123I with as favourable imaging characteristics as 99Tcm, several new agents are emerging for cardiac imaging. One such agent is 123I-labelled meta-iodobenzylguanidine (MIBG). MIBG is a norepinephrine (noradrenaline) analogue that is concentrated and stored in the myocardial pre-synaptic adrenergic nerve terminals in the myocardium [28]. Sympathetic innervation of the myocardium can be assessed by measuring uptake and washout of MIBG, giving insight to the state of autonomic innervation of the myocardium. The ratio of uptake in the myocardium (heart) to the mediastinum is used to semiquantitatively assess sympathetic innervation of the myocardium [28,29]. This measurement has incremental value for the assessment of symptoms and left ventricular ejection fraction in the risk stratification and prognostication of patients with cardiac failure and assessment of sympathetic innervation in various cardiac diseases [30,31]. It has been shown to predict response to β-adrenergic blockade and has been shown to be useful in better selection of patients for implantable cardioverter defibrillators [29].
Another exciting new development is the introduction of 123I-labelled 15-(p-iodophenyl)-3R,S-methylpentadecanoic acid (BMIPP), an iodinated fatty acid with high uptake and retention in myocardium suitable for SPECT imaging. Iodinated fatty acids are particularly useful for imaging because they are taken up by myocardium as normal, but are metabolised with relatively long retention in the myocardium [32]. Fatty acid is the normal substrate for energy production in the myocardium. During ischaemia, fatty acid utilisation is reduced as the myocardial metabolism shifts from fatty acid to glucose utilisation for the production of energy. This shift persists for up to 12 h after the ischaemic event has subsided; thus, BMIPP injection after the ischaemic event can be used to image the past ischaemic event (ischaemic memory imaging) [33,34]. Fatty acid imaging together with rest perfusion imaging can be used to diagnose ischaemia in suspected acute coronary syndrome presenting in emergency departments. A recent study demonstrated a sensitivity of 74% and a specificity of 92% in patients presenting to emergency units with acute coronary syndrome [35]. In theory, it is possible to perform both rest (using 99Tcm agents) and 123I fatty acid (ischaemic memory) imaging without the need for stressing the patient during the same acquisition (different energy settings) in acute coronary syndrome [36-39].
Cardiac PET and PET/CT
Cardiac PET has been in use for more than 35 years, mainly within a research setting. However, in recent years, there has been an increase in the use of cardiac PET and PET/CT for assessment of myocardial perfusion, function and viability in a clinical setting.
Its principle is based on concomitant detection of γ-rays emitted from matter/antimatter (electron/positron) annihilation, which results in 511 keV γ-rays being emitted in almost exactly opposite directions. This enables accurate localisation with better resolution and less radiation to patients. PET imaging has had a big impact on the management of patients with certain types of cancer. This has led to wider availability in the UK; however, because of cost and logistics, it is mainly centred in larger imaging departments and is not as widely available.
Radiopharmaceuticals
There are only four clinical cardiac PET tracers that are currently widely in use. Three of the tracers are used for myocardial perfusion and function assessment and only one of the tracers is for assessment of myocardial viability (Table 1) [40].
Table 1. Clinical tracers used in cardiac positron emission tomography imaging.
| Tracer | Tissue positron range (mm) | Half-life | Myocardial uptake mechanism |
| H215O | 1.1 | 2 min | Free diffusion |
| 13NH3 | 0.7 | 10 min | Diffusion/metabolic trapping |
| 82Rb | 2.6 | 78 s | Na/K-ATPase |
| 18F-FDG | 0.2 | 110 min | Glucose transport/hexokinase |
18F-FDG, fluorine-18 fluorodeoxyglucose.
Adapted from Le Guludec et al [40].
H215O (water) is a clinically inert tracer with free diffusion across capillary and cell membranes. This property makes it the ideal tracer in myocardial blood flow measurement, which will be accurate because of its high myocardial extraction across a wide range of myocardial blood flows. Unfortunately, this also leads to a high concentration in the blood pool, thus causing difficulty in visualising the myocardium. Furthermore, as it is produced in a cyclotron and has a short half-life, it is limited to centres with a readily available cyclotron.
13NH3 (ammonia) is another perfusion tracer that requires an on-site cyclotron. Nonetheless, it has an excellent first-pass extraction of 80%, with a linear uptake over a wide range of myocardial blood flows. It also produces high-quality images.
82Rb (rubidium), on the other hand, is produced in a generator, and thus is more readily available and more economical. It is a potassium analogue and thus functions similarly to thallium-201. It has a non-linear extraction pattern with increasing coronary blood flow but remains superior to 99Tcm-labelled myocardial perfusion tracers. This is the most widely used cardiac PET perfusion tracer in clinical practice. There are now two centres in the UK using this tracer in clinical practice.
Fluorine-18 fluorodeoxyglucose (18F-FDG) is a PET tracer used mainly in oncology PET imaging. However, owing to its property as a glucose analogue, it is an ideal agent for the assessment of viable myocardium. The availability of the tracer for oncology PET imaging because of its longer half-life also allows its more extensive use for cardiac PET in viability assessment despite being a cyclotron-produced tracer.
Clinical impact
The evidence for the use of cardiac PET and PET/CT in a clinical setting for the assessment of myocardial perfusion, function and viability is increasing. Cardiac PET perfusion imaging has an overall sensitivity of 92% and a specificity of 85% for the detection of coronary artery disease [41]. Sampson et al [42] have also demonstrated similar results for cardiac PET/CT perfusion with 82Rb, showing a sensitivity of 93%, a specificity of 83% and a normalcy rate of 100%. Cardiac PET/CT myocardial perfusion imaging with 82Rb also allows the assessment of left ventricular systolic function and wall motion at peak hyperaemia during the stress procedure. This further improves the diagnostic accuracy for coronary artery disease [43]. Equally, patients with a normal cardiac PET myocardial perfusion image have an excellent prognosis, whereas patients with increasing size and severity of perfusion defects demonstrate an increasing cardiac event rate [44-46].
Absolute myocardial blood flow quantification can be performed with all three cardiac PET myocardial perfusion tracers. The use of absolute quantification of myocardial blood flow enables the detection of early endothelial and vascular changes affecting flow before overt disease develops as well as detecting multivessel or left main stem coronary artery disease in situations where there is balanced flow reduction to all coronary arteries [47].
The integrated PET/CT cameras now allow anatomical as well as functional imaging of the coronary arteries to aid in the detection of coronary artery disease. Coronary calcium scoring and CT coronary angiography can now be performed in one sitting in combination with cardiac PET myocardial perfusion imaging. The CT also allows a more accurate attenuation correction to be performed on the myocardial perfusion images.
Finally, 18F-FDG PET cardiac imaging has a high sensitivity in detecting viable myocardium [48]. This has a role in clinical practice in influencing patient management and improving clinical outcome [49,50].
There is the worry of increasing cost with the use of cardiac PET and PET/CT for assessment of coronary artery disease with the more expensive PET tracers and PET/CT machines. Nonetheless, both cardiac PET and PET/CT for myocardial perfusion imaging with 82Rb and myocardial viability assessment with 18F-FDG have been shown to be equally cost-effective for the management of patients with suspected coronary artery disease [51-53].
Myocardial perfusion scintigraphy and NICE guidelines
In 2003, the NICE in the UK recommended myocardial perfusion scintigraphy as a gatekeeper for angioplasty [54]. It was shown to be cost-effective both by reducing the number of unnecessary angiographies and by directing the planning for appropriate angioplasty. The use of myocardial perfusion scintigraphy has spiralled in the UK since the recommendation despite relatively limited availability of funding and equipment for this procedure. The more recent NICE guidelines [2] for patients who present for the first time with acute chest pain have incorporated the new developments in imaging, in particular coronary CT calcium scoring, streamlining the management of these patients. Coronary calcium scoring for initial stratification is the first-line investigation for these patients, rather than the exercise tolerance test, which has traditionally been the most common first investigation. Functional imaging remains an important step in further stratification of some of these patients with positive calcium scoring. Functional imaging remains crucial for the diagnosis of a large group of these patients. The new guidelines do not address patients with known coronary disease, and functional imaging continues to be invaluable in assessment of this group and has not changed.
There are now several modalities for assessment of functional imaging. All have their advantages and disadvantages and all are in constant development. It is now possible to assess myocardial perfusion using cardiac magnetic resonance (CMR) technology, or indeed contrast CT coronary angiographic (CTCA) imaging. During the last decade, CMR has developed from a research tool to a practical diagnostic tool. Its strength is superb structural detail, with growing functional information, and it has the advantage of not using ionising radiation. However, it is still relatively labour intensive and has long procedure times. CTCA has meanwhile developed into a very fast technique (imaging the heart in one heart beat or in a breath-hold), with a reduced radiation dose for structural imaging. These technologies have great promise and will no doubt soon be incorporated in the routine management of patients with ischaemia and heart failure.
Myocardial perfusion scintigraphy is a well-established technique that has a tremendous wealth of literature to support its use in management, stratification and prognostication of patients with ischaemia and heart failure. With the use of new technology, it is now possible to obtain more functional information, with better resolution and less radiation, and with faster scintigraphy (less than 5 min) it is now possible to offer the test with same-day results on demand in the clinic. Unlike the other modalities in which stressing and contrast injection is performed on the camera systems, the radiopharmaceuticals can be injected before the patient comes to the camera, thus minimising the camera time, increasing patient turnover and also minimising patient discomfort. New pharmaceuticals will see the development of this technology for imaging heart failure and in emergency admissions. Hybrid cameras, on the other hand, will enable better streamlining of ischaemic patient investigations with a one-stop diagnostic visit. It is conceivable to envisage diagnostic algorithms for selected patients to have calcium scoring and, if necessary, perfusion CTCA or both in one “sitting”.
Cardiac imaging is developing fast. There are now several options available, all with their own strengths and weaknesses. The role of each modality will be changing and evolving. Nuclear (molecular) cardiac imaging is also evolving and, hand-in-hand with the other technologies, will continue to play a major role in the management of patients with cardiac disease.
References
- 1.Vitola JV, Shaw LJ, Allam AH, Orellana P, Peix A, Ellmann A, et al. Assessing the need for nuclear cardiology and other advanced cardiac imaging modalities in the developing world. J Nucl Cardiol 2009;16:956–61 [DOI] [PubMed] [Google Scholar]
- 2.National InstituteforHealthandClinicalExcellence Chest pain of recent onset: assessment and diagnosis of recent onset. NICE Clinical Guideline 95. London, UK: NICE, 2010 [Google Scholar]
- 3.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarctions. Circulation 1998;97:535–43 [DOI] [PubMed] [Google Scholar]
- 4.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–7 [DOI] [PubMed] [Google Scholar]
- 5.Underwood SR, Godman B, Salyani S, Ogle J, Ell PJ. Economics of myocardial perfusion imaging in Europe: the EMPIRE study. Eur Heart J 1999;20:157–66 [DOI] [PubMed] [Google Scholar]
- 6.Anagnostopoulos C, Harbinson M, Kelion A, Kundley K, Loong CY, Notghi et al. Procedure guidelines for radionuclide myocardial perfusion imaging. Heart 2004;90(Suppl. 1):i1–i10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesse B, Tägil K, Cuocolo A, Anagnostopoulos C, Bardiés M, Bax J, et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging 2005;32:855–97 [DOI] [PubMed] [Google Scholar]
- 8.Strauss HW, Miller DD, Wittry MD, Cerqueira MD, Garcia EV, Iskandrian AS, et al. Procedure guideline for myocardial perfusion imaging 3.3. J Nucl Med Tech 2008;36:155–61 [DOI] [PubMed] [Google Scholar]
- 9.Garcia EV. SPECT attenuation correction: an essential tool to realize nuclear cardiology's manifest destiny. J Nucl Cardiol 2007;14:16–24 [DOI] [PubMed] [Google Scholar]
- 10.Lebowitz E, Greene MV, Fairchild R, Bradley-Moore PR, Atkins HL, Ansari AN, et al. Thallium-201 for medical use. I. J Nucl Med 1975;16:151–5 [PubMed] [Google Scholar]
- 11.Beller GA, Watson DD, Ackell P, Pohost GM. Time course of thallium-201 redistribution after transient myocardial ischemia. Circulation 1980;61:791–7 [DOI] [PubMed] [Google Scholar]
- 12.Dahlber ST. Assessment of myocardial perfusion with Tc-99m: Image is everything. J Nucl Cardiol 2009;16:493–6 [DOI] [PubMed] [Google Scholar]
- 13.Watson DD, Campbell NP, Read EK. Spatial and temporal quantification of plane thallium myocardial images. J Nucl Med 1981;22:577–84 [PubMed] [Google Scholar]
- 14.Garcia E, Maddahi J, Berman D, Waxman A. Space/time quantification of thallium-201 myocardial scintigraphy. J Nucl Med 1981;22:309–17 [PubMed] [Google Scholar]
- 15.Iskandrian AS, Verani MS, Heo JY. Pharmacologic stress testing: mechanism of action, haemodynamic responses, and results in detection of coronary disease. J Nucl Cardiol 1994;1:94–111 [DOI] [PubMed] [Google Scholar]
- 16.Sharir T, Slomka PJ, Berman DS. Solid-state SPECT technology: fast and furious. J Nucl Cardiol 2010;17:890–6 [DOI] [PubMed] [Google Scholar]
- 17.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, et al. Single photon-emission computed tomography. J Nucl Cardiol 2010;17:941–73 [DOI] [PubMed] [Google Scholar]
- 18.Gaemperli O, Kaufmann P. 2006 Image of the Year: focus on cardiac SPECT/CT. J Nucl Med 2006;47:14N. [PubMed] [Google Scholar]
- 19.Di Carli MF, Dorbala S, Meserve J, El-Fakhri G, Sitek A, Moore SC. Clinical myocardial perfusion PET/CT. J Nucl Med 2007;48:783–93 [DOI] [PubMed] [Google Scholar]
- 20.Garcia EV, Cooke CD, Folks RD, Santana CA, Krawczynska EG, De Braal L, et al. Diagnostic performance of an expert system for the interpretation of myocardial perfusion SPECT studies. J Nucl Med 2001;42:1185–91 [PubMed] [Google Scholar]
- 21.Sciagrà R. The expanding role of left ventricular functional assessment using gated myocardial perfusion SPECT: the supporting actor is stealing the scene. Eur J Nucl Med Mol Imaging 2007;34:1107–22 [DOI] [PubMed] [Google Scholar]
- 22.Schindler TH, Quercioli A. Left ventricular dyssynchrony assessment by phase analysis from gated myocardial perfusion SPECT: moving beyond conventional criteria. Heart 2011;97:4–5 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Boogers MM, Bax JJ, Soman P, Garcia EV. The use of nuclear imaging for cardiac resynchronisation therapy. Curr Cardiol Rep 2010;12:185–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boogers MJ, Chen J, van Bommel RJ, Borleffs CJW, Dibbets-Schneider P, van derHiel B, et al. Optimal left ventricular lead position assessed with phase analysis on gated myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging 2011;38:230–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uebleis C, Ulbrich M, Tegtmeyer R, Schuessler F, Haserueck N, Siebermair J, et al. Electrocardiogram-gated 18F-FDG PET/CT hybrid imaging in patients with unsatisfactory response to cardiac resynchronisation therapy: initial clinical results. J Nucl Med 2011;52:67–71 [DOI] [PubMed] [Google Scholar]
- 26.Bilchick KC. Single photon emission computed tomography (SPECT) techniques for resynchronisation: phase analysis and equilibrium radionuclide angiocardiography. J Nucl Cardiol 2011;18:16–20 [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Chen L, Liu S, Barber C, Stevenson GD, Furenlid LR, et al. Kinetic characterization of a novel cationic 99mTc(I)-tricarbonyl complex, 99mTc-15C5-PNP, for myocardial perfusion imaging. J Nucl Cardiol 2010;17:858–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel AD, Iskandrian AE. MIBG imaging. J Nucl Cardiol 2002;9:75–94 [DOI] [PubMed] [Google Scholar]
- 29.Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, et al. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging 2008;35:535–46 [DOI] [PubMed] [Google Scholar]
- 30.Flotats A, Carrió I. Cardiac neurotransmission SPECT imaging. J Nucl Cardiol 2004;11:587–602 [DOI] [PubMed] [Google Scholar]
- 31.Henneman MM, Bengel FM, van derWall EE, Knuuti J, Bax JJ. Cardiac neuronal imaging: application in the evaluation of cardiac disease. J Nucl Cardiol 2008;15:442–55 [DOI] [PubMed] [Google Scholar]
- 32.Goodman MM, Kirsch G, Knapp FF., Jr Synthesis and evaluation of radioiodinated terminal p-iodophenyl-substituted alpha- and beta-methyl-branched fatty acids. J Med Chem 1984;27:390–7 [DOI] [PubMed] [Google Scholar]
- 33.Herrero P, Gropler RJ. Imaging of myocardial metabolism. J Nucl Cardiol 2005;12:345–58 [DOI] [PubMed] [Google Scholar]
- 34.Dilsizian V, Bateman TM, Bergmann SR, Des Prez R, Magram MY, Goodbody AE, et al. Metabolic imaging with β-methyl-p-[123I]-iodophenyl-pentadecanoic acid identifies ischemic memory after demand ischemia. Circulation 2005;112:2169–74 [DOI] [PubMed] [Google Scholar]
- 35.Kawai Y, Tsukamoto E, Nozaki Y, Morita K, Sakurai M, Tamaki N. Significance of reduced uptake of iodinated fatty acid analogue for the evaluation of patients with acute chest pain. J Am Coll Cardiol 2001;38:1888–94 [DOI] [PubMed] [Google Scholar]
- 36.Mahmarian JJ. Myocardial metabolic imaging in the acute care setting. J Nucl Cardiol 2007;14:S139–44 [DOI] [PubMed] [Google Scholar]
- 37.Tamaki N, Yoshinaga K. Novel iodinated tracers, MIBG and BMIPP, for nuclear cardiology. J Nucl Cardiol 2011;18:135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastore CJ, Babich JW, Udelson JE. Future directions of myocardial fatty acid imaging. J Nucl Cardiol 2007;14:S153–63 [DOI] [PubMed] [Google Scholar]
- 39.Tamaki N, Morita K, Kawai Y. The Japanese experience with metabolic imaging in the clinical setting. J Nucl Cardiol 2007;14:S145–52 [DOI] [PubMed] [Google Scholar]
- 40.Le Guludec D, Lautamäki R, Knuuti J, Bax J, Bengel F. Present and future of clinical cardiovascular PET imaging in Europe: a position statement by the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging 2008;35:1709–24 [DOI] [PubMed] [Google Scholar]
- 41.Kiran RN, Ben AD, Asim FC, Sirisha RN, Priya R, Ruth CC. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol 2008;15:444–51 [DOI] [PubMed] [Google Scholar]
- 42.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol 2007;49:1052–8 [DOI] [PubMed] [Google Scholar]
- 43.Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med 2007;48:349–58 [PubMed] [Google Scholar]
- 44.Chow BJW, Wong JW, Yoshinaga K, Ruddy TD, Williams K, deKemp RA, et al. Prognostic significance of dipyridamole-induced ST depression in patients with normal 82Rb PET myocardial perfusion imaging. J Nucl Med 2005;46:1095–101 [PubMed] [Google Scholar]
- 45.Marwick TH, Shan K, Patel S, Go RT, Lauer MS. Incremental value of rubidium-82 positron emission tomography for prognostic assessment of known or suspected coronary artery disease. Am J Cardiol 1977;80:865–70 [DOI] [PubMed] [Google Scholar]
- 46.Yoshinaga K, Chow BJW, Williams K, Chen L, deKemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol 2006;48:1029–39 [DOI] [PubMed] [Google Scholar]
- 47.Knuuti J, Kajander S, Mäki M, Ukkonen H. Quantification of myocardial blood flow will reform the detection of CAD. J Nucl Cardiol 2009;16:497–506 [DOI] [PubMed] [Google Scholar]
- 48.Schinkel AFL, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Prob Cardiol 2007;32:375–410 [DOI] [PubMed] [Google Scholar]
- 49.Tarakji KG, Brunken R, McCarthy PM, Al-Chekakie MO, Abdel-Latif A, Pothier CE, et al. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation 2006;113:230–7 [DOI] [PubMed] [Google Scholar]
- 50.Beanlands RSB, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. F-18-Fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol 2007;50:2002–12 [DOI] [PubMed] [Google Scholar]
- 51.Merhige ME, Breen WJ, Shelton V, Houston T, D'Arcy BJ, Perna AF. Impact of myocardial perfusion imaging with PET and 82Rb on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. J Nucl Med 2007;48:1069–76 [DOI] [PubMed] [Google Scholar]
- 52.Patterson RE, Eisner RL, Horowitz SF. Comparison of cost-effectiveness and utility of exercise ECG, single photon emission computed tomography, positron emission tomography, and coronary angiography for diagnosis of coronary artery disease. Circulation 1995;91:54–65 [DOI] [PubMed] [Google Scholar]
- 53.Jacklin PB, Barrington SF, Roxburgh JC, Jackson G, Sariklis D, West PA, et al. Cost-effectiveness of preoperative positron emission tomography in ischemic heart disease. Ann Thorac Surg 2002;73:1403–9 [DOI] [PubMed] [Google Scholar]
- 54.National InstituteforHealthandClinicalExcellence Myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Technology Appraisal 73. London, UK: NICE, 2003 [Google Scholar]