Abstract
Objectives
The purpose of this study was to characterise dose distribution in linear accelerator-based intracranial stereotactic radiosurgery using the dynamic conformal arc technique, and to validate the pertinence of dose prescription to the specific percentage isodose surface (IDS).
Methods
73 plans for brain metastases were reviewed and replanned with a uniform method for target definition and treatment planning.
Results
In all cases except 1 the dose prescription to the 80% IDS satisfied the criteria of the standardised prescription IDS as previously proposed. However, both of the planning target volume (PTV) coverage values for the 80% and 90% IDSs and the PTV D99 and D95 (IDS receiving at least 99% or 95% of the PTV) were inconsistent and significantly increased as a function of the PTV size. The 80% IDS for a PTV of more than 5 cm3 achieved adequate PTV coverage without a leaf margin. The dose conformity for 80% IDS gradually worsened as the PTV increased, whereas that for the PTV D99 or D95 improved as a function of the PTV size. The addition of a leaf margin attained 100% PTV coverage for 80% IDS, while leading to a poorer dose conformity.
Conclusion
The dose prescription to the specific percentage IDS does not necessarily guarantee consistent target coverage, D99 and D95, and desirable dose conformity in proportion to the target volume. The dose prescription and evaluation at the specific target coverage would therefore be preferable as an objective method in order to report the “marginal dose” and to clearly compare the planning parameters with those from other modalities.
Linear accelerator-based stereotactic radiosurgery (SRS) for intracranial lesions has increased in sophistication with the incorporation of the micro-multileaf collimator (mMLC) and the advent of the dynamic conformal arc (DCA) technique, which simplified treatment planning and improved both dose conformity and homogeneity [1,2]. The DCA technique is a forward-planning method using multiple non-coplanar arcs rotating around a single isocentre, in which the mMLC continuously changes its aperture to conform the beam to the target with every 10° of arc [1,2].
In the DCA technique, the dose distribution can be influenced by several factors, including the number, length and table position of the arcs, the collimator angle, the leaf margin and the position of the reference point as well as the target shape and its proximity to an organ-at-risk (OAR) [3,4]. The proximity of the target to an OAR may influence the target coverage for the intended marginal dose to maintain the dose constraint for the OAR. The dose prescription has been commonly defined at the specific percentage isodose surface (IDS) (e.g. 80% or 90%) normalised to 100% at the isocentre in many institutions [5-12]. Because the planning methods and the selection of the prescription IDS have been left to the discretion of each institution, a substantial variability has been observed in the method of dose prescription and/or in the assessment of a “marginal dose” [1,5-12]. Therefore, the optimal methods for dose prescription or treatment planning have remained uncharacterised. In general, the intended marginal dose for SRS planning does not necessarily correspond to the dose that indicates the specific target coverage (e.g. D95). There remains some doubt whether treatment planning based on the dose prescription to the specific percentage IDS in DCA planning would guarantee a uniform dose distribution to the target periphery.
We herein describe the dose distribution characteristics and the inclination in proportion to the target volume and the depth from the skin surface in the DCA planning, under the stipulation that the uniform methods were applied to the target definition and the treatment planning for subjects that were relatively simple in shape. More specifically, we evaluated the planning target volume (PTV) and clinical target volume (CTV) coverage values for the specific percentage IDSs, the dose indicating the specific target coverage, the dose conformity for each IDS selection and the dose homogeneity in proportion to the target volume and the depth. The propriety with the routine application of a leaf margin was also examined. Through these analyses, we validated the pertinence of dose prescription to the specific percentage IDS and sought an objective method of marginal dose evaluation in DCA planning.
Methods and materials
Study population
We selected 73 lesions from a database of patients harbouring brain metastases who had been treated with the DCA technique between 2005 and 2009 (Table 1). The selected lesions were intended to represent the wide range of PTVs between 0.5 cm3 and 20 cm3, and of depths from the skin surface, which were nearly spherical or ellipsoidal in shape, in order to elucidate the fundamental dosimetric characteristics of the DCA plans. All lesions were regarded as those that did not abut any critical structure (Table 1). Treatment cases for the post-operative tumour cavity or tumours concomitant with the leptomeningeal spread were excluded because of the complexity in shape.
Table 1. Descriptive statistics of treatment parameters.
| Median (IQR) |
Range | Normalitya | |
| (Mean ± SD)b | |||
| CTV | 3.11 (1.27, 5.73) | 0.14–12.23 | <0.001 |
| PTV | 5.80 (2.89, 9.91) | 0.53–19.42 | <0.001 |
| Average tissue depth (mm) | 73.50 (59.60, 94.80) | 39.70–119.90 | 0.011 |
| PTV coverage (%) by 80% IDS | 98.9 (98.46, 99.73) | 93.64–99.98 | <0.001 |
| PTV coverage (%) by 90% IDS | 84.88 (75.81, 89.13) | 58.80–94.72 | <0.001 |
| Difference (%)c | 14.53 (10.68, 21.72) | 5.26–36.20 | <0.001 |
| PTV D99 (%) | 82.00 (79.00, 83.00) | 76.00–86.00 | 0.035 |
| PTV D95 (%) | 86.00 (83.00, 87.00) | 79.00–90.00 | 0.006 |
| CTV coverage (%) by 90% IDS | 99.93 (99.79, 100) | 98.74–100 | <0.001 |
| CTV D99 (%) | 92.00 (91.00, 92.50) | 90.00–94.00 | 0.004 |
| CTV D95 (%) | 93.50 (93.00, 94.00) | 92.00–95.50 | 0.001 |
| Difference (%) of D99d | 10.77 ± 2.26b | 7.00–17.00 | 0.056 |
| Difference (%) of D95d | 7.50 (6.38, 10.25) | 4.50–14.25 | 0.001 |
| Dmax (%) | 102.00 (101.00, 103.00) | 101.00–106.00 | <0.001 |
| HI (Dmax/D99) | 1.26 ± 0.03b | 1.17–1.33 | 0.416 |
| PITV (80% IDS) | 1.31 ± 0.06b | 1.16–1.46 | 0.303 |
| PITV (D99) | 1.26 (1.23, 1.32) | 1.15–1.47 | 0.007 |
| PITV (D95) | 1.11 (1.07, 1.14) | 1.02–1.24 | 0.016 |
CTV, clinical target volume; D99, D95, IDS (%) receiving at least 99% or 95% of the target volume; Dmax, maximum dose; HI (Dmax/D99), homogeneity index defined as the ratio of Dmax to D99; IDS, isodose surface; IQR, interquartile range; PITV, ratio of prescription isodose volume/target volume; PTV, planning target volume; SD, standard deviation.
aThe p-values were the results from the Shapiro–Wilk tests using to examine the normality of distribution of variables. Significant results are shown in bold, in which the hypothesis of normal distribution was dismissed.
bVariables with normal distribution.
cDifference of the PTV coverage of the 80% IDS minus that of the 90% IDS.
dDifference of the D99 or D95 between CTV and PTV.
Treatment system and planning technique
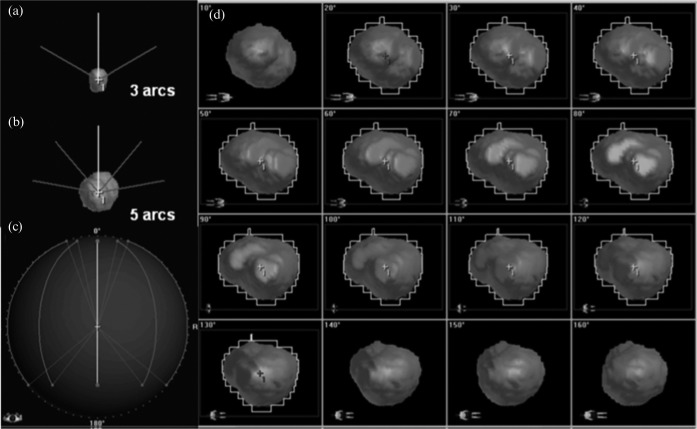
The treatment system included a 3 mm central leaf width mMLC (m3: BrainLAB AG, Feldkirchen, Germany) as an add-on device on the non-dedicated linear accelerator (Clinac 21EX, Varian, Palo Alto, CA) with 6 MV photon energy (dose rate 600 MU min–1) [13]. The treatment planning system (TPS) used was the BrainSCAN version 5.3 (BrainLAB). For purposes of maintaining study consistency, all target definition and DCA planning were reviewed and reperformed using the same methods as follows. Stereotactically localised CT scans were obtained with contiguous 2 mm slices. T1 weighted post-contrast MRI was acquired with 2 mm slices without fiducial markers, and co-registered with the CT scans using a mutual information-based algorithm implemented in the TPS. The CTV was defined as an enhanced lesion on MRI. The CTV was expanded to a PTV with a 2 mm isotropic margin considering the practical set-up uncertainty [14]. The number of arcs per plan used was 3 for a PTV of less than 5 cm3 and 5 for those of more than or equal to 5 cm3, in which the table position was set at 30°, 90° and 300° for 3 arcs (the modified default), and 10°, 50°, 90°, 310°, and 350° for 5 arcs (Figure 1a, b). The arc length (i.e. the range between the start and stop angles of the gantry) was set at 110° (20–130° or 230–340°) (Figure 1c). The collimator angle in all arcs was set at 90° in order to secure the clearance between the mMLC and the patient (Figure 1d). The leaf edge was adapted to the outline of the PTV without any leaf margin between the leaf edge and the outline of the PTV (Figure 1d), unless otherwise indicated. All treatment plans were normalised to the 100% IDS at the geometric isocentre of the PTV. To circumvent any dose interference for the simultaneous treatment of multiple targets, all cases were planned as a single lesion. To evaluate of the effect of leaf margin, we arbitrarily selected 30 lesions from the aforementioned 73 lesions and generated plans with the addition of a 1 mm leaf margin.
Figure 1.
The arc arrangement and the example of the beam's eye view (BEV) for the dynamic conformal arc plans. Frontal views of the three-arc plan (a) and five-arc plan (b) reveal the table position, and the superior view of the five-arc plan (c) denotes the arc length. The BEV (d) from the arc with a table position of 90° in the plan for planning target volume of 15.66 cm3 demonstrated the aperture of the micro-multileaf collimator that changed for each 10° interval along with the altered projection of the orientation figures displayed at the left lower corners.
Dose volume histogram analyses
The dose calculation was based on a pencil-beam algorithm with the radiological path length for tissue heterogeneity correction. The grid size for the dose volume histogram (DVH) calculation was set to 1.0 mm and the adaptive grid size was applied to small lesions to ensure at least 10 voxels for each dimension inside the PTV.
The average tissue depth (ATD) was the average distance from the skin surface to the isocentre along the beam path, in which the variation in the density of the tissue along the beam path was taken into account.
The PTV coverage was evaluated as a percentage of the PTV encompassed by the 80% and 90% IDSs, respectively. The CTV coverage for the 90% IDS was also measured. The D99 and D95 corresponded to the IDS (%) encompassing at least 99% and 95% of the PTV or CTV, respectively.
The dose homogeneity index (HI) within the PTV was defined as the ratio of the maximum dose within the PTV (Dmax) to the PTV D99, and referred to here as the HI (Dmax/D99).
To evaluate the dose conformity, the PITV (prescription isodose volume/target volume) value from the Radiation Therapy Oncology Group (RTOG) [15,16] was calculated for the 80% IDS, D99 and D95. The PITV was defined as the ratio of the total volume encompassed by the reference IDS to the target volume (PTV), in which the values of 1.0, 0.99 and 0.95 were considered to be perfect for the 80% IDS, D99 and D95, respectively.
Statistical analyses
Statistical analyses were performed using the PASW Statistics 18 (SPSS Inc, Chicago, IL) unless otherwise noted. The Shapiro–Wilk test was used to examine the normality of the data distribution. Many variables were observed to depart substantially from a normal distribution, and the hypotheses of normal distribution were dismissed by the Shapiro–Wilk test (Table 1). Therefore, we used non-parametric tests for the following analyses. Spearman's rank correlation coefficient was applied to evaluate any correlations between the variables. In selected cases, linear or logarithmic curve fitting was also applied according to the data distribution on the scatter plots using Microsoft Excel (Microsoft, Redmond, WA). The correlation coefficient R2 values were used to evaluate the goodness-of-fit for each function. The Wilcoxon signed rank test was applied to compare paired variables. All p-values reported were calculated with two-sided tests, and a p-value of less than 0.05 was considered to be statistically significant.
Results
Sensitivity of dosimetric parameters to the target volume and the average tissue depth
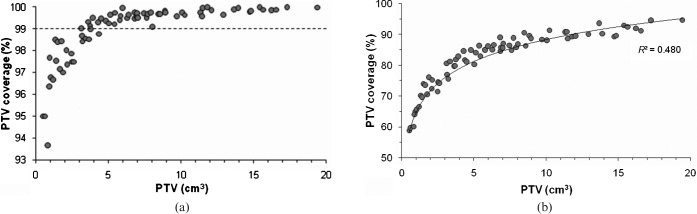
No significant correlation was found between the PTV and the ATD. The 80% IDSs without a leaf margin achieved adequate PTV coverage (>99%) in cases with a PTV of more than 5 cm3, whereas those with a PTV of less than 5 cm3 decreased as the PTV decreased (Figure 2a). Therefore, the PTV coverage for the 80% IDS were sensitive to the PTV (Table 2).
Figure 2.
Planning target volume (PTV) vs the PTV coverage for the 80% (a) or 90% (b) isodose surface. The dashed line indicates the level of the 99% PTV coverage. The solid lines denote the fit of the logarithmic curves with the correlation coefficient R2.
Table 2. Spearman's rank correlation coefficients for planning target volume (PTV) and other parameters.
| Parameters | rho | p-value |
| Average tissue depth (mm) | 0.051 | 0.668 |
| PTV coverage (%) by 80% IDS | 0.923 | <0.001 |
| PTV coverage (%) by 90% IDS | 0.973 | <0.001 |
| Difference (%)a | −0.969 | <0.001 |
| PTV D99 (%) | 0.932 | <0.001 |
| PTV D95 (%) | 0.959 | <0.001 |
| CTV coverage (%) by 90% IDS | −0.006 | 0.962 |
| CTV D99 (%) | 0.116 | 0.328 |
| CTV D95 (%) | 0.185 | 0.118 |
| Difference (%) of D99b | −0.969 | <0.001 |
| Difference (%) of D95b | −0.971 | <0.001 |
| Dmax (%) | 0.731 | <0.001 |
| HI (Dmax/D99) | −0.737 | <0.001 |
| PITV (80% IDS) | 0.731 | <0.001 |
| PITV (D99) | −0.584 | <0.001 |
| PITV (D95) | −0.524 | <0.001 |
CTV, clinical target volume; Dmax, maximum dose; HI (Dmax/D99), homogeneity index defined as the ratio of Dmax to D99; IDS, isodose surface; PITV, ratio of prescription isodose volume/target volume; PTV, planning target volume.
Significant results are shown in bold.
aDifference of the PTV coverage of the 80% IDS minus that of the 90% IDS.
bDifference of the D99 or D95 between CTV and PTV.
When the dose prescription was assumed to the 80% IDS, the only case with a PTV of 0.84 cm3 could not satisfy the criteria of the standardised prescription IDS as recently proposed by Hazard et al [,1] and Chern et al [17], in which the target coverage for the reference dose should be greater than or equal to the D95, and the D99 should be at least 95% of the reference dose. In the only case with a PTV of 0.84 cm3, the PTV coverage for the 80% IDS was found to be 93.64% (<95%), while the PTV D99 was only 95% of the 80% IDS (76%).
The PTV coverage for the 90% IDS also significantly increased as a function of PTV (Figure 2b). No significant correlation was observed between the ATD and the PTV coverage values for the 80% or 90% IDSs (Table 3). The differences of the PTV coverage between the 80% and 90% IDS decreased as the PTV increased (Table 2).
Table 3. Spearman's rank correlation coefficients for the average tissue depth and other parameters.
| Parameters | rho | p-value |
| PTV coverage (%) by 80% IDS | 0.156 | 0.189 |
| PTV coverage (%) by 90% IDS | 0.167 | 0.159 |
| PTV D99 (%) | 0.204 | 0.083 |
| PTV D95 (%) | 0.224 | 0.057 |
| CTV coverage (%) by 90% IDS | 0.488 | <0.001 |
| CTV D99 (%) | 0.711 | <0.001 |
| CTV D95 (%) | 0.700 | <0.001 |
| Dmax (%) | −0.492 | <0.001 |
| HI (Dmax/D99) | −0.482 | <0.001 |
| PITV (80% IDS) | 0.115 | 0.334 |
| PITV (D99) | −0.212 | 0.072 |
| PITV (D95) | −0.365 | 0.001 |
CTV, clinical target volume; Dmax, maximum dose; HI (Dmax/D99), homogeneity index defined as the ratio of Dmax to D99; IDS, isodose surface; PITV, ratio of prescription isodose volume/target volume; PTV, planning target volume.
Significant results are shown in bold.
Strongly significant correlations were observed between PTV D99 or D95 and the PTV, in which both the PTV D99 and D95 were directly proportional to the PTV (Table 2). Figure 3 shows the scatter plots of the PTV D99 and D95 vs the PTV, into which the logarithmic curves fitted with high correlation coefficients of 0.9147 and 0.8628, respectively.
Figure 3.
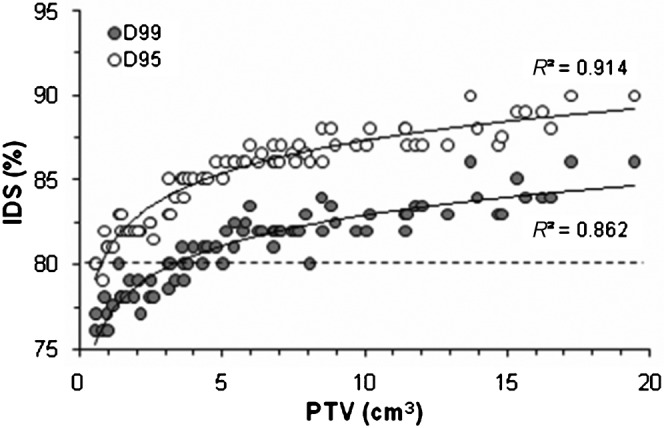
Planning target volume (PTV) vs the isodose surface (IDS) indicating the PTV D99 and D95. The dashed line denotes the 80% IDS. The solid lines indicate the fit of the logarithmic curves with the correlation coefficient R2.
The differences of the D99 or D95 between the PTV and CTV were significantly correlated with the PTV, and these values decreased as the PTV increased (Table 2). The CTV coverage for the 90% IDS and the CTV D99 and D95 significantly correlated with the ATD, but did not with the PTV (Table 3). Figure 4 shows the scatter plots of the CTV D99 vs the ATD, thus indicating that the CTV D99 values were directly proportional to the ATD.
Figure 4.
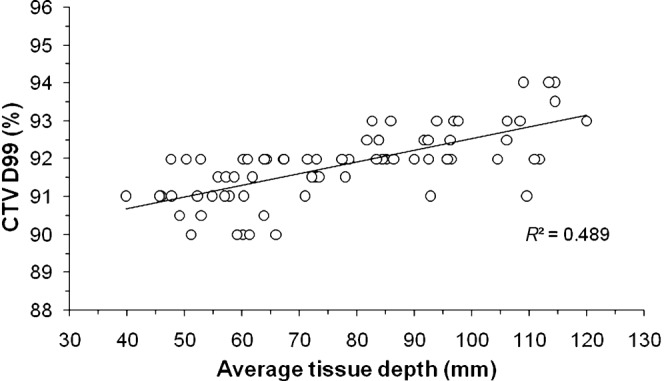
The average tissue depth vs the clinical target volume D99. The solid line represents the linear curve fit with the correlation coefficient R2.
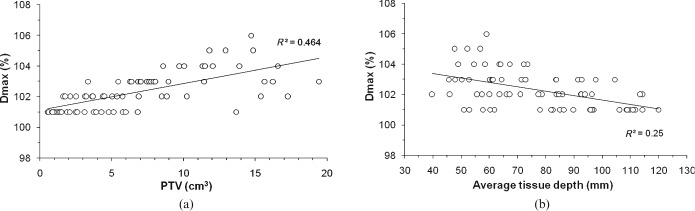
Factors influencing dose homogeneity
Significant correlations were observed between Dmax and the PTV or the ATD (Tables 2, 3). Dmax increased as the PTV increased, whereas Dmax decreased as a function of the ATD (Figure 5). The HI (Dmax/D99) values were inversely proportional to the PTV and the ATD (Tables 2, 3).
Figure 5.
Planning target volume (PTV) (a) and the average tissue depth (b) vs the PTV Dmax. The solid lines indicate the fit of the linear curves with the correlation coefficient R2.
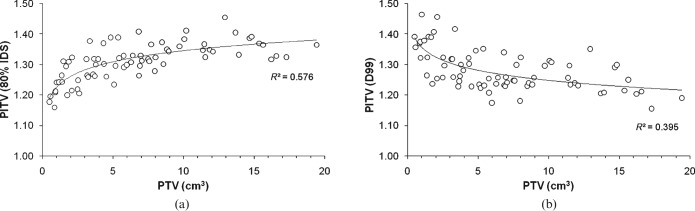
The influence of the IDS selection on the correlation of dose conformity with PTV or the average tissue depth
The PITV values calculated at the 80% IDS, D99, and D95 significantly correlated with the PTV (Table 1). The PITV values for the 80% IDS worsened as the PTV increased, whereas those for the PTV D99 and D95 improved as the PTV increased (Figure 6). The PITV values for the D95 were also significantly correlated with the ATD, whereas no significant correlation was found between the ATD and the PITV values for the 80% IDS or D99 (Table 3).
Figure 6.
Planning target volume (PTV) vs ratio of prescription isodose volume/target volume (PITV) for the 80% isodose surface (a) and D99 (b). The solid lines represent the logarithmic curves fit with the correlation coefficient R2.
Significance of leaf margin for the target coverage and conformity
To examine the effect of the leaf margin, we compared the 30 plans with or without a 1 mm leaf margin (LM 1 plans vs LM 0 plans) (Table 4). The 80% IDS for the LM 1 plans achieved 100% PTV coverage in all cases. The PTV coverage for the 90% IDS and the PTV D99 and D95 in the LM 1 plans were significantly higher than those for the LM 0 plans. Although the median PTV coverage for the 90% IDS in the LM 1 plans was higher than the PTV D95, a wide range in the values was still observed. The interquartile ranges of the PTV D99 and D95 in the LM 1 plans were smaller than those for the LM 0 plans.
Table 4. Differences of several dosimetric parameters for plans with or without a 1 mm leaf margin.
| LM 0 mm |
LM 1 mm |
||
| Median (IQR) |
Median (IQR) |
Difference (p-value)a | |
| Range | Range | ||
| PTV coverage (%) by 80% IDS | 99.58 (98.54, 99.84) | (—) | NA |
| 95.00–99.97 | 100 | ||
| PTV coverage (%) by 90% IDS | 85.78 (79.07, 90.43) | 96.57 (94.22, 98.17) | 10.79 (<0.001) |
| 58.80–94.66 | 91.86–99.64 | ||
| D99 (%) | 82.00 (79.75, 83.50) | 88.00 (87.00, 89.00) | 6 (<0.001) |
| 76.00–86.00 | 86.00–91.00 | ||
| D95 (%) | 86.00 (83.75, 88.00) | 90.50 (89.88, 91.63) | 4.5 (<0.001) |
| 80.00–90.00 | 89.00–93.50 | ||
| PITV (80%) | 1.32 (1.28, 1.35) | 1.71 (1.66, 1.80) | 0.39 (<0.001) |
| 1.18–1.41 | 1.56–2.12 | ||
| PITV (D99) | 1.26 (1.23, 1.31) | 1.33 (1.26, 1.38) | 0.07 (<0.001) |
| 1.15–1.47 | 1.17–1.56 | ||
| PITV (D95) | 1.10 (1.07, 1.13) | 1.17 (1.13, 1.21) | 0.07 (<0.001) |
| 1.02–1.24 | 1.06–1.27 |
IDS, isodose surface; IQR, interquartile range; LM, leaf margin; NA, not assessed; PITV, ratio of prescription isodose volume/target volume; PTV, planning target volume.
Significant results are shown in bold.
aDifferences between the variables are represented as the median value for the 1 mm leaf margin plan minus that without leaf margin. The p-values were the results from Wilcoxon signed-rank test.
Despite adequate PTV coverage, the PITV values at the 80% IDS for the LM 1 plans were significantly worse than for the LM 0 plans, and in contrast to the LM 0 plans, the PITV values at the 80% IDS for the LM 1 plans decreased as a function of the PTV (Figure 7). In addition, the PITV values at the PTV D99 and D95 for the LM 1 plans were significantly inferior to those for the LM 0 plans.
Figure 7.
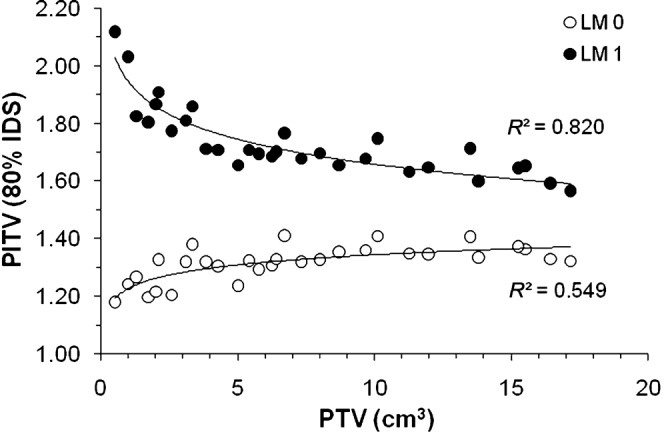
Planning target volume (PTV) vs ratio of prescription isodose volume/target volume (PITV) for the 80% isodose surface in plans with or without a 1 mm leaf margin. The solid lines indicate the fit of the logarithmic curves with the correlation coefficient R2. LM 0, plans without leaf margin; LM 1, plans with a 1 mm leaf margin.
Discussion
Is dose prescription to the specific percentage IDS pertinent in the DCA?
The present study demonstrated that the PTV coverage values for the specific percentage IDSs (80% and 90%) were inconsistent and varied in proportion to the PTV, on the stipulation that uniform planning methods were applied. Because the minimum dose (Dmin) derived from the DVH for the PTV or CTV can occur in single voxels of clinically insignificant volume, the D99 is considered to be a sufficient alternative to Dmin [18]. Considering the above, the PTV coverage values for the 80% IDS in plans without a leaf margin are regarded to be adequate (>99%) for a PTV of more than 5 cm3, whereas those for a PTV of less than 5 cm3 achieved at least 95% in most cases. Regarding the dose conformity, it is undesirable that the dose conformity for the 80% IDS worsens as a function of PTV (Figure 6a). There is still room for improvement in the PITV values, especially for a PTV value of more than 5 cm3. Meanwhile, the dose conformity for the IDS indicating the PTV D99 improved as a function of PTV (Figure 6b). The IDS indicating the PTV D99 may therefore be more suitable than the 80% IDS between the adequate PTV coverage and conformity. Several planners may infer that the IDS denoting the PTV D95 is pertinent for dose prescription, considering the 2 mm PTV margin. Of note, the IDSs indicating the PTV D99 or D95 also significantly varied as a function of the PTV (Figure 3, Table 4). The IDS for constant PTV coverage should be selected in proportion to the PTV. Because the PTV D95 has been also an objective measure for the dose prescription, especially in the intensity-modulated radiotherapy (IMRT) plans [18], the dose prescription and evaluation at the PTV D99 or D95 for the DCA plans is also useful to compare the planning parameters with the IMRT plans.
Although the addition of a 1 mm leaf margin led to 100% PTV coverage for the 80% IDS in all cases, the dose conformity significantly worsened compared with those without leaf margin. Although the addition of the leaf margin may be suitable for a PTV of less than 5 cm3 to ensure adequate PTV coverage (>99%), planners should be more discreet in routinely applying a leaf margin. The application and the size of the leaf margin should be prudently determined on an individual basis, considering the target volume and the balance among the target coverage, dose conformity and homogeneity.
Considering the simultaneous treatment of multiple lesions, the PTV coverage for the 80% IDS would differ even more (increase) from that for a single lesion treatment, when the same dose was applied to the isocentre, most likely as a result from the dose interference.
Taken together, the only stipulation for the dose prescription to the specific IDS percentage does not necessarily guarantee the uniform dose prescription to the target periphery and the definitive plan quality. Although the dose prescription to the specific IDS percentage may be advantageous for uniform dose homogeneity, dose prescription to the D99 or D95 rather than the specific IDS percentage appears to be more suitable for the consistent plan quality, regardless of the target volume, location or the planning method. The D99 or D95 would be also pertinent to the marginal dose evaluation in terms of the objectivity. For lesions that abut or are closely localised to the OAR, several planners may decide the intentional partial coverage for the target with the intended marginal dose in order to reduce the dose to the OAR [19]. In such cases, the PTV D90 may be appropriate for the marginal dose evaluation.
Consideration for the peripheral dose to the CTV apart from the PTV
The present study demonstrated that the transition of the CTV D99 and D95 in proportion to the PTV did not parallel that for the PTV D99 and D95, respectively (Table 2). The difference of the marginal dose between the PTV and CTV increased as the PTV decreased. The CTV coverage for the 90% IDS and the CTV D99 and D95 were significantly correlated with the ATD, but not with the PTV (Figure 4, Table 3). These findings suggest that the location of the target significantly affects the peripheral dose for the CTV, and the arc arrangement is also important for the marginal dose adjustment for the CTV.
Based on the premise that treatment set-up accuracy is nearly perfect, the marginal dose for the CTV appears to be more important for lesion control than that for the PTV. Given the recent progress in the treatment precision with image-guided systems, planners should also be also concerned with the marginal dose for the CTV. Whether the treatment planning for boosting the marginal dose for the CTV would lead to an improved treatment outcome warrants further investigation.
Factors influencing dose homogeneity and the implication for planning method
The present study confirmed the sensitivity of the PTV Dmax to the PTV and the ATD (Figure 5). The correlation of Dmax with the ATD appears to be explained by the percent depth dose profile of a 6 MV photon beam [20]. The ATD is affected by the target location and the arc arrangement, especially the number of arcs, the table position, and the range. These suggest that the selection of the table angle which takes the shorter distance between the skin and the target along the beam path may be disadvantageous for dose homogeneity. The PTV D99 was much more susceptible to the PTV and it may be also significantly increased by the addition of a leaf margin (Table 4). Therefore, planners must consider not only the IDS selection and the addition of a leaf margin, but the optimisation of the arc arrangement in order to attain a homogeneous dose distribution.
Study limitations
In the present study, the consistent planning method was applied according to the PTV with a boundary of 5 cm3 in order to explore the fundamental dosimetric characteristics as a baseline for more complex cases and its inclination to the target volume and the depth. In practice, the PTV margin, the arc arrangement, the collimator angle and the leaf margin as well as other conditions have been optimised for individual cases in each institution. The resulting dosimetric parameters may differ from the present results. Considering these situations, the marginal dose designation at the specific target coverage appears to be more important for the definitive plan evaluation.
The present study focused on the marginal dose evaluation for the PTV and CTV. Given the inevitable dose heterogeneity within the target, the evaluation of the equivalent uniform dose for the target appears to be a better method to consider the absorbed energy to the target in its entirety [2].
The present study was a TPS-based planning study and did not attempt to investigate the isodose distributions that are actually delivered in practice. Regarding the dose calculation algorithm, the loss of lateral secondary electron equilibrium was not taken into account. For lesions that are located in the vicinity of the air cavity or bony structure, the actual dose distribution may differ from the present results. The application of a more precise dose calculation algorithm such as the Monte Carlo method may be expected to clarify this issue in the future [21].
Conclusions
We herein described the dose distribution characteristics and the inclinations in proportion to the target volume or location in intracranial linear accelerator-based SRS/stereotactic radiotherapy using the mMLC with the DCA technique by applying the uniform planning method to targets relatively simple in shape. For the dose prescription to the specific percentage IDS, it is difficult to attain consistent target coverage, the D99 or D95 and the desirable dose conformity in proportion to the target volume. The prescribed IDS selection and the planning method should be individually optimised in proportion to the target volume. The tissue depth along the beam path should be also considered to optimise the peripheral dose to the CTV and the dose homogeneity. Although the intended marginal dose is estimable by planners, objective evaluation for the marginal dose at the specific level of target coverage (e.g. D99, D95, or D90) is nevertheless recommended to designate the treatment contents and to compare the planning parameters with those of other modalities. The selection of the target coverage for evaluation should be individually determined according to either the degree of the PTV margin or the proximity of the lesion to an OAR.
References
- 1.Hazard LJ, Wang B, Skidmore TB, Chern SS, Salter BJ, Jensen RL, et al. Conformity of LINAC-based stereotactic radiosurgery using dynamic conformal arcs and micro-multileaf collimator. Int J Radiat Oncol Biol Phys 2009;73:562–70 [DOI] [PubMed] [Google Scholar]
- 2.Wiggenraad RG, Petoukhova AL, Versluis L, van Santvoort JP. Stereotactic radiotherapy of intracranial tumors: a comparison of intensity-modulated radiotherapy and dynamic conformal arc. Int J Radiat Oncol Biol Phys 2009;74:1018–26 [DOI] [PubMed] [Google Scholar]
- 3.Monk JE, Perks JR, Doughty D, Plowman PN. Comparison of a micro-multileaf collimator with a 5-mm-leaf-width collimator for intracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2003;57:1443–9 [DOI] [PubMed] [Google Scholar]
- 4.Jin JY, Yin FF, Ryu S, Ajlouni M, Kim JH. Dosimetric study using different leaf-width MLCs for treatment planning of dynamic conformal arcs and intensity-modulated radiosurgery. Med Phys 2005;32:405–11 [DOI] [PubMed] [Google Scholar]
- 5.Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol 2006;81:18–24 [DOI] [PubMed] [Google Scholar]
- 6.Grabenbauer GG, Ernst-Stecken A, Schneider F, Lambrecht U, Oliver Ganslandt O. Radiosurgery of functioning pituitary adenomas: Comparison of different treatment techniques including dynamic and conformal arcs, shaped beams, and IMRT. Int J Radiat Oncol Biol Phys 2006;66:33–9 [Google Scholar]
- 7.Ding M, Newman F, Kavanagh BD, Stuhr K, Johnson TK, Gaspar LE. Comparative dosimetric study of three-dimensional conformal, dynamic conformal arc, and intensity-modulated radiotherapy for brain tumor treatment using Novalis system. Int J Radiat Oncol Biol Phys 2006;66:82–6 [Google Scholar]
- 8.Clark B, McKenzie M, Robar J, Vollans E, Candish C, Toyota B, et al. Does intensity modulation improve healthy tissue sparing in stereotactic radiosurgery of complex arteriovenous malformations? Med Dosim 2007;32:172–80 [DOI] [PubMed] [Google Scholar]
- 9.Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg 2009;23:170–8 [DOI] [PubMed] [Google Scholar]
- 10.Chen JC, Bugoci DM, Girvigian MR, Miller MJ, Arellano A, Rahimian J. Control of brain metastases using frameless image-guided radiosurgery. Neurosurg Focus 2009;27:E6. [DOI] [PubMed] [Google Scholar]
- 11.Lawson JD, Fox T, Waller AF, Davis L, Crocker I. Multileaf collimator-based linear accelerator radiosurgery: five-year efficiency analysis. J Am Coll Radiol 2009;6:190–3 [DOI] [PubMed] [Google Scholar]
- 12.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996–1001 [DOI] [PubMed] [Google Scholar]
- 13.Cosgrove VP, Jahn U, Pfaender M, Bauer S, Budach V, Wurm RE. Commissioning of a micro multi-leaf collimator and planning system for stereotactic radiosurgery. Radiother Oncol 1999;50:325–36 [DOI] [PubMed] [Google Scholar]
- 14.Coscia G, Vaccara E, Corvisiero R, Cavazzani P, Ruggieri FG, Taccini G. Fractionated stereotactic radiotherapy: a method to evaluate geometric and dosimetric uncertainties using radiochromic films. Med Phys 2009;36:2870–80 [DOI] [PubMed] [Google Scholar]
- 15.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993;27:1231–9 [DOI] [PubMed] [Google Scholar]
- 16.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys 2006;64:333–42 [DOI] [PubMed] [Google Scholar]
- 17.Chern SS, Leavitt DD, Jensen RL, Shrieve DC. Is smaller better? Comparison of 3-mm and 5-mm leaf size for stereotactic radiosurgery: a dosimetric study. Int J Radiat Oncol Biol Phys 2006;66:76–8116765534 [Google Scholar]
- 18.IMRT Documentation Working Group Holmes T, Das R, Low D, Yin FF, Balter J, et al. American Society of Radiation Oncology recommendations for documenting intensity-modulated radiation therapy treatments. Int J Radiat Oncol Biol Phys 2009;74:1311–18 [DOI] [PubMed] [Google Scholar]
- 19.Rowe JG, Walton L, Vaughan P, Malik I, Radatz M, Kemeny A. Radiosurgical planning of meningiomas: compromises with conformity. Stereotact Funct Neurosurg 2004;82:169–74 [DOI] [PubMed] [Google Scholar]
- 20.Yin FF, Zhu J, Yan H, Gaun H, Hammoud R, Ryu S, et al. Dosimetric characteristics of Novalis shaped beam surgery unit. Med Phys 2002;29:1729–38 [DOI] [PubMed] [Google Scholar]
- 21.Theodorou K, Stathakis S, Lind B, Kappas C. Dosimetric and radiobiological evaluation of dose distribution perturbation due to head heterogeneities for Linac and Gamma Knife stereotactic radiotherapy. Acta Oncol 2008;47:917–27 [DOI] [PubMed] [Google Scholar]