Abstract
A 53-year-old male with a remote history of colon adenocarcinoma presented with weakness, severe anaemia and an actively bleeding ulcerated lesion in the stomach. An 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT showed FDG-avid masses in the stomach and mesentery, which were biopsied to reveal an unsuspected diagnosis of plasmacytoma. The original colon tumour pathology was identical and this prompted its re-evaluation to a primary colon plasmacytoma. The patient was treated with chemotherapy and a follow-up PET/CT scan showed complete resolution of the gastric and mesenteric masses. 18F-FDG PET/CT is useful in the restaging and follow-up of this very rare extramedullary plasmacytoma.
Extramedullary plasmacytomas (EMP) represent approximately 3% of all plasma cell neoplasms. Involvement of the colon is extremely rare, with only 32 cases reported in the literature. CT and MRI can be useful in the staging of EMPs; however, in most institutions a whole-body MRI is not feasible. 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT has been shown to be very useful in evaluating multiple myeloma and other plasma cell dyscrasias. The literature regarding the use of 18F-FDG PET/CT in EMPs is limited owing to the small number of cases, although isolated reports suggest that it is very useful. 18F-FDG uptake in a primary colon plasmacytoma has been reported in one case; however, the staging and follow-up of a primary colon plasmacytoma recurrence in the abdomen by 18F-FDG PET/CT has not been previously described. This report expands on the potential uses of 18F-FDG PET/CT in the evaluation of EMPs.
Case report
A 53-year-old male was referred for an 18F-FDG PET/CT scan after presenting with weakness and severe anaemia (haemoglobin 64 g l−1, normal levels 120–160 g l−1). An endoscopy showed a fungating, ulcerated and actively bleeding lesion in the greater curvature of the stomach. The patient had a poorly differentiated colon adenocarcinoma 4 years earlier that had been treated with a right hemicolectomy, chemotherapy and radiation therapy. The patient had been followed-up with regular colonoscopies and was in remission.
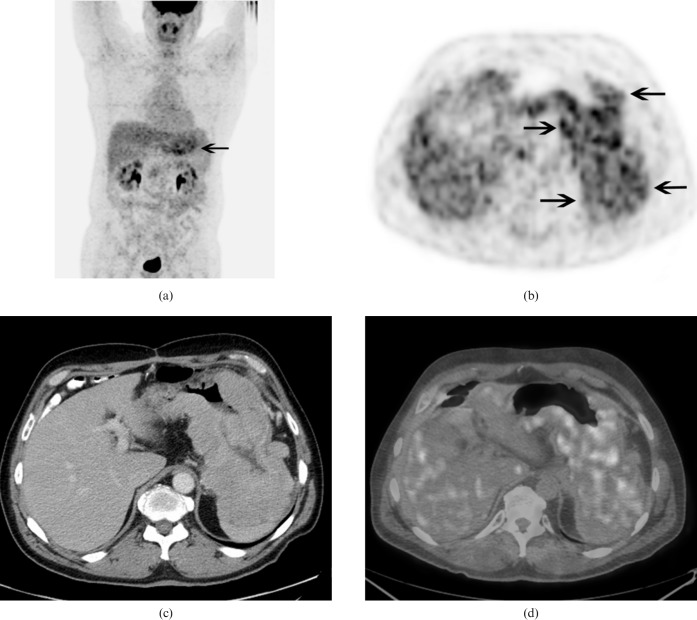
The 18F-FDG PET/CT (Discovery ST, GE Healthcare, Waukesha, WI) showed extensive lobulated thickening of the stomach with intense 18F-FDG uptake throughout with a maximum standardised uptake value (SUVmax) of 7.8 (Figure 1). It also showed moderate uptake in a 4.2 cm mesenteric lymph node with a SUVmax of 4.7 (Figure 2).
Figure 1.
(a) 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT maximum intensity projection anterior image shows intense FDG uptake throughout the stomach (arrow). (b) Transaxial PET, (c) contrast enhanced CT and (d) PET/CT fusion images show intense FDG uptake in the lobulated masses involving most of the stomach [arrows (b)].
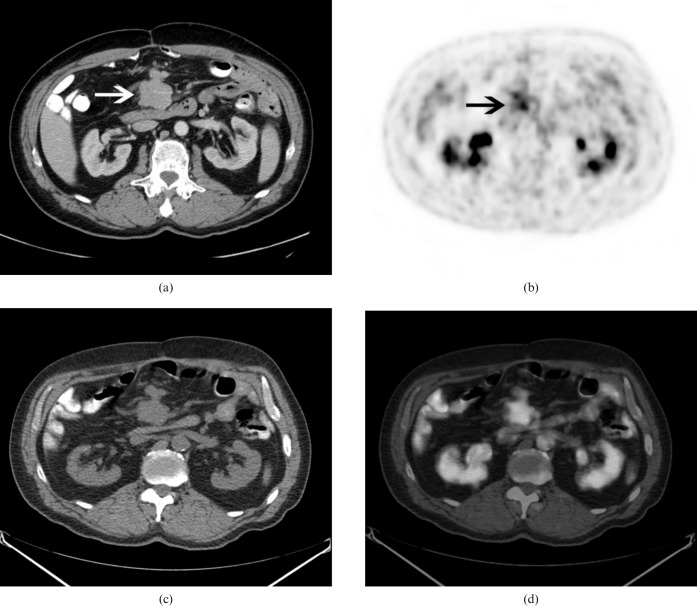
Figure 2.
Transaxial views of the (a) contrast-enhanced CT, (b) positron emission tomography (PET) show moderately intense fluorodeoxyglucose uptake in a 4.2 cm mesenteric lymph node (arrows), (c) CT portion of the PET/CT and (d) PET/CT fusion images.
Multiple biopsies of the stomach showed a gastric mucosa with a prominent diffuse infiltrate of atypical plasmacytoid cells in the lamina propria. The majority of the cells were medium to large pleomorphic cells with mildly irregular nuclei, moderately clumped chromatin and inconspicuous to distinct nucleoli. There was no invasion of epithelium or formation of lymphoepithelial lesions. No Helicobacter pylori was identified. Immunohistochemistry showed MUM-1, strongly positive in atypical cells; EMA, positive in majority of cells; CD138, strongly positive; CD43, positive; lambda, strongly positive; kappa, negative; Ki67, positive in 60–70% of the atypical cells; Cyclin-D1, positive; CD117, faintly positive; CD3, negative; CD5, negative; CD20, negative; CD34, negative; CD38, negative; CD45, negative; CD56, negative; PAX-5, negative; EBER, negative; and HSV8, negative. The histopathological and immunohistochemical results were compatible with a poorly differentiated plasma cell myeloma. These findings prompted a re-evaluation of the original poorly differentiated colon adenocarcinoma from 4 years earlier. The histology and immunohistochemistry were found to be identical to the gastric tumour, leading to a revision of the original diagnosis to a primary colon plasmacytoma. A bone marrow biopsy did not show any abnormalities and there was no monoclonal paraprotein identified in the serum or urine.
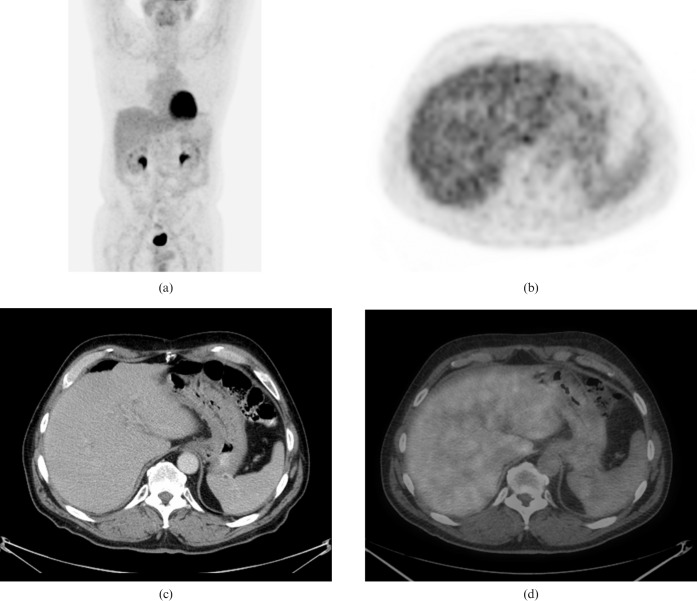
The patient was treated with one cycle of ESHAP chemotherapy (etoposide, methylprednisolone, high-dose cytarabine, platinum) for rapid debulking, followed by five cycles of bortezomib (Velcade®, Millenium, Cambridge, MA) and dexamethasone. A follow-up 18F-FDG PET/CT scan done 3 months following the completion of chemotherapy did not show any abnormal 18F-FDG uptake in the stomach (Figure 3). Transaxial views of the abdomen showed full resolution of the mesenteric lymph node mass compatible with a complete response to therapy (Figure 4). The resolution of the imaging abnormalities was considered sufficient to confirm remission.
Figure 3.
An 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan performed 3 months following the completion of chemotherapy. (a) Maximum intensity projection anterior image, (b) PET, (c) contrast enhanced CT and (d) PET/CT fusion images show a complete resolution of the stomach masses with no residual abnormal FDG uptake.
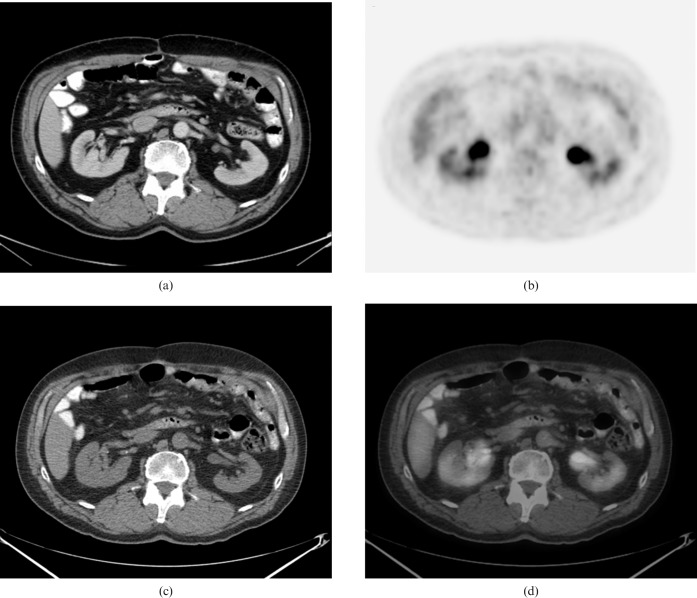
Figure 4.
Transaxial views of the (a) contrast enhanced CT, (b) positron emission tomography (PET), (c) CT portion of the PET/CT and (d) PET/CT fusion images show complete resolution of the mesenteric lymph node with no residual abnormal fluorodeoxyglucose uptake.
Discussion
According to the World Health Organization, plasma cell tumours are classified into two main groups: multiple myeloma and plasmacytoma. Plasmacytoma includes solitary plasmacytoma of the bone and solitary EMP [1]. EMPs represent about 3% of all plasma cell neoplasms and approximately 80% of these occur in the aerodigestive tract, while only a small percentage is found in the gastrointestinal tract (usually in the stomach or small intestine). Primary colon plasmacytomas are extremely rare, with only 32 cases reported in the literature [2]. Diagnosis of a solitary EMP requires the exclusion of associated multiple myeloma. Lesions of EMP are histologically characterised by monoclonal proliferation of plasma cells, which can be confirmed by demonstrating a single type of paraprotein in the tumour cells using immunoperoxidase staining (lambda chain in our case). The plasma cells are arranged in clusters or sheets with a scant, delicate supportive connective tissue stroma [3,4].
Colon plasmacytomas have very different clinical presentations from those of other sites in the body. Males are more commonly affected than females (24 vs 8 of the known 32 cases), with a mean age of presentation of 50.9 years and a younger age distribution than multiple myeloma or colon adenocarcinoma. The most common presenting symptom is abdominal pain. Other symptoms can include rectal bleeding, change in bowel habits, large bowel obstruction and intussusception [2,5].
EMPs are generally well-defined soft-tissue masses on a CT scan, isointense to muscle and white matter on T1 weighted images and iso- to hyperintense to muscle and white matter on T2 weighted images with heterogeneous enhancement, especially on MRI. Larger plasmacytomas can show aggressive traits such as invasion of adjacent fat, bone erosion or vascular encasement. CT is superior to MRI in delineating subtle bone erosions. Although MRI of the spine is a useful staging modality in multiple myeloma, whole body MRI for the staging of an apparent solitary EMP is not a feasible option at most institutions [6,7].
18F-FDG PET/CT has been found to be useful in staging, identifying optimal sites for biopsy, restaging, and monitoring response to treatment for multiple myeloma and related plasma cell dyscrasias [8]. Its use in the evaluation of EMPs appears promising, although the literature is limited owing to the small number of cases [9,10]. 18F-FDG uptake in a primary colon plasmacytoma has been described in one case [11]; however, the staging and follow-up of a primary colon plasmacytoma recurrence in the abdomen (gastric and mesenteric regions) by 18F-FDG PET/CT has not been previously reported. This report expands on the potential uses of 18F-FDG PET/CT in EMPs. 18F-FDG uptake in the stomach is a non-specific finding. The differential diagnosis of 18F-FDG uptake in the stomach includes more common benign entities such as physiological uptake [12]; gastritis (with or without H. pylori involvement) [13]; and malignant entities. These malignant entities include gastric adenocarcinoma [14], signet ring cell carcinoma [15], diffuse large B-cell lymphoma [16], MALT lymphoma [17], primary mediastinal B-cell lymphoma [18], neuroendocrine tumour [19], gastrointestinal stromal tumour (GIST) [20] and metastases [21].
Conclusion
Referring physicians should be aware of the usefulness of 18F-FDG PET/CT in the restaging and evaluation of response to therapy of an EMP of the colon.
References
- 1.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting — Airlie House, Virginia, November 1997. J Clin Oncol 1999;17:3835–49 [DOI] [PubMed] [Google Scholar]
- 2.Doki T, Takeuchi O, Kaiho T, Tsuchiya S, Matsuzaki O, Miyazaki M. Primary isolated extramedullary plasmacytoma of the colon. Int J Colorectal Dis 2008;23:719–20 [DOI] [PubMed] [Google Scholar]
- 3.Gupta V, Nahak B, Sakhuja P, Agarwal AK, Kumar N, Mishra PK. Primary isolated extramedullary plasmacytoma of the colon. World J Surg Oncol 2007;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohmori T, Kono H, Kajiwara S, Arita N, Tabei R. Primary colonic plasmacytoma associated with acute myelogenous leukemia. Am J Gastroenterology 1984;79:265–9 [PubMed] [Google Scholar]
- 5.Islam SR, Attaya MN, Parupudi S, Islam EA, D'Cunha N, Labib S, et al. Sigmoid plasmacytoma mimicking colon cancer in a patient with multiple myeloma: case report and review of literature. Gastrointest Endosc 2010;71:655–7 [DOI] [PubMed] [Google Scholar]
- 6.Ooi GC, Chim J, Au WY, Khong PL. Radiologic manifestations of primary solitary extramedullary and multiple solitary plasmacytomas. AJR 2006;186:821–7 [DOI] [PubMed] [Google Scholar]
- 7.Kaneko Y, Satoh H, Haraguchi N, Imagawa S, Sekizawa K. Radiologic findings in primary pulmonary plasmacytoma. J Thorac Imaging 2005;20:53–4 [DOI] [PubMed] [Google Scholar]
- 8.Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 2009;114:2068–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schirrmeister H, Buck AK, Bergmann L, Reske SN, Bommer M. Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm 2003;18:841–5 [DOI] [PubMed] [Google Scholar]
- 10.Salaun PY, Gastinne T, Frampas E, Bodet-Milin C, Moreau P, Bodere-Kraeber F. FDG-positron emission tomography for staging and therapeutic assessment in patients with plasmacytoma. Haematologica 2008;93:1269–71 [DOI] [PubMed] [Google Scholar]
- 11.Tajima T, Mukai M, Fukasawa M, Yazawa N, Fukumitsu H, Nakamura M, et al. A case report of laparoscopic ileocecal resection for extramedullary plasmacytoma originating from the large intestine. Jpn J Gastroenterol Surg 2010;43:277–81 [Google Scholar]
- 12.Takahashi H, Ukawa K, Ohkawa N, Kato K, Hayashi Y, Yoshimoto K, et al. Significance of (18)F-2-deoxy-2-fluoro-glucose accumulation in the stomach on positron emission tomography. Ann Nucl Med 2009;23:391–7 [DOI] [PubMed] [Google Scholar]
- 13.McDivitt JD, Arluk GM, Turton DB. Incidental detection of helicobacter pylori gastritis during FDG PET scanning for lymphoma. Clin Nucl Med 2008;33:113–14 [DOI] [PubMed] [Google Scholar]
- 14.Hur H, Kim SH, Kim W, Song KY, Park CH, Jeon HM. The efficacy of preoperative PET/CT for prediction of curability in surgery for locally advanced gastric carcinoma. World J Surg Oncol 2010;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickey A, Hrom J. A rare case of signet ring cell gastric adenocarcinoma. J Miss State Med Assoc 2007;48:271–5 [PubMed] [Google Scholar]
- 16.Chihara D, Oki Y, Ine S, Kato H, Onoda H, Taji H, et al. Primary gastric diffuse large B-cell lymphoma (DLBCL): analyses of prognostic factors and value of pretreatment FDG-PET scan. Eur J Haematol 2010;84:493–8 [DOI] [PubMed] [Google Scholar]
- 17.Suga K, Yasuhiko K, Hiyama A, Takeda K, Matsunaga N. F-18 FDG PET/CT findings in a patient with bilateral orbital and gastric mucosa-associated lymphoid tissue lymphomas. Clin Nucl Med 2009;34:589–93 [DOI] [PubMed] [Google Scholar]
- 18.Makis W, Derbekyan V, Hickeson M. Primary mediastinal large B-cell lymphoma (thymic lymphoma) imaged with F-18 FDG PET-CT. Clin Nucl Med 2010;35:421–4 [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N, Kato H, Shimizu M, Murakami J, Kawabe H, Kamisaki Y, et al. F-18 FDG PET imaging in gastric neuroendocrine carcinoma. Clin Nucl Med 2006;31:345–6 [DOI] [PubMed] [Google Scholar]
- 20.Otomi Y, Otsuka H, Morita N, Terazawa K, Furutani K, Harada M, et al. Relationship between FDG uptake and the pathological risk category in gastrointestinal stromal tumors. J Med Invest 2010;57:270–4 [DOI] [PubMed] [Google Scholar]
- 21.Mesa H, Rawal A, Rezcallah A, Iwamoto C, Niehans GA, Druck P, et al. “Burned out” testicular seminoma presenting as a primary gastric malignancy. Int J Clin Oncol 2009;14:74–7 [DOI] [PubMed] [Google Scholar]