Abstract
Objectives
The purpose of this study was to compare interfraction prostate displacement data between electronic portal imaging (EPI) and kilovoltage imaging (KVI) treatment units and discuss the impact of any difference on margin calculations for prostate cancer image-guided radiotherapy (IGRT).
Methods
Prostate interfraction displacement data was collected prospectively for the first 4 fractions in 333 patients treated with IGRT with daily pre-treatment EPI or KVI orthogonal imaging. Displacement was recorded in the anteroposterior (AP), left–right (LR) and superoinferior (SI) directions. The proportion of displacement <3 mm and the difference in median absolute displacements were calculated in all directions.
Results
1088 image pairs were analysed in total, 448 by EPI and 640 by KVI. There were 23% (95% confidence interval [CI] 18–28%) more displacements under 3 mm for EPI than for KVI in the AP direction, 14% (95% CI 10–19%) more in the LR direction and 10% (95% CI 5–15%) more in the SI direction. The differences in absolute median displacement (KVI>EPI) were AP 1 mm, LR 1 mm and SI 0.5 mm. Wilcoxon rank-sum test showed that distributions were significantly different for all three dimensions (p<0.0001 for AP and LR and p=0.02 for SI).
Conclusion
EPI has a statistically significant smaller set-up error distribution than KVI. We would expect that, because fiducial marker imaging is less clear for EPI, the clinical target volume to planning target volume margin would be greater when using IGRT; however, relying wholly on displacement data gives the opposite result. We postulate that this is owing to observer bias, which is not accounted for in margin calculation formulas.
The integration of medical imaging and radiotherapy treatment has the potential to improve pre-treatment localisation of the target and therefore the accuracy of delivery of radiotherapy. Image-guided radiotherapy (IGRT) using implanted gold seeds and pre-treatment orthogonal imaging is increasingly being used as a treatment option for prostate cancer [1]. Orthogonal imaging can be conducted either with megavoltage or with kilovoltage imaging (KVI). Electronic portal imaging (EPI) is a system that uses a few monitor units from the megavoltage treatment beam to capture the pre-treatment position of fiducial markers implanted in the prostate, thus correcting for day-to-day variations in set-up and organ displacement. The set-up error is accounted for by a margin around the target called the planning target volume (PTV) [2]. By reducing set-up error, EPI-IGRT for prostate cancer has been studied in target (or clinical target volume [CTV]) to PTV margin reduction [3-10]. Recently, linear accelerators with gantry-mounted KVI have also become available [11]. Investigators are beginning to publish on margin reduction in prostate cancer IGRT using KVI [12].
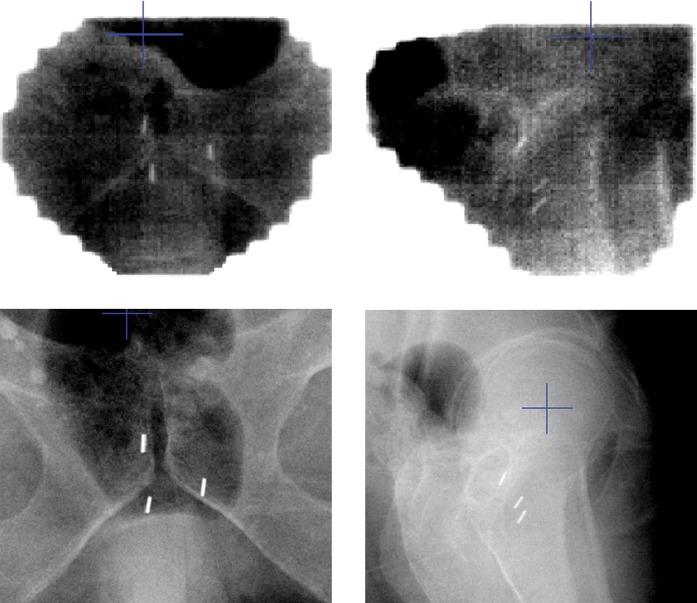
Compared with EPI, contrast for fiducial markers and bone is better using KVI (Figure 1). Kilovoltage and megavoltage photons interact with matter predominantly through photoelectric and Compton effects, respectively. This difference in atomic interaction results in a greater absorption of kilovoltage energy photons by high atomic number materials such as gold compared with megavoltage energy photons. The result is better visibility of gold seeds on radiographs taken with kilovoltage energy compared with megavoltage energy.
Figure 1.
Example of anteroposterior and lateral electronic portal imaging (above) and kilovoltage imaging (below) of fiducial markers on the same patient.
The primary aim of this study is to quantify interfraction displacement differences between megavoltage and kilovoltage imaging used in prostate cancer IGRT. Because both groups come from the same population of prostate cancer patients, the average amount of day-to-day prostate displacement should be the same. Any difference in displacement is probably attributable to the difference in observer assessment. Observer inaccuracy in assessing displacement using EPI in IGRT has implications on margin calculations for future studies.
Methods and materials
Between 1 March 2007 and 29 November 2008, data for fiducial marker displacement were collected from the daily IGRT pre-treatment images of 333 prostate cancer patients. All patients were treated on Varian linear accelerators (Varian Medical Systems, Palo Alto, CA) either with EPI or with an on-board kilovoltage imager. All treatment units underwent regular and systematic physics quality assurance to check the accuracy of couch and isocentre positions. Although patients were not randomised, they were treated according to the availability of either type of treatment unit. Only the first four fractions were considered in our analysis because patients underwent a permanent isocentre move after the fourth fraction to correct for systematic error.
Details of the marker implantation procedure, CT scan for simulation, creation of digitally reconstructed images (DRRs) and registration have previously been published by our group [13]. In summary, three fiducial markers made of 24-carat gold and measuring 1 mm by 5 mm were implanted into the prostate gland at least 1 week before CT simulation. CT slices were 3 mm thick with 3-mm spacing. Patients were set up and immobilised identically on EPI and KVI units. Immobilisation was supine with a bolster under the knees and foot rests, which slotted into an immobilisation board (Combifix-Sinmed; Civco, Kalona, IA). Set-up was checked with laser lights matched to three skin tattoos: one midline and two lateral on either side of the pelvis. Pre-treatment images were taken either with 6-mV photons using two monitor units for each exposure on EPI treatment units or with 75 kV for anterior to posterior and 120 kV for lateral images on the KVI treatment unit. Isocentre shifts were made if there was a difference in the fiducial position on the pre-treatment images above the predetermined action threshold. The action threshold was 3 mm on the EPI treatment units. If the displacement was 0, 1 or 2 mm, displacement was recorded but a couch shift was not made. If displacement was 3 mm or more, the radiation therapist would enter the treatment room, manually make a shift and re-image to check that the shift had corrected the displacement. On the KVI treatment units, the action threshold was >0 mm and any required shift was applied from the treatment console without re-entering the treatment room [14].
Ascertainment of displacement data
Image registration was done on Impac Software (IMPAC Medical Systems Inc., Sunnyvale, CA) on the EPI treatment units and on the on-board imager console on KVI treatment units. On both units, any value of 0 mm and above was recorded as a displacement. The EPIs were matched using the Impac record and verify system and when the match was accepted the displacement data were automatically recorded into Impac's third party offset list. For KVIs, the images were matched on the on-board imager computer screen, which gave three displacement figures along the anteroposterior (AP) axis, left–right (LR) axis and superoinferior (SI) axis, which were then entered manually into Impac. Reports were created from the Impac database using Crystal Reports software (Crystal Reports 11; Business Objects Software, San Jose, CA). Institutionally developed software was then used to write this information to an Access database (Microsoft Corporation, Redmond, WA) for further analysis. Details of the data extraction have been published by our group [15].
Statistical methods
A z-test was used to compare EPI and KVI, the difference in the proportion of fractions that would have had a shift if a 3-mm threshold were used for KVI and the proportion of fractions that had a set-up with no recorded displacement (0-mm displacement). The differences in median absolute displacements were calculated for AP, LR and SI directions. The Wilcoxon rank-sum test was used to test for a difference in the distribution of displacements between KVI and EPI for each direction. The distribution of KVI displacements was considered the best estimate of the true underlying distribution of set-up displacements. The absolute value of the displacements was used. A 95% confidence interval for the number of displacements in the EPI group that were <3 mm, which should have been ≥3 mm, was calculated using a maximum likelihood approach with probabilities from the binomial distribution. A two-sided p-value for the null hypothesis that the proportion of displacements <3 mm for EPI was the same as that for KVI was calculated using the same approach. Statistical significance was set at p=0.05.
Results
For 333 patients, 1088 image pairs were analysed in total, 448 on EPI and 640 on KVI linear accelerators. As a result of the difference in action threshold of 3 mm for EPI and 0 mm for KVI, the number of actual couch shifts made on each unit was 53% and 98%, respectively. If the action threshold for KVI shift were raised to 3 mm, a couch shift would have been made in 80% of fractions (27% more shifts on KVI than on EPI; p<0.0001). This is unusual, because we would have expected the same number of shifts on both units for the same action threshold.
Anteroposterior
There is strong evidence that the proportion of displacements 3 mm in the EPI group is greater than from that in the KVI group (one-sided; p<0.0001).
If the EPI displacements really were the same as the KVI displacements, we would have expected to see somewhere between 82 and 123 of the displacements (out of a total of 448), which were <3 mm, being ≥3 mm. Hence, we estimate that somewhere between 18% and 28% of the EPI displacements were recorded as being <3 mm when they should have been recorded as ≥3 mm.
Left to right
There is strong evidence that the proportion of displacements <3 mm in the EPI group is greater than from that in the KVI group (one-sided; p<0.0001).
If the EPI displacements really were the same as the KVI displacements, we would have expected to see somewhere between 45 and 85 of the displacements (out of a total of 448), which were <3 mm, being ≥3 mm. Hence, we estimate that somewhere between 10% and 19% of the EPI displacements were recorded as being <3 mm when they should have been recorded as ≥3 mm.
Superoinferior
There is strong evidence that the proportion of displacements <3 mm in the EPI group is greater than from that in the KVI group (one-sided; p<0.0001).
If the EPI displacements really were the same as the KVI displacements, we would have expected to see somewhere between 24 and 65 of the displacements (out of a total of 448), which were <3 mm, being ≥3 mm. Hence, we estimate that somewhere between 5% and 15% of the EPI displacements were recorded as being <3 mm when they should have been recorded as ≥3 mm.
Figure 2 shows the difference in distribution of absolute displacement in AP, LR and SI dimensions, respectively. The vast majority of displacements were <5 mm for both EPI and KVI. A larger proportion of patients had a smaller displacement on EPI compared with KVI. The crossover point occurred between 2 mm and 3 mm. The difference in distributions was statistically significant (p<0.0001 for AP and LR and p=0.02 for SI). With larger displacements (>5 mm) the proportion of prostate displacements between EPI and KVI tend to be similar, and the largest discrepancy is for displacements <3 mm (Table 1).
Figure 2.
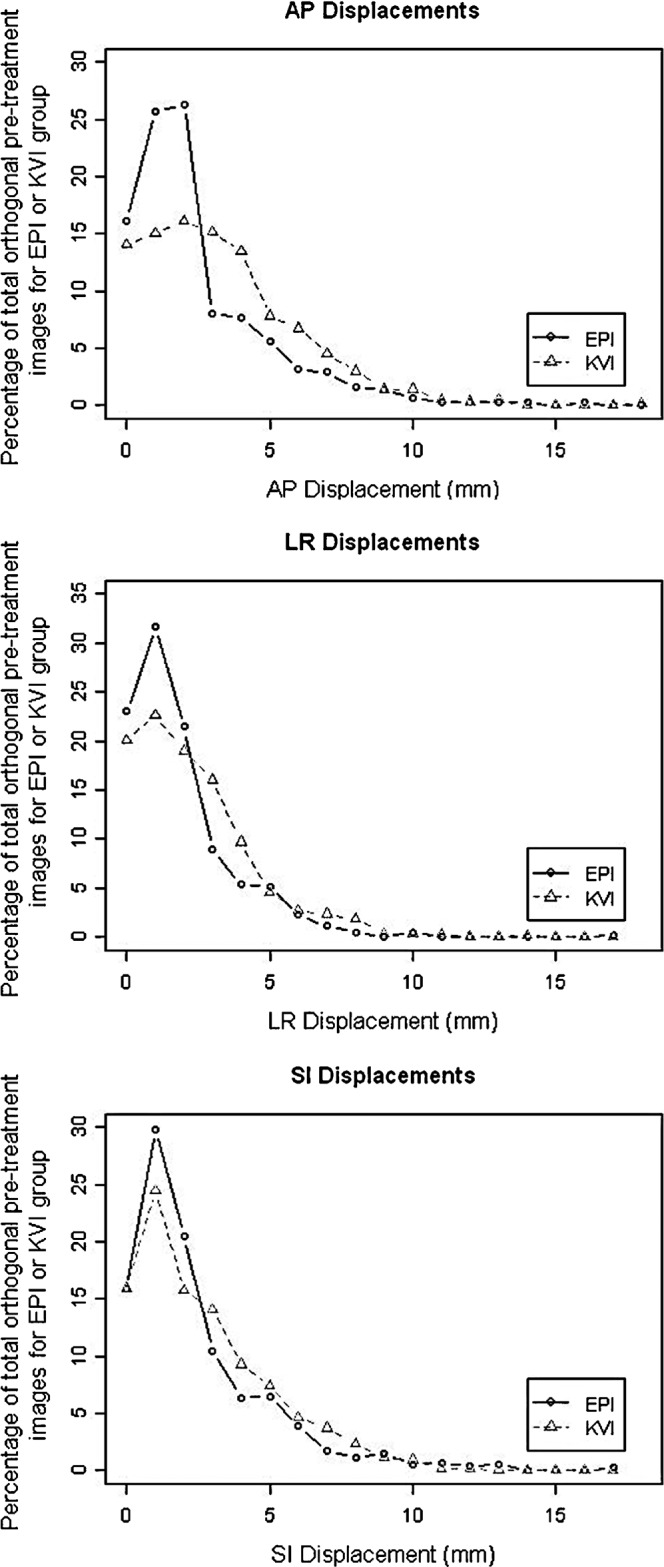
Anteroposterior (AP), left–right (LR) and superoinferior (SI) absolute displacements for electronic portal imaging (EPI) and kilovoltage imaging (KVI).
Table 1. Proportion of displacements <3 mm, <5 mm and <7 mm for KVI and EPI.
| Dimension | Proportion of displacements <3 mm (%) |
Proportion of displacements <5 mm (%) |
Proportion of displacements <7 mm (%) |
|||
| KVI | EPI | KVI | EPI | KVI | EPI | |
| AP | 45 | 68 | 74 | 84 | 88 | 92 |
| LR | 62 | 76 | 88 | 90 | 95 | 98 |
| SI | 56 | 66 | 79 | 84 | 92 | 94 |
| Average difference | 16 | 6 | 3 | |||
AP, anteroposterior; EPI, electronic portal imaging; KVI, kilovoltage imaging; LR, left–right; SI, superoinferior.
For KVI, the median values for absolute displacement were 3 mm for AP, 2 mm for LR and 2 mm for SI. For EPI, the median values for absolute displacement were 2 mm for AP, 1 mm for LR and 1.5 mm for SI. Therefore, the differences in median absolute displacements were AP 1 mm, LR 1 mm and SI 0.5 mm, respectively.
Discussion
For each of the AP, LR and SI dimensions, the proportion of displacements recorded as <3 mm in the EPI group was significantly greater than that in the KVI group (p-values all <0.0001). Our results indicate that the data set for EPI is shifted to the left (right-skewed) compared with the data set for KVI because the median value representing a large number of patients is less on EPI in all three dimensions (Figure 2). Assuming KVI displacement data are the true estimates for the overall population, this implies that EPI data are an underestimate of the true displacement figures for the population overall. This finding was most prominent in the AP direction, where there were 23% more displacements under 3 mm for EPI than expected. AP displacement is the most difficult to judge because it is assessed on the lateral image, which is the direction of greatest patient separation and most soft tissue, pelvic and hip bone interference (Figure 1). This is more difficult to see on megavoltage imaging than on KVI. This has clinical relevance because the CTV to PTV margin is smallest in the posterior direction in most clinical protocols (7 mm vs 10 mm in our department protocol compared with SI and LR CTV to PTV margin). Furthermore, the sub-capsular portion of the posterior aspect of the prostate gland harbours approximately 70% of prostate cancers [16]. Underestimating AP displacement could lead to a geographical tumour miss in a significant proportion of patients.
One of the main reasons for the disparity between EPI and KVI displacement data is likely to be that the image quality for assessing bone and fiducial markers with megavoltage imaging is inferior. Pisani et al [17] studied the interobserver alignment of an anthomorphic phantom with a known translational shift when imaged with both kilovoltage and megavoltage beams. They found that interobserver alignment was more variable with megavoltage imaging than KVI. With KVI, radiation therapists have greater confidence when comparing DRR and pre-treatment images and therefore smaller differences in the position of the fiducial markers are detected and corrected for. It is possible that in a situation where the radiation therapist is not confident about making a couch shift, owing to poor quality imaging, they may be more likely to record the observation as below the action threshold. However, our findings reflect the interobserver bias within our department and may not necessarily reflect interobserver bias in other departments. For example, imaging quality may be affected by differences in energy or monitor units used when imaging in different departments, differences in size of fiducial markers, differences in amorphous silicone detector sensitivity on linear accelerators made by different manufacturers, and differences in the shape (spherical vs cylindrical) and materials (gold vs tungsten) used to make fiducial markers, which may make a difference to the variability in interobserver assessment across departments. For example, in a phantom study conducted at our centre, 6 observers looked at 10 lateral EPIs and the visualisation rate of fiducial markers of 1.2 mm, 1.0 mm and 0.8 mm in diameter were 97.8%, 83.9% and 73.3%, respectively. Compared with the known positions of the fiducial markers in the phantom, the average observer discrepancy was 0.3 mm (±0.17 mm) in the LR direction, 0.2 mm (±0.2 mm) in the AP direction and 0.23 mm (±0.83 mm) in the SI direction [18]. In addition, some departments use a system of automated fiducial marker detection. Automated systems rely on grey levels when searching for pixel clusters, which the computer algorithm uses to detect fiducial markers automatically [19]. Therefore, radiation therapist input, in deciding whether an observation is above or below an action threshold, is limited when this system is used.
If a 3-mm action threshold were to be applied to both EPI and KVI units, there would still be 27% more actual couch shifts on KVI units than on EPI units. The difference in automation between EPI and KVI units may be a contributing factor to this disparity. Automation of couch shifts on the KVI treatment unit is likely to reduce observer bias when it comes to recording a displacement value. On EPI units, 3 mm was taken as a pragmatic balance between slowing down treatment overall and clinical benefit of correction. Other authors have also reported using a 3-mm threshold when EPI is used in prostate cancer IGRT [20].
Using in vivo dosimetry measurements, Walter et al [21] compared the dose at skin and in the rectum for five patients. For a five monitor unit, megavoltage exposure the dose for AP imaging was 57.8 mGy at the surface and 33.9 mGy in the rectum, and for lateral imaging, 69.4 mGy and 31.7 mGy, respectively. The dose for AP KVI was 0.8 mGy at the surface and 0.2 mGy in the rectum, and for lateral imaging it was 1.1 mGy and 0.1 mGy, respectively. Although the dose to the rectum with KVI was less, megavoltage imaging exposure dose can be, and usually is, taken into consideration in the planning process as part of the summed dose delivered to organs at risk. However, the dose to the skin and rectum for a 39-fraction course of IGRT with KVI is relatively small.
Margin calculations using a margin recipe, such as the van Herk formula, assumes that the displacement data collected are accurate [22]. If the displacement data are not accurate, calculated random and systematic error would also be incorrect. In one study, IGRT with grossly reduced margins was a predictor of a poorer biochemical outcome, leading to recurrence in 42% vs 9% in non-IGRT patients at 5 years [23]. In our study, if displacement data from EPI were used to calculate random and systematic errors, we would get a smaller CTV to PTV margin than with calculations from KVI data. An additional margin would be required to cover the uncertainty in the position of fiducial markers created by observer bias. We found the uncertainty in observer assessment occurred more for smaller displacements under 5 mm. The 3-mm threshold on the EPI units is not only there for the pragmatic reason of keeping overall treatment times low, but also because of reduced confidence in making couch shifts below that level owing to reduced clarity of imaging in our department. A visual assessment of Figure 2 shows that the absolute displacements for KVI and EPI converge at approximately 5 mm in the LR and SI directions, and at about 8 mm in the AP direction. The additional margin required to cover the uncertainty because of imaging quality should be balanced against the possible benefits of margin reduction in terms of toxicity reduction, and the possible risk of missing a tumour through margin reduction, which can also be individualised. Owing to interdepartmental differences in action thresholds and methods used to conduct IGRT as discussed above, we recommend that individual consultants should be aware of the potential for interobserver bias when calculating margin reduction, and individual departments should carry out their own quality assurance process to assess inter- and intra-observer bias to be factored in when calculating margin reduction.
There are limitations to our study that should be addressed. Firstly, a total of 1332 images were taken for 4 fractions from 333 patients, and only 1088 images were analysed. The reason for the shortfall in the number of image pairs analysed compared with those taken was because the automated system of data extraction from Impac only considered complete sets of data available and abandoned data if one of the displacement figures was missed out [15]. For example, if there was only one image taken, or if the displacement data had not been entered for all three directions of displacement, then it did not include data for the entire imaging session. Overall, 18% of data for the first 4 fractions was abandoned owing to the way it was extracted from Impac; however, in total there was a very large number of image sessions considered. Secondly, the impact of non-randomisation in patient selection for EPI and KVI treatment units must be considered. Although patients were not randomised, the only factor affecting which unit they were treated on was its availability. If both EPI and KVI were available, staff would preferentially put patients receiving IGRT on the KVI units, which explains why there were 30% less patients treated with EPI than with KVI in our study. Therefore, staff on the KVI units may be more skilled at assessing fiducial marker position compared with staff on EPI units; they may also be more confident in making shifts. However, in reality the staff rotate between treatment units and the impact of this is likely to be minimal.
Factors that affect margin reduction in IGRT include clarity of imaging and possibly automation of couch shift, both of which are not accounted for in margin formulas. Other factors not considered in margin formulas include uncertainty in GTV to CTV expansion, inter- and intraclinician variation in defining the CTV, delay between imaging and treatment and organ movement in that time, and the possibility of relative movement of fiducial markers within the prostate gland between simulation and treatment [24]. Observer bias on EPI units should be considered as one more uncertainty adding to the PTV margin. In the future, it is likely that an increasing number of prostate cancer patients will be radically treated with dose-escalated IGRT and intensity-modulated radiotherapy (IMRT), and there are clinical trials running currently looking at hypofractionation [25]. It is crucial that small geometrical errors are detected and corrected for as the steep dose gradients outside the intended target area in IMRT leads to a reduced fringe dose and therefore more likelihood of geographical miss if margins are calculated incorrectly. The development of clearer imaging solutions for targeted radiotherapy is to be encouraged.
Conclusion
In summary, our study quantifies the accuracy of EPI in IGRT of prostate cancer compared with KVI in a large clinical population. This information is relevant to physicians considering information on margin reduction, especially if their own unit uses one imaging modality and the information quoted uses the other. It highlights that IGRT is a complex decision-making process and radiotherapy margins in IGRT should take into consideration the influence of image quality, action thresholds and automation of couch shifts on operator confidence, and potentials for bias. The differences in median absolute displacement between EPI and KVI were no more than 1 mm, validating EPI as an accurate tool in IGRT for prostate cancer. However, our study supports the use of a slightly larger CTV to PTV margin in EPI-based IGRT in prostate cancer for similar protocols, owing to observer bias.
Acknowledgments
The Peter MacCallum Cancer Centre has a Research Collaborative Agreement with Varian Medical Systems.
References
- 1.Kupelian PA, Langen KM, Willoughby TR, Zeidan OA, Meeks SL. Image-guided radiotherapy for localized prostate cancer: treating a moving target. Semin Radiat Oncol 2008;18:58–66 [DOI] [PubMed] [Google Scholar]
- 2.International Commission on Radiation Units report 62: Prescribing, recording and reporting photon beam therapy Supplement to ICRU Report 50. Bethseda, MD: International Commission on Radiation Units and Measurement, 1999 [Google Scholar]
- 3.Greer PB, Dahl K, Ebert MA, Wratten C, White M, Denham JW. Comparison of prostate set-up accuracy and margins with off-line bony anatomy corrections and online implanted fiducial-based corrections. J Med Imaging Radiat Oncol 2008;52:511–16 [DOI] [PubMed] [Google Scholar]
- 4.Alasti H, Petric MP, Catton CN, Warde PR. Portal imaging for evaluation of daily on-line setup errors and off-line organ motion during conformal irradiation of carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001;49:869–84 [DOI] [PubMed] [Google Scholar]
- 5.Reinhold G, Peter W, Volker B, Dirk B. Potentials of on-line repositioning based on implanted fiducial markers and electronic portal imaging in prostate cancer radiotherapy. Radiat Oncol 2009;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Haycocks T, Alasti H, Ottewell G, Middlemiss N, Abdolell M, et al. Positioning errors and prostate motion during conformal prostate radiotherapy using on-line isocentre set-up verification and implanted prostate markers. Radiother Oncol 2001;61:127–33 [DOI] [PubMed] [Google Scholar]
- 7.Nederveen AJ, Dehnad H, van derHeide UA, van Moorselaar RJA, Hofman P, Lagendijk JJW. Comparison of megavoltage position verification for prostate irradiation based on bony anatomy and implanted fiducials. Radiother Oncol 2003;68:81–8 [DOI] [PubMed] [Google Scholar]
- 8.Schallenkamp JM, Herman MG, Kruse JJ, Pisansky TM. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys 2005;63:800–11 [DOI] [PubMed] [Google Scholar]
- 9.Graf R, Wust P, Budach V, Boehmer D. Potentials of on-line repositioning based on implanted fiducial markers and electronic portal imaging in prostate cancer radiotherapy. Radiat Oncol 2009;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bel A, Van Herk M, Lebesque JV. Target margins for random geometrical treatment uncertainties in conformal radiotherapy. Med Phys 1996;23:1537–45 [DOI] [PubMed] [Google Scholar]
- 11.Jaffray DA, Drake DG, Moreau M, Martinez AA, Wong JW. A radiographic and tomographic imaging system integrated into a medical linear accelerator for localization of bone and soft-tissue targets. Int J Radiat Oncol Biol Phys 1999;45:773–89 [DOI] [PubMed] [Google Scholar]
- 12.Poulsen PR, Muren LP, Høyer M. Residual set-up errors and margins in on-line image-guided prostate localization in radiotherapy. Radiother Oncol 2007;85:201–6 [DOI] [PubMed] [Google Scholar]
- 13.Thompson A, Fox C, Foroudi F, Styles C, Tai KH, Owen R, et al. Planning and implementing an implanted fiducial programme for prostate cancer radiation therapy. J Med Imaging Radiat Oncol 2008;52:419–24 [DOI] [PubMed] [Google Scholar]
- 14.Bel A, Petrascu O, Van deVondel I, Coppens L, Linthout N, Verellen D, et al. A computerized remote table control for fast on-line patient repositioning: Implementation and clinical feasibility. Med Phys 2000;27:354–8 [DOI] [PubMed] [Google Scholar]
- 15.Fox C, Fisher R, Kron T, Tai KH, Thompson A, Owen R, et al. Extraction of data for margin calculations in prostate radiotherapy from a commercial record and verify system. J Med Imaging Radiat Oncol 2010;54:161–70 [DOI] [PubMed] [Google Scholar]
- 16.Haffner J, Potiron E, Bouyé S, Puech P, Leroy X, Lemaitre L, et al. Peripheral zone prostate cancers: Location and intraprostatic patterns of spread at histopathology. Prostate 2009;69:276–82 [DOI] [PubMed] [Google Scholar]
- 17.Pisani L, Lockman D, Jaffray D, Yan D, Martinez A, Wong J. Setup error in radiotherapy: on-line correction using electronic kilovoltage and megavoltage radiographs. Int J Radiat Oncol Biol Phys 2000;47:825–39 [DOI] [PubMed] [Google Scholar]
- 18.Owen R, Kron T, Foroudi F, Cox J, Zhu L, Cramb J, et al. The detectability and localization accuracy of implanted fiducial markers determined on in-room computerized tomography (CT) and electronic portal images (EPI). Med Dosim 2008;33:226–33 [DOI] [PubMed] [Google Scholar]
- 19.Buck D, Alber M, Nusslin F. Potential and limitations of the automatic detection of fiducial markers using an amorphous silicon flat-panel imager. Phys Med Biol 2003;48:763–74 [DOI] [PubMed] [Google Scholar]
- 20.Chung PWM, Haycocks T, Brown T, Cambridge Z, Kelly V, Alasti H, et al. On-line aSi portal imaging of implanted fiducial markers for the reduction of interfraction error during conformal radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 2004;60:329–34 [DOI] [PubMed] [Google Scholar]
- 21.Walter C, Boda-Heggemann J, Wertz H, Loeb I, Rahn A, Lohr F, et al. Phantom and in-vivo measurements of dose exposure by image-guided radiotherapy (IGRT): megavoltage portal images vs. kilovoltage portal images vs. cone-beam CT. Radiother Oncol 2007;85:418–23 [DOI] [PubMed] [Google Scholar]
- 22.van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1121–35 [DOI] [PubMed] [Google Scholar]
- 23.Engels B, Soete G, Verellen D, Storme G. Conformal arc radiotherapy for prostate cancer: increased biochemical failure in patients with distended rectum on the planning computed tomogram despite image guidance by implanted markers. Int J Radiat Oncol Biol Phys 2009;74:388–91 [DOI] [PubMed] [Google Scholar]
- 24.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol 2004;14:52–64 [DOI] [PubMed] [Google Scholar]
- 25.PROFIT–Prostate Fractionated Irradiation Trial [web page on the Internet] Accessed August 2010. Available from: http://clinicaltrials.gov/ct2/show/NCT00304759.