Abstract
A 58-year-old female who presented with a lower gastrointestinal bleed was referred for an 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT after a colonoscopy revealed a submucosal mass in the ascending colon. The PET/CT confirmed the presence of an FDG-avid mass in the ascending colon with no other FDG-avid abnormalities. Dual time-point imaging was performed and showed a significant increase in FDG uptake in the mass, which raised strong suspicion of a colon malignancy. Although an initial biopsy of the mass did not show evidence of neoplasia, a decision was made to proceed with a right hemicolectomy based on high clinical and imaging suspicion of malignancy. Histological evaluation of the hemicolectomy revealed a benign colon desmoid tumour.
The role of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT in the post-therapy evaluation of colorectal cancer is well established. PET/CT has been found to be very useful in assessing response to chemotherapy and monitoring for local recurrence or distant metastases [1-5]. However, several recent studies have re-examined PET/CT's role in the staging of colorectal cancer and have found it to be significantly more useful than was previously reported in the literature [6-8]. Dual time-point imaging is a technique that has been proposed by several authors to increase the confidence of differentiating benign colon processes from malignant ones. Malignant processes show on average a 20% increase in maximum standardised uptake value (SUVmax) on the delayed acquisition, while benign processes show either no change or a reduced SUVmax [9,10]. However, currently, dual time-point imaging is not routinely used in most institutions.
Certain types of desmoid tumours are known to show 18F-FDG uptake and cause difficulty in the interpretation of soft tissue tumours on PET/CT [11-13], although 18F-FDG uptake in a colon desmoid tumour has not been previously described. We present a case that highlights a potentially serious pitfall, in which a colon desmoid tumour mimicked a primary colon malignancy on dual time-point 18F-FDG PET/CT imaging.
Case report
A 58-year-old female presented with acute-onset abdominal pain and 24 h of bright red blood per rectum associated with diarrhoea. The patient had a history of pulmonary hypertension and chronic ascites secondary to right-sided heart failure, which had been treated with continuous ambulatory peritoneal dialysis for 3 years. On presentation to the emergency department, the patient's haemoglobin level was 84 g l−1 (normal, 120–160 g l−1). A colonoscopy showed polyps in the hepatic flexure and descending colon, as well as an approximately 3-cm submucosal mass in the ascending colon. All of these were biopsied, but none showed evidence of malignancy. The patient had no history of familial adenomatous polyposis or any prior abdominal surgery.
An 18F-FDG PET/CT scan (Discovery ST, GE Healthcare, Waukesha, WI) was performed 6 weeks after the colonoscopy and biopsies. The patient fasted overnight prior to the examination and in the morning waited in a quiet, dark room. Her blood glucose level was 4.5 mmol l−1. An 18F-FDG emission scan extending from the head to the mid-thigh was obtained 60 min after intravenous injection of 17.9 mCi of 18F-FDG. Dual time-point imaging was performed with a set of delayed abdominal images taken 115 min post-18F-FDG injection. The emission scans were acquired for 5 min per field of view, each covering 14.9 cm, at an axial sampling thickness of 3.75 mm per slice. The 16-slice helical CT acquisition was performed prior to a full-ring dedicated PET scan of the same axial range. The CT acquisition parameters were an X-ray tube voltage peak of 140 kVp, 80 mA, a 1.75:1 pitch, a slice thickness of 3.75 mm and a rotational speed of 0.8 s per rotation. The patient was allowed to breathe normally during the PET and CT acquisitions. PET images were reconstructed with CT-derived attenuation correction using ordered subset expectation maximisation software (21 subsets, 2 iterations). Only the SUVmax was reported, which was corrected for body weight. Maximum intensity projection (MIP) images showed heterogeneous FDG uptake throughout the large bowel. There was also FDG uptake around the peritoneal dialysis catheter (Figure 1).
Figure 1.
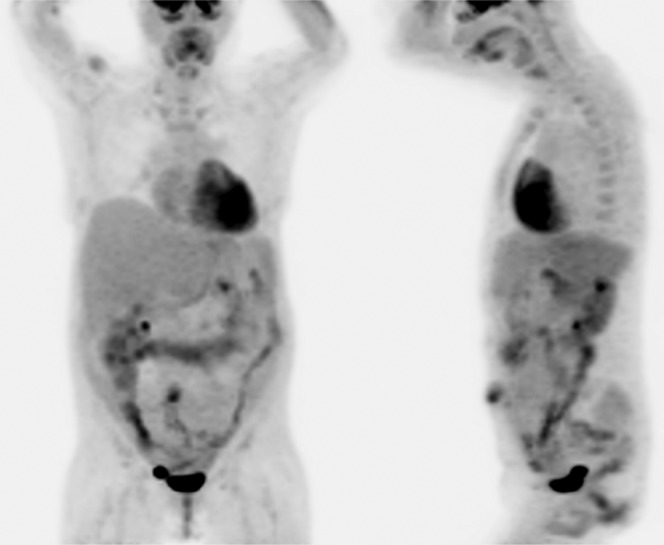
18F-fluorodeoxyglucose (FDG) positron emission tomography/CT (Discovery ST, GE Healthcare, Waukesha, WI) maximum intensity projection images with anterior and left lateral views show extensive heterogeneous 18F-FDG uptake throughout the large bowel, most of which is physiological.
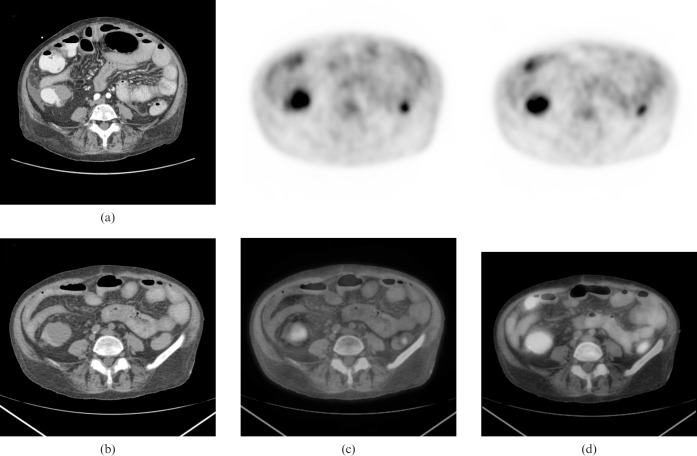
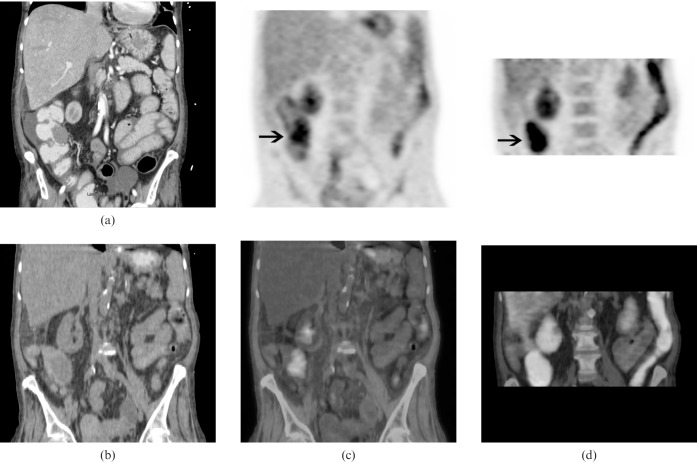
A contrast-enhanced CT scan of the lower abdomen (for abdominal pain to rule out an incarcerated umbilical hernia) was performed 2 months prior to the PET/CT and showed a 3-cm mass in the ascending colon, which was not investigated further at the time (Figures 2a and 3a). Corresponding 18F-FDG PET/CT images showed intense 18F-FDG uptake in a 4-cm ascending colon mass with an SUVmax of 4.2 (Figures 2b,c and 3b,c). The initial set of images was taken 60 min post-18F-FDG injection (Figures 2c and 3c) and dual time-point imaging was performed with a set of delayed images taken at 115 min (Figures 2d and 3d). The SUVmax of the ascending colon mass increased from 4.2 to 7.4 (76% increase). There was a noticeable clearance of activity from the surrounding bowel, which raised strong suspicion of a primary colon malignancy.
Figure 2.
(a) Transaxial view of a contrast-enhanced CT of the abdomen. (b) Corresponding CT portion of the positron emission tomography (PET)/CT, (c) initial PET (top row) and PET/CT fusion (bottom row) images taken at 60 min post-fluorodeoxyglucose (FDG) injection, (d) dual time-point PET (top row) and PET/CT fusion (bottom row) images taken at 115 min post-FDG injection.
Figure 3.
(a) Coronal view of a contrast-enhanced CT of the abdomen. (b) Corresponding CT portion of the positron emission tomography (PET)/CT, (c) initial PET (top row) and PET/CT fusion (bottom row) images, (d) dual time-point PET (top row) and PET/CT fusion (bottom row) images show significantly increased 18F-fluorodeoxyglucose uptake in the colon mass on delayed (dual time-point) images compared with the initial images (arrows).
The biopsy results of the initial colonoscopy showed tubular adenomas in the hepatic flexure and descending colon. However, the biopsy of the submucosal ascending colon mass showed only colonic mucosa with increased intra-epithelial lymphocytes consistent with lymphocytic colitis and lamina propria haemorrhage, but no evidence of malignancy. The maximum diameter of the mass grew rapidly from 3 cm to 4 cm in two months and the dual time-point PET/CT imaging was suspicious for a malignant lesion; therefore, a decision was made to proceed with a right hemicolectomy. Histopathological evaluation of the excised colon segment showed a well-circumscribed, white-grey, firm, solid submucosal mass. The surrounding mucosa showed patchy haemorrhagic areas. The tumour consisted of a spindle cell proliferation (without cytological atypia) with focally abundant collagen deposition involving the colonic wall and pericolonic adipose tissue. The margins of the tumour were infiltrative. The beta catenin was positive in tumour cell nuclei; other markers (CD34, CD117, S100, actin and desmin) were negative. The histopathological findings were consistent with deep fibromatosis (desmoid tumour) involving the colonic wall and pericolonic adipose tissue, measuring 4 cm in the greatest dimension.
A follow-up 18F-FDG PET/CT was performed 1 year after the right hemicolectomy. MIP images with anterior and left lateral views are shown in Figure 4. There was no suspicious FDG uptake at the anastomosis site or in the remainder of the study.
Figure 4.
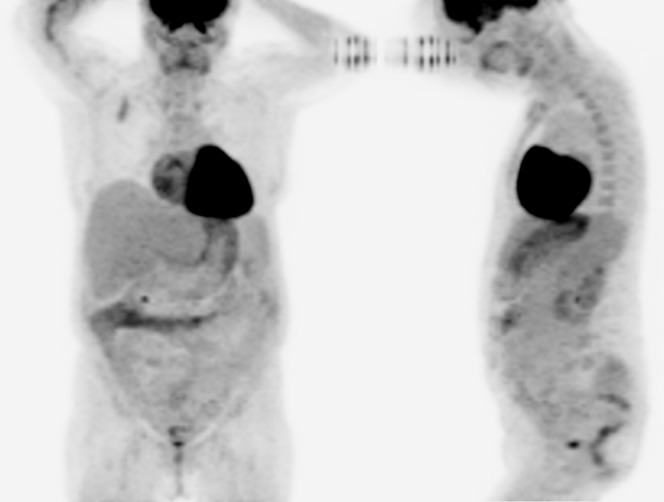
An 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT was performed 1 year after the right hemicolectomy. Maximum intensity projection images with anterior and left lateral views are shown. There was no suspicious 18F-FDG uptake at the anastomosis site or in the remainder of the study.
Discussion
Originally described by Stout in 1954 [14], desmoid tumours, also called aggressive fibromatosis, are rare benign soft-tissue tumours that arise from the connective tissue of the muscle, fascia or aponeurosis. They can occur anywhere in the body and account for 0.03% of all neoplasms and <3% of all soft-tissue tumours. Despite a benign appearance and lack of propensity to metastasise, they display local aggressiveness to surrounding structures. Although the exact aetiology is unknown, genetic abnormalities, trauma and steroid sex hormones may contribute to their oncogenesis [15-17]. Most desmoid tumours localise to the abdominal wall or are extra-abdominal, with only 6% classified as intra-abdominal. However, intra-abdominal desmoids have the highest local recurrence rates of up to 77% [18]. Solitary intestinal desmoids are extremely rare, with only 17 cases reported in the literature and only 5 cases localised to the colon [19,20].
18F-FDG uptake in desmoid tumours has been described in various locations in the body including the chest wall [11], abdominal wall [12], muscles of the extremities, mesentery and retroperitoneum [13]. 18F-FDG PET/CT imaging has shown promise in the evaluation of response to therapy [21]; however, the role of PET/CT in the evaluation of desmoid tumours remains undefined. Recently, 18F-FDG uptake was described in a segment of colon affected by intussusception (caused by a colonic desmoid), although the inflammation associated with the intussusception precluded any possible evaluation of FDG avidity of the colon desmoid itself [20].
Dual time-point 18F-FDG PET/CT imaging has been proposed by several authors to increase the confidence of differentiating benign colon processes from malignant ones. A standardised dual time-point imaging protocol does not exist at this time, with several studies suggesting a second (delayed) acquisition anywhere from 50 min to 4.5 h following the initial acquisition [9,10]. Our patient was imaged at 55 min following the initial acquisition, with the colon mass showing a 76% increase in SUVmax. In one study, the malignant lesions showed an average 20% increase in SUVmax on 4-h delayed images [9]. This finding contributed to the strong suspicion of a primary colon malignancy.
The differential diagnosis of malignant colon lesions that are 18F-FDG avid includes adenocarcinoma [22], gastrointestinal stromal tumour [23], leiomyosarcoma [24], plasmacytoma [25] and lymphoma [26]. This report highlights a potentially serious pitfall in the evaluation of colon masses by PET/CT whereby a desmoid tumour of the colon can mimic a primary colon malignancy on dual time-point 18F-FDG PET/CT imaging.
References
- 1.de Geus-Oei LF, Vriens D, van Laarhoven HW, van derGraaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med 2009;50:43S–54S [DOI] [PubMed] [Google Scholar]
- 2.de Geus-Oei LF, van Laarhoven HW, Visser EP, Hermsen R, van Hoorn BA, Kamm YJ, et al. Chemotherapy response evaluation with FDG-PET in patients with colorectal cancer. Ann Oncol 2008;19:348–52 [DOI] [PubMed] [Google Scholar]
- 3.Kong G, Jackson C, Koh DM, Lewington V, Sharma B, Brown G, et al. The use of 18F-FDG PET/CT in colorectal liver metastases—comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging 2008;35:1323–9 [DOI] [PubMed] [Google Scholar]
- 4.Lubezky N, Metser U, Geva R, Nakache R, Shmueli E, Kalusner JM, et al. The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastroint Surg 2007;11:472–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Chen Y, Xue H, Zheng P, Tong J, Liu J, et al. Diagnostic value of FDG-PET in recurrent colorectal carcinoma: A meta-analysis. Int J Cancer 2009;124:167–73 [DOI] [PubMed] [Google Scholar]
- 6.Shin SS, Jeong YY, Min JJ, Kim HR, Chung TW, Kang HK. Preoperative staging of colorectal cancer: CT vs. integrated PET/CT. Abdom Imaging 2008;33:270–7 [DOI] [PubMed] [Google Scholar]
- 7.Tsunoda Y, Ito M, Fujii H, Kuwano H, Saito N. Preoperative diagnosis of lymph node metastases of colorectal cancer by FDG-PET/CT. Jpn J Clin Oncol 2008;35:347–53 [DOI] [PubMed] [Google Scholar]
- 8.Llamas-Elvira JM, Rodriguez-Fernandez A, Gutierrez-Sainz J, Gomez-Rio M, Bellon-Guardia M, Ramos-Font C, et al. Fluorine-18 fluorodeoxyglucose PET in the preoperative staging of colorectal cancer. Eur J Nucl Med Mol Imaging 2007;34:859–67 [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Lim ST, Jeong YJ, Kim DW, Jeong HJ, Sohn MH. The clinical value of dual time point F-18 FDG PET/CT imaging for the differentiation of colonic focal uptake lesions. Nucl Med Mol Imaging 2009;43:309–16 [Google Scholar]
- 10.Yoshida K, Umehara I, Narumi H, Toriihara A, Nakagawa T, Toda K. Normal variants in bowel FDG uptake in dual-time point PET imaging. J Nucl Med 2007;48:65P. [DOI] [PubMed] [Google Scholar]
- 11.Souza FF, Fennessy FM, Yang Q, van denAbbeele AD. PET/CT appearance of desmoid tumor of the chest wall. Br J Radiol 2010;83:e39–e42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Font C, Santiago Chinchilla A, Rebollo Aguirre AC, Rodríguez Fernández A, Medina Benítez A, Llamas Elvira JM. Desmoid tumor of the thoraco-abdominal wall characterized with 18F-fluorodeoxyglucose PET/CT scan. Correlation with magnetic resonance and bone scintigraphy. Review of the literature. Rev Esp Med Nucl 2009;28:70–3 [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Nair N, Banavali S. Uptake characteristics of fluorodeoxyglucose (FDG) in deep fibromatosis and abdominal desmoids: potential clinical role of FDG-PET in the management. Br J Radiol 2007;80:750–6 [DOI] [PubMed] [Google Scholar]
- 14.Stout AP. Juvenile fibromatoses. Cancer 1954;7:953–78 [DOI] [PubMed] [Google Scholar]
- 15.Huang K, Fu H, Shi YQ, Zhou Y, Du CY. Prognostic factors for extra-abdominal and abdominal wall desmoids: a 20-year experience at a single institution. J Surg Oncol 2009;100:563–9 [DOI] [PubMed] [Google Scholar]
- 16.Yantiss RK, Spiro IJ, Compton CC, Rosenberg AE. Gastrointestinal stromal tumor versus intra-abdominal fibromatosis of the bowel wall: a clinically important differential diagnosis. Am J Surg Pathol 2000;24:947–57 [DOI] [PubMed] [Google Scholar]
- 17.Kreuzberg B, Koudelova J, Ferda J, Treska V, Spidlen V, Mukensnabl P. Diagnostic problems of abdominal desmoid tumors in various locations. Eur J Radiol 2007;62:180–5 [DOI] [PubMed] [Google Scholar]
- 18.Easter DW, Halasz NA. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann Surg 1989;210:765–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Numanoglu A, Davies J, Millar AJ, Rode H. Congenital solitary intestinal fibromatosis. Eur J Pediatr Surg 2002;12:337–40 [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z, Li F, Zhuang H, Yan J, Wu C, Cheng W. FDG PET/CT detection of intussusception caused by aggressive fibromatosis. Clin Nucl Med 2010;35:370–3 [DOI] [PubMed] [Google Scholar]
- 21.Kasper B, Dimitrakopoulou-Strauss A, Strauss LG, Hohenberger P. Positron emission tomography in patients with aggressive fibromatosis/desmoid tumours undergoing therapy with imatinib. Eur J Nucl Med Mol Imaging 2010;37:1876–82 [DOI] [PubMed] [Google Scholar]
- 22.Kantorova I, Lipska L, Belohlavek O, Visokai V, Trubac M, Schneiderova M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med 2003;44:1784–8 [PubMed] [Google Scholar]
- 23.Reddy MP, Reddy P, Lilien DL. F-18 FDG PET imaging in gastrointestinal stromal tumor. Clin Nucl Med 2003;28:677–9 [DOI] [PubMed] [Google Scholar]
- 24.Tatlidil R, Jadvar H, Bading JR, Conti PS. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology 2002;224:783–7 [DOI] [PubMed] [Google Scholar]
- 25.Kakati BR, Krishna K, Krishna SG, Sharma SG, Sanathkumar N, Rego RF. Extensive extramedullary disease involving the colon in multiple myeloma: a case report and review of literature. J Gastrointest Cancer. doi: 10.1007/s12029-010-9199-z. in press. Epub ahead of print Aug 2010. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann M, Vogelsang H, Kletter K, Zettinig G, Chott A, Raderer M. 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) for assessment of enteropathy-type T cell lymphoma. Gut 2003;52:347–51 [DOI] [PMC free article] [PubMed] [Google Scholar]