Abstract
mRNA-dependent cell-free protein synthesis systems were prepared from a heme-deficient ole3 mutant of the yeast Saccharomyces cerevisiae grown either in the absence or in the presence of the heme precursor δ-aminolevulinate. When supplemented with total yeast mRNA, the two systems—from heme-deficient and from heme-containing cells—translate most mRNAs with comparable efficiencies. mRNAs coding for the hemoproteins catalase T and catalase A, however, are translated at a low rate by the system from heme-deficient cells whereas their translation in the system from heme-containing cells is comparable to that observed in a heterologous in vitro system from wheat germ. Addition of 10 μM hemin to the system from heme-deficient cells stimulates translation of catalase mRNAs significantly. Control experiments showed that the results obtained cannot be explained by specific proteinase or nuclease action. Together with previous findings indicating lack of translation of catalase T mRNA in heme-deficient cells in vivo, the results demonstrate that specific control of yeast catalase formation occurs at the level of translation.
Keywords: catalase T, catalase A, homologous mRNA-dependent translation system, heme-deficient ole3 mutant
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Translational control of protein synthesis during the early stages of differentiation of the slime mold Dictyostelium discoideum. Cell. 1977 Sep;12(1):301–310. doi: 10.1016/0092-8674(77)90208-2. [DOI] [PubMed] [Google Scholar]
- Ammerer G., Richter K., Hartter E., Ruis H. Synthesis of Saccharomyces cerevisiae catalase A in vitro. Eur J Biochem. 1981 Jan;113(2):327–331. doi: 10.1111/j.1432-1033.1981.tb05070.x. [DOI] [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Bard M., Woods R. A., Haslam J. M. Porphyrine mutants of Saccharomyces cerevisiae: correlated lesions in sterol and fatty acid biosynthesis. Biochem Biophys Res Commun. 1974 Jan 23;56(2):324–330. doi: 10.1016/0006-291x(74)90845-6. [DOI] [PubMed] [Google Scholar]
- Benoff S., Bruce S. A., Skoultchi A. I. Negative control of hemoglobin production in somatic cell hybrids due to heme deficiency. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4354–4358. doi: 10.1073/pnas.75.9.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. J., Jr, Murnane M. J., Tonkonow B. L., Berman B. W., Mazur E. M., Cavallesco C., Jenko T., Snyder E. L., Forget B. G., Hoffman R. Embryonic-fetal erythroid characteristics of a human leukemic cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3509–3513. doi: 10.1073/pnas.77.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chen J. J., London I. M. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981 Oct;26(1 Pt 1):117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gasior E., Herrera F., Sadnik I., McLaughlin C. S., Moldave K. The preparation and characterization of a cell-free system from Saccharomyces cerevisiae that translates natural messenger ribonucleic acid. J Biol Chem. 1979 May 25;254(10):3965–3969. [PubMed] [Google Scholar]
- Haslam J. M., Astin A. M. The use of heme-deficient mutants to investigate mitochondrial function and biogenesis in yeast. Methods Enzymol. 1979;56:558–568. doi: 10.1016/0076-6879(79)56054-6. [DOI] [PubMed] [Google Scholar]
- Hofbauer R., Fessl F., Hamilton B., Ruis H. Preparation of a mRNA-dependent cell-free translation system from whole cells of Saccharomyces cerevisiae. Eur J Biochem. 1982 Feb;122(1):199–203. doi: 10.1111/j.1432-1033.1982.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Hörlein D., McPherson J., Goh S. H., Bornstein P. Regulation of protein synthesis: translational control by procollagen-derived fragments. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6163–6167. doi: 10.1073/pnas.78.10.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay F. T., Laughlin C. A., Carter B. J. Eukaryotic translational control: adeno-associated virus protein synthesis is affected by a mutation in the adenovirus DNA-binding protein. Proc Natl Acad Sci U S A. 1981 May;78(5):2927–2931. doi: 10.1073/pnas.78.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
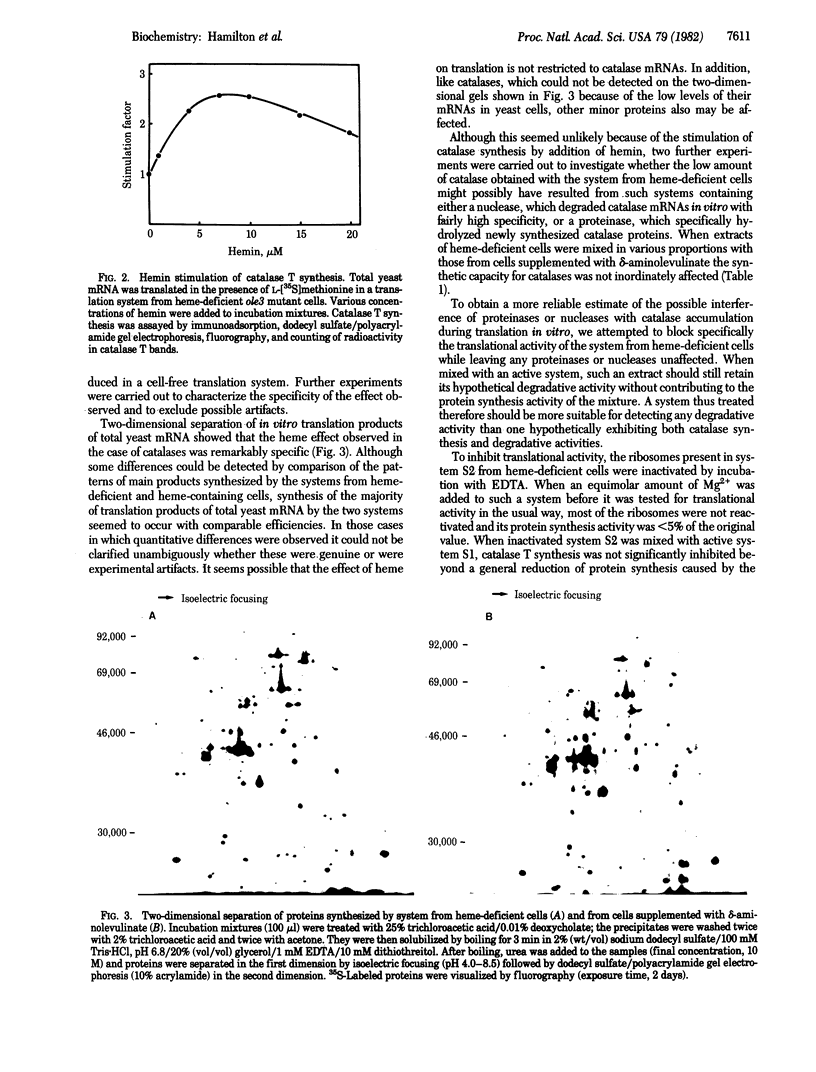
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ochoa S., de Haro C. Regulation of protein synthesis in eukaryotes. Annu Rev Biochem. 1979;48:549–580. doi: 10.1146/annurev.bi.48.070179.003001. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Richter K., Ammerer G., Hartter E., Ruis H. The effect of delta-aminolevulinate on catalase T-messenger RNA levels in delta-aminolevulinate synthase-defective mutants of Saccharomyces cerevisiae. J Biol Chem. 1980 Sep 10;255(17):8019–8022. [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Ross J., Sautner D. Induction of globin mRNA accumulation by hemin in cultured erythroleukemic cells. Cell. 1976 Aug;8(4):513–520. doi: 10.1016/0092-8674(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Rovera G., Vartikar J., Connolly G. R., Magarian C., Dolby T. W. Hemin controls the expression of the beta minor globin gene in Friend erythroleukemic cells at the pretranslational level. J Biol Chem. 1978 Nov 10;253(21):7588–7590. [PubMed] [Google Scholar]
- Rutherford T. R., Harrison P. R. Globin synthesis and erythroid differentiation in a Friend cell variant deficient in heme synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5660–5664. doi: 10.1073/pnas.76.11.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G., Brooker J. D., May B. K., Elliott W. H. Haem control in experimental porphyria. The effect of haemin on the induction of delta-aminolaevulinate synthase in isolated chick-embryo liver cells. Biochem J. 1980 Jun 15;188(3):781–788. doi: 10.1042/bj1880781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Godefroy-Colburn T., Thach R. E. The role of mRNA competition in regulating translation. I. Demonstration of competition in vivo. J Biol Chem. 1981 Nov 25;256(22):11739–11746. [PubMed] [Google Scholar]
- Zimniak P., Hartter E., Woloszczuk W., Ruis H. Catalase biosynthesis in yeast: formation of catalase A and catalase T during oxygen adaptation of Saccharomyces cerevisiae. Eur J Biochem. 1976 Dec 11;71(2):393–398. doi: 10.1111/j.1432-1033.1976.tb11126.x. [DOI] [PubMed] [Google Scholar]