Abstract
Malignant tumours of the sacrum may be primary or secondary. While sacral metastases are frequently encountered, a diagnostic dilemma can present when there is a single sacral bone tumour with no history or evidence of malignancy elsewhere in the body. Familiarity with the imaging features and clinical presentations of primary malignant bone tumours is helpful in narrowing the differential. This pictorial review will illustrate with both common and uncommon malignant sacral tumours CT, MRI and positron emission tomography/CT, highlighting the specific features of each.
The sacrum is composed of bone, cartilage and bone marrow, as well as notochord remnants. Malignant sacral tumours can arise from any one of its components. Since the sacrum contains haematopoietic bone marrow, it is a common site of metastases and is also susceptible to haematological malignancies such as lymphoma, multiple myeloma or plasmacytoma. Metastases are, in fact, the most common malignant tumours of the sacrum. Primary malignant sacral tumours are uncommon and include chordoma and sarcomas such as chondrosarcoma, osteosarcoma and Ewing sarcoma.
In this pictorial review, we will outline with illustrations both primary and secondary sacral malignant bone tumours, and highlight the clinical and imaging features of each tumour.
Primary malignant bone tumours of the sacrum
Chordoma
Chordomas are most common in individuals aged 40–70 years, and occur twice as often in males as in females [1]. They are the most common primary tumour of the sacrum, arising from notochordal rests. 50–60% of chordomas occur in the sacrococcygeal region [1]. Chordoma is a low-grade tumour but can cause significant morbidity and mortality with local recurrence. Metastatic disease is uncommon but is seen more frequently in sacral chordomas than in skull base chordomas [2]. Complete surgical resection is the mainstay of therapy. The most important predictor of lack of recurrence and improved survival is a wide tumour-free margin achieved at initial surgical resection, with a 10-year survival of 52% [3]. Chordoma is insensitive to chemotherapeutic agents. The role of radiation therapy is not yet clearly defined; however, it can be used in the setting of inoperable chordomas [4].
Sacral chordoma usually arises from the third, fourth or fifth sacral vertebra in the midline or paramedian location, and is often seen as a large destructive osteolytic lesion with extraosseous extension (Figure 1) [1]. When there is extraosseous extension, the tumour can be seen to extend exophytically into the pre-sacral region or sacral canal, resulting in a “mushroom” or “dumbbell” shape (Figure 1). Internal calcifications are frequently seen on plain radiographs and CT [1]. The tumour can spread over several segments, with or without involvement of the intervertebral discs. In >50% of cases, there are areas of decreased attenuation within the tumour on CT, which reflect myxoid-type tissue presented pathologically [5]. A fibrous pseudocapsule is common, which is of increased attenuation relative to the rest of the tumour [5].
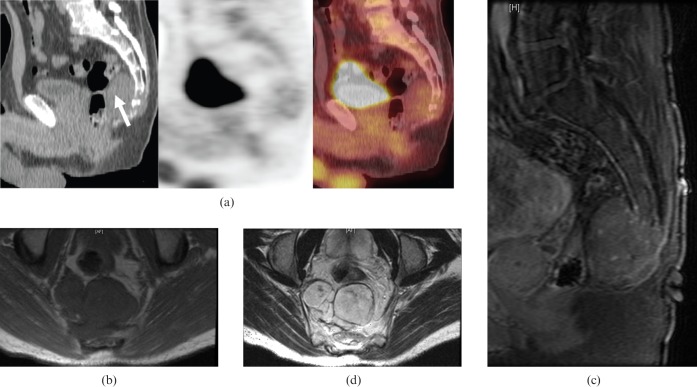
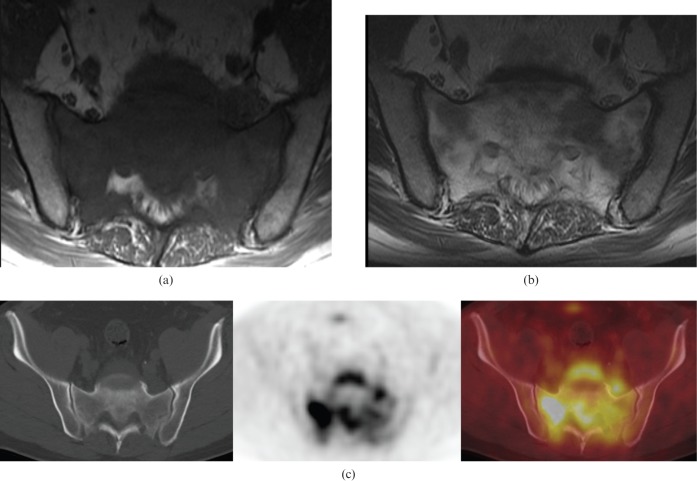
Figure 1.
55-year-old male with sacral chordoma. (a) Sagittal 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT images showing low FDG uptake, similar to blood pool/background. (b) Axial T1 weighted MR image showing a lobulated hypointense mass arising from the lower sacrum. (c) Sagittal post-contrast fat-saturated T1 weighted MR image demonstrating nodular enhancement of the sacral mass with destruction of the lower sacrum. (d) Axial T2 weighted MR image showing a lobulated hyperintense mass with hypointense internal septations arising from the lower sacrum extending into the pre-sacral space.
On MRI, chordomas are hypointense or isointense on T1 weighted images (Figure 1b) and hyperintense on T2 weighted images (Figure 1d). Hyperintense foci can be seen on T1 weighted imaging, representing either haemorrhage or proteinaceous material within the tumour. Enhancement of the extraosseous component varies from mild to moderate. The septations, which divide the gelatinous components of this tumour, are hypointense on T2 weighted images. Hypointense haemosiderin from previous haemorrhage is seen more frequently in sacral than in spinal chordomas on T2 weighted imaging [6]. To date, there have been only two case reports evaluating 18F-fluorodeoxyglucose (18F-FDG) uptake in chordomas on positron emission tomography (PET)/CT, which describe either low or intermediate uptake of FDG (Figure 1a) [7,8].
Primary sacral sarcoma
Primary sarcomas of the sacrum are rare and include chondrosarcoma, Ewing sarcoma and osteosarcoma, in order of decreasing incidence. Only Ewing sarcoma is seen in children and young adults, with the others presenting in individuals aged 30–70 years [5,9].
Primary chondrosarcoma of the sacrum predominantly affects men aged between 30 and 70 years [9]. Males are affected two to four times more than females [10]. Conventional chondrosarcoma can be categorised according to its location in the bone; central chondrosarcomas are located in the medullary cavity and peripheral chondrosarcomas arise from the surface of the bone [11]. Chondrosarcoma can arise either de novo, in which case it is termed a primary chondrosarcoma, or as a result of malignant transformation of an enchondroma (central) or osteochondroma (peripheral), termed a secondary chondrosarcoma. All types can affect the pelvic bones, including the sacrum; however, peripheral secondary chondrosarcoma is seen more commonly in younger patients than in central primary chondrosarcoma, which predominantly affects patients >50 years of age [11]. The thoracolumbar spine is affected more frequently than the sacrum [12]. It is a low-grade tumour, which produces cartilage matrix and tends to recur if incompletely resected at surgery.
Imaging features of chondrosarcoma include a destructive lytic tumour with a lobulated contour, which results in endosteal scalloping and can demonstrate extraosseous extension [10]. Destruction of the surrounding trabecular bone is a common radiological feature of this tumour [10]. “Ring and arc” calcifications of the chondroid matrix are typically seen, which can be appreciated on radiographs or CT [5]. The non-mineralised portion of the tumour is hypodense on CT relative to skeletal muscle, owing to the high water content of hyaline cartilage. On MRI, the hyaline cartilage nodules are iso- to hypointense on T1 weighted images and hyperintense on T2 weighted images (Figure 2a,b). Calcification is seen as areas of signal void on all MRI sequences. The degree of contrast enhancement is usually mild, with varying patterns of enhancement: nodular (Figure 2c), septal or diffuse. The tumour can extend through the intervertebral disc in up to 35% of cases [5]. The imaging features of chondrosarcoma can be similar to those of chordoma, and can pose a clinical problem, for which biopsy is frequently the only solution.
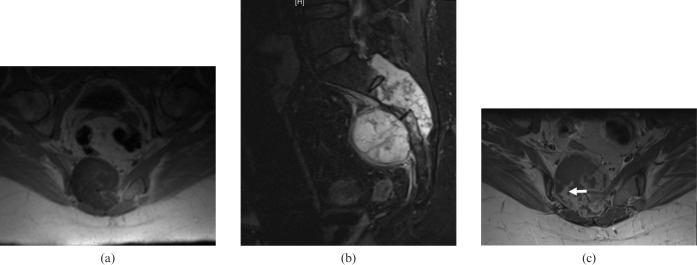
Figure 2.
52-year-old female with primary sacral chondrosarcoma. (a) Axial T1 weighted pre-contrast MR image showing a hypointense mass with intracanalicular extension. (b) Sagittal short-inversion-time inversion recovery MR image showing a large destructive hyperintense mass arising from the second sacral vertebra. The posterior cortex of the involved vertebra is destroyed, with preservation of the intervertebral disc between S1 and S2. (c) Axial T1 weighted contrast-enhanced MR image showing peripheral nodular enhancement (arrow). The central portion of the tumour is poorly enhancing, owing to the presence of myxoid/chondral tissue.
Primary vertebral Ewing sarcoma is usually seen in the second decade of life, and is more common in males than in females (62% vs 38% of cases, respectively) [9]. Ewing sarcoma is a high-grade malignancy with a proliferation of undifferentiated small, round cells. Primary Ewing sarcoma of the spine is uncommon, representing only 3–10% of cases, with metastatic disease from extraspinal Ewing sarcoma seen more frequently [13]. The sacral ala is the most common site for primary Ewing sarcoma of the spine [9]. The prognosis is worse for sacrococcygeal Ewing sarcoma than for extraspinal Ewing sarcoma, usually owing to to larger tumour size at presentation because of delayed clinical presentation [5]. If tumours are small, complete surgical resection may be possible; chemotherapy and radiation therapy are the mainstay of treatment with large unresectable tumours.
Ewing sarcoma presents as a destructive osteolytic lesion, frequently with a dominant extraosseous component [5]. Invasion of the spinal canal is common, particularly with a large mass [9]. The extraosseous component is often larger than the intraosseous component [9]. On MRI, the tumour is iso- to hypointense on T1 weighted images and iso- to hyperintense on T2 weighted images, with variable contrast enhancement that is relatively non-specific [5].
Patients with primary spinal osteosarcoma are older at presentation than those with appendicular lesions; the condition occurs most often in the fourth decade of life, compared with the second for appendicular lesions [5]. It is more common in males than in females and most frequently affects the lumbosacral spine [5]. Secondary sacral osteosarcoma occurs in patients with previous radiation treatment or a history of Paget's disease. Elderly patients with polyostotic Paget's disease are most at risk of sarcomatous degeneration.
Most spinal osteosarcomas are pathologically osteoblastic, resulting in densely mineralised lesions on radiographs and CT (Figure 3). MRI features of osteosarcoma are non-specific; the tumour is typically hypointense on T1 weighted imaging and hyperintense on T2 weighted imaging with contrast enhancement. Foci of bone formation are usually seen as foci of signal void on all sequences. MRI is useful in guiding biopsy of the lesion, identifying skip lesions and surgical planning. On PET/CT, there is increased 18F-FDG uptake within the tumour, which decreases following effective neoadjuvant therapy [14]. Bone scintigraphy is still recommended for the staging of osteosarcoma; however, whole-body MRI and PET/CT are currently being evaluated for the detection of metastatic disease [15].
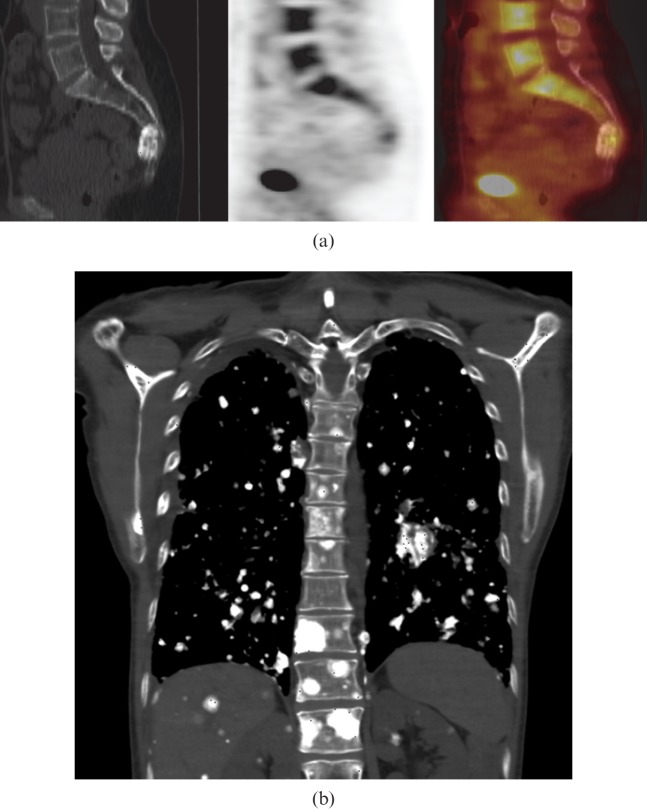
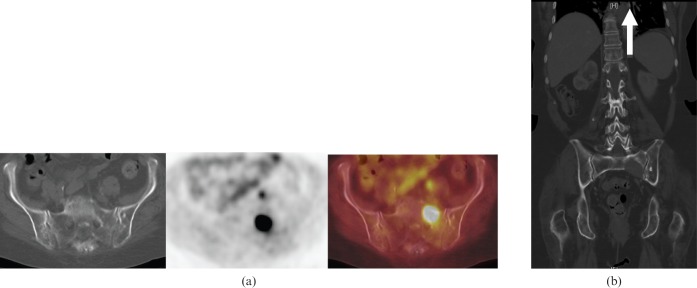
Figure 3.
54-year-old female with primary sacral osteosarcoma. (a) Sagittal 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/CT images showing mild 18F-FDG uptake in a sclerotic mass involving the fourth sacral vertebra at presentation. This is not typical of osteosarcomas, which usually demonstrate more FDG uptake. (b) Coronal CT of the chest shows multiple calcified nodules in lungs, liver, kidneys, ribs, right scapula and thoracic vertebra, in keeping with diffuse metastatic osteosarcoma 3 years after presentation.
Other sarcomas can arise from the sacrum, including the very rare clear cell sarcoma (Figure 4), which usually arises from tendons or aponeuroses of the extremities. It is pathologically identical to malignant melanoma, but has a specific chromosomal translocation that is not found in cutaneous malignant melanoma [16].
Figure 4.
25-year-old female with recurrent primary clear cell sarcoma of the sacrum. (a) Sagittal short-inversion-time inversion recovery MR image showing a hyperintense mass involving the second and third sacral vertebra with extension of the mass anteriorly into the pre-sacral space and also posteriorly. (b) Axial T1 weighted fat-suppressed contrast-enhanced MR images showing enhancement of the mass with intracanalicular extension. (c) Axial 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/CT images showing a mixed lytic and sclerotic lesion of the sacrum. Increased 18F-FDG uptake is asymmetrical, with more uptake on the left correlating with the site of less severe bone destruction.
Haematological malignancies involving the sacrum
Multiple myeloma and plasmacytoma
The majority of patients with multiple myeloma and plasmacytoma are >60 years of age at presentation, with a male predominance [9]. A plasmacytoma is a focal proliferation of malignant plasma cells, without bone marrow involvement elsewhere. A solitary plasmacytoma is seen in only 3–7% of patients with plasma cell neoplasms [9]. It is often considered the early form of multiple myeloma, so has a better prognosis.
CT often demonstrates a predominantly lytic pattern with peripheral sclerosis or purely lytic lesions with a “soap bubble” appearance [9]. Cortical bone is partly preserved or can become more sclerotic [9]. On MRI, a plasmacytoma is hypointense to healthy marrow on T1 weighted images, is hyperintense on T2 weighted images and enhances avidly post-contrast (Figure 5). PET/CT demonstrates increased FDG uptake in the plasmacytoma and can rule out distant disease and, therefore, the diagnosis of myeloma.
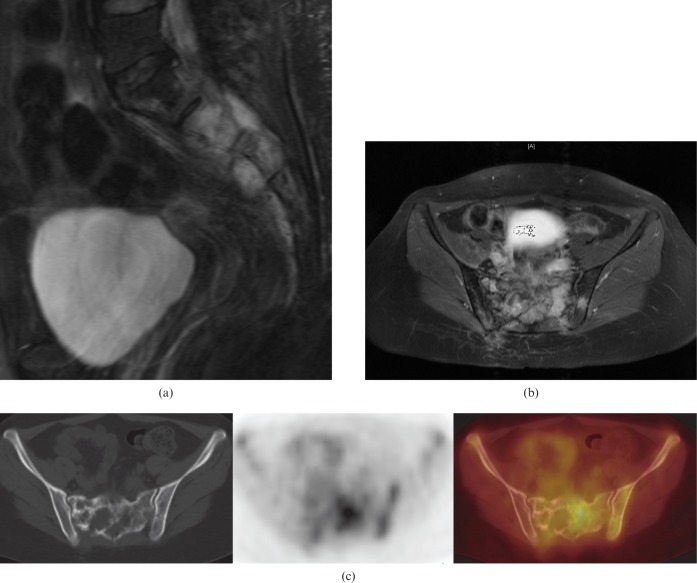
Figure 5.
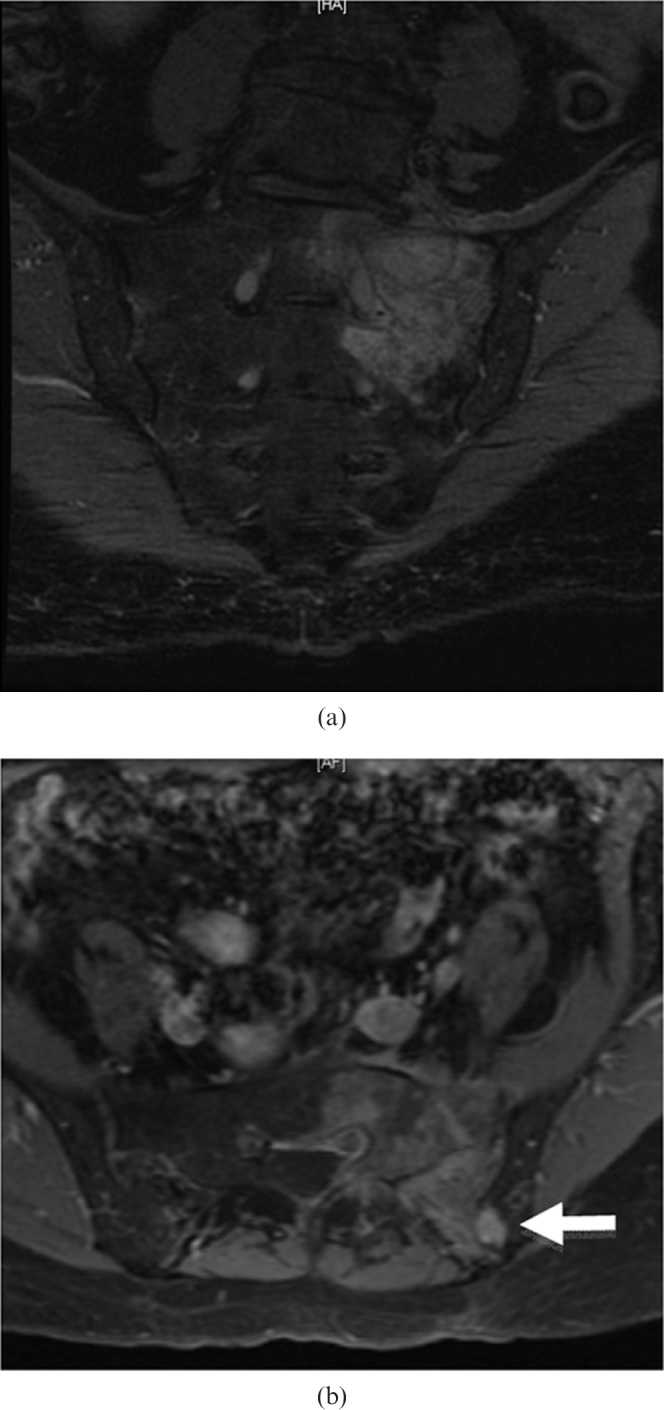
78-year-old male with sacral plasmacytoma. (a) Coronal oblique short-inversion-time inversion recovery MR image showing a hyperintense mass in the left sacral ala centred on S1 extending into the left sacral neural exit foramen of S1. There is no evidence of spread across the L5/S1 intervertebral disc. (b) Axial T1 weighted fat-suppressed contrast-enhanced MR image showing heterogeneous diffuse enhancement of the mass. There is contiguous spread of this mass across the left sacroiliac joint into the left iliac bone (arrow).
Lymphoma
Primary lymphoma of the sacrum has a peak incidence during the second and third decades of life, affecting more males than females at a ratio of 2:1 [17]. Lymphoma involving the sacrum may be due to either primary bone lymphoma or secondary involvement of the bone in disseminated disease where viscera, lymph nodes and bones are involved. Primary bone lymphoma, by definition, is the lymphomatous involvement of a single bone, and persists for longer than 6 months, with no evidence of disseminated disease [18]. It is extremely rare, accounting for only 1% of cases of non-Hodgkin lymphoma [18].
Frequently, radiographic findings are absent; however, when they are present, a permeative lytic lesion is most often seen, with minimal cortical destruction. On CT, there can be minimal, if any, abnormality present (Figure 6). MRI demonstrates involvement of the bone marrow, with well-delineated hypointense areas on T1 weighted imaging and hyperintense areas on T2 weighted imaging, which enhance after contrast administration at the sites of disease (Figure 6). PET/CT shows increased FDG uptake at the site of lymphomatous involvement (Figures 6c and 7d). In primary bone lymphoma, 18F-FDG PET/CT can be particularly useful after treatment with chemoradiation, because a focal abnormality in the bone usually persists on MRI following adequate treatment. The absence of FDG uptake in the abnormal bone may help to exclude residual or recurrent tumours (Figure 7e) [19].
Figure 6.
53-year-old male with primary sacral lymphoma. (a) Axial T1 weighted MR image showing complete replacement of the normal bone marrow with a hypointense mass. A small pre-sacral soft tissue mass is also present. (b) Axial T1 weighted contrast-enhanced MR image showing diffuse heterogeneous enhancement of the sacral mass without involvement of the sacroiliac joints. (c) Axial 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/CT images showing increased 18F-FDG uptake in the sacrum, particularly on the right sacrum. Note how the sacrum is relatively normal on CT, despite the extensive abnormality on MR.
Figure 7.
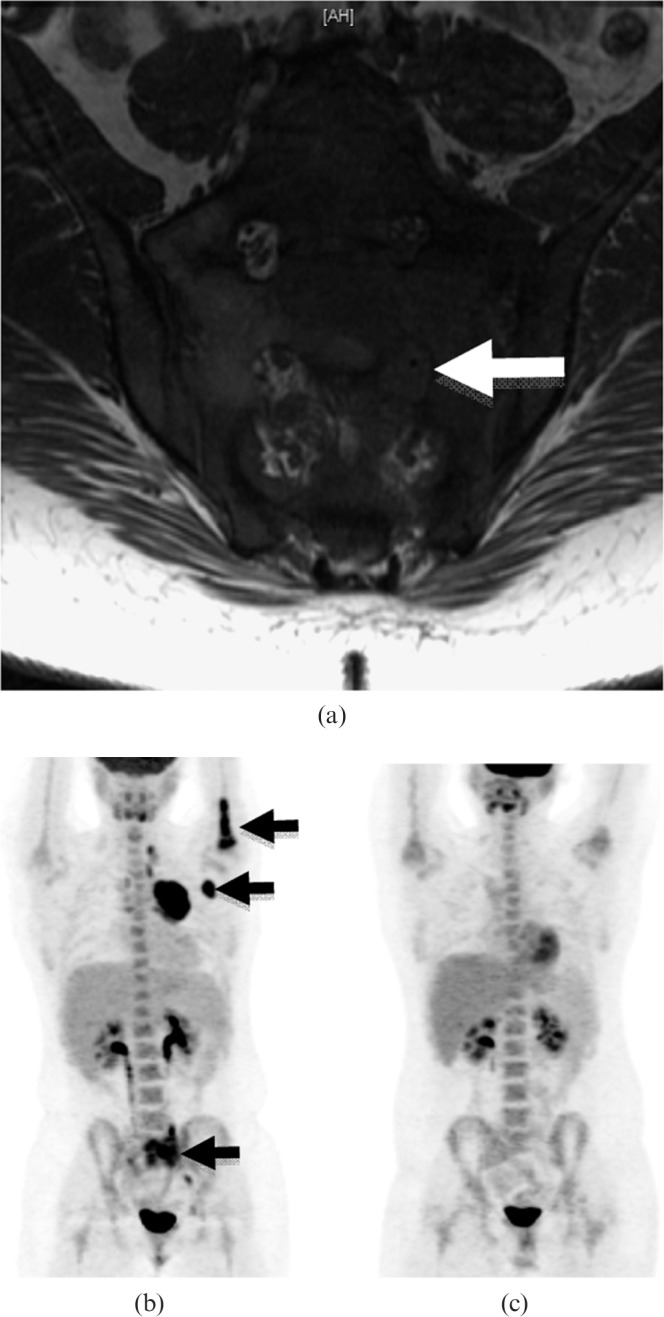
16-year-old female with secondary sacral lymphoma. (a) Coronal oblique T1 weighted unenhanced MR image showing that the infiltrative mass is hypointense. There is obliteration of the fat within the neural exit foramina of S1 and S2 on the left due to extension of the tumour into the foramen, surrounding the nerve roots (arrow). (b) 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT coronal maximum intensity projection demonstrating increased FDG uptake in the sacrum and the proximal left humerus, as well as in the left axillary lymph nodes (arrows), small left pelvic and left cervical lymph nodes. (c) Axial 18F-FDG PET/CT images showing a photopenic area at the site of the previous lymphoma following radiation treatment. There is no longer increased FDG uptake in the humerus and previously FDG-avid lymph nodes following treatment with chemotherapy.
Secondary malignant bone tumours of the sacrum from epithelial malignancies
Metastases from lung, breast, prostate, kidney, head and neck; melanoma; and gastrointestinal cancers are the most common malignant sacral tumours [17]. Metastases are usually osteolytic (Figure 8), except in prostate and breast cancer, where they are frequently osteoblastic. Bone lesions elsewhere in the skeleton further indicate metastatic disease.
Figure 8.
75-year-old female with sacral lytic bone metastasis from primary non-small cell lung cancer. (a) Axial 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/CT images showing a lytic lesion in the left sacrum, which is 18F-FDG-avid. (b) Coronal CT of the abdomen and pelvis 6 months later showing a larger lytic lesion in the left sacrum with a soft tissue component. Note the surgical suture material in the left infrahilar region from previous left lower lobectomy (arrow).
Bone scintigraphy with radiographs or cross-sectional imaging can be used to diagnose bone metastases, with increased radiotracer uptake in the metastases. The role of PET/CT in the detection of bone metastases is not yet clear and appears to be disease-specific [20]. PET/CT demonstrates increased FDG uptake at the site of bone metastases in some but not all of the malignancies named above. In particular, bone scintigraphy has been shown to be more sensitive than PET/CT in the detection of bone metastases in prostate cancer [21].
Approach to differentiating malignant sacral tumours
Although imaging findings of malignant sacral tumours overlap, certain features may be helpful in narrowing the differential. We recommend evaluating where the epicentre of the lesion lies (midline vs eccentric and upper vs lower sacral vertebra), and looking for the presence or absence of a matrix as well as the presence or absence of extraosseous extension.
Chordomas lie predominantly in the midline, unlike chondrosarcomas which lie eccentrically [1,2]. Chordomas tend to arise from the lower sacral segments or sacrococcygeal region; by contrast, chondrosarcomas generally arise from the mid to upper sacrum [5]. When calcification is present in chordomas, it is amorphous and predominates in the periphery of the lesion, as opposed to the ring-and-arc calcification seen in chondrosarcomas from the chondroid matrix [5]. When present, dense amorphous osteoid matrix is a characteristic finding in osteosarcomas [5].
Ewing sarcoma is seen in young adults, with a permeative lesion without a definite matrix, and may be associated with disproportionately large extraosseous extension [5]. A sclerotic variant of Ewing sarcoma is rare but when it happens it typically affects only the pelvis [9]. Sacral lymphomas usually diffusely involve the entire sacrum, with frequent extraosseous extension [2]. The tumour may extend into the pre-sacral region and neural exit foramina, with no visible cortical defects [2]. Lymphadenopathy, when present, may provide an additional clue. Plasmacytomas tend more often to be eccentric than midline. These tumours may be infiltrating and slightly expansile, with no internal matrix [22]. The overall structure of the sacrum is frequently preserved in cases of sacral plasmacytoma [22].
In a patient with a solitary sacral mass who has no history of primary malignancy, a biopsy should be performed to identify the exact pathology.
Management of malignant sacral tumours
A pathological diagnosis should be obtained in all cases. CT-guided core needle biopsy using a posterior midline entry portal at the appropriate level is most commonly performed [23]. This approach minimises local contamination and ensures that the biopsy tract is excised at the time of definitive surgery. Consultation with the surgeon prior to biopsy is recommended to ensure that the tract proposed by the radiologist will be excised at the time of surgery. In cases of non-calcified necrotic tumours, enhancing soft tissue or the FDG-avid component seen on PET/CT may increase the yield of the biopsy. If an adequate sample is not obtained following percutaneous biopsy, an open biopsy should be pursued.
Primary surgical resection is the treatment of choice for localised primary malignant sacral tumours, except in cases of lymphomatous involvement of the sacrum and plasma cell tumours. Pre-operative planning with both CT and MRI is vital to assess the exact extent of the tumour; particular attention should be paid to the specific vertebrae, muscles, nerves, joints and pelvic viscera that are involved by the tumour and would need to be resected at surgery.
En bloc resection with wide surgical margins is the optimal technique for most tumours, resulting in the lowest rates of recurrence [23]. Excision through the vertebral body cephalad to the uppermost diseased level is recommended [23]. If the tumour lies at or below the level of S4, a posterior approach alone is required [23]. Tumours lying above S4 require both an anterior and a posterior approach [23]. When >50% of either sacroiliac joint is resected, lumbosacral stabilisation is required [23]. If the tumour is spread to the lumbar spine, hemicorporectomy is required [24]. With eccentric tumours, lateral sacrectomy is performed [24]. If pelvic viscera are involved, pelvic exenteration may be required for tumour-free margins.
Contraindications to surgery
There is no absolute contraindication for surgical resection. However, given the mortality and morbidity associated with extensive pelvic/sacral surgeries, the decision for surgical resection is dictated by local practice and the surgical expertise available. The level of sacral amputation in tumour resection correlates with the expected neurological deficit [25]. With a high sacrectomy, when S1–S5 are sacrificed, patients should be advised pre-operatively of expected post-operative motor paresis, the loss of sphincter control, saddle anaesthesia and sexual dysfunction [25]. With a middle sacrectomy, when S2–S5 are sacrificed, the loss of motor function is unlikely; however, the loss of sphincter control as well as perineal anaesthesia and sexual dysfunction are highly likely [25]. Low amputations (distal–S3) and unilateral sacrificing of S1–S5 usually lead to minimal deficits, with preservation of sphincter control and motor function but possible sexual dysfunction [25].
Conclusion
While secondary malignant tumours of the sacrum are more common than primary malignant tumours, it is important that radiologists are familiar with the imaging features of both, especially in the setting of a solitary sacral mass.
CT, PET/CT and MRI are complementary imaging modalities, each with its individual strength: CT demonstrates excellent spatial resolution of bone lesions and allows for assessment of tumour matrix, PET allows for metabolic evaluation of lesions and MRI demonstrates superior soft tissue resolution.
References
- 1.Diel J, Ortiz O, Losada RA, Price DB, Hayt MW, Katz DS. The sacrum: pathologic spectrum, multimodality imaging, and subspecialty approach. Radiographics 2001;21:83–104 [DOI] [PubMed] [Google Scholar]
- 2.Ha S. Imaging of sacral masses: self-assessment module. AJR Am J Roentgenol 2010;195:S32–6 [DOI] [PubMed] [Google Scholar]
- 3.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am 2005;87:2211–16 [DOI] [PubMed] [Google Scholar]
- 4.Gerber S, Ollivier L, Leclere J, Vanel D, Missenard G, Brisse H, et al. Imaging of sacral tumours. Skeletal Radiol 2008;37:277–89 [DOI] [PubMed] [Google Scholar]
- 5.Murphey MD, Andrews CL, Flemming DJ, Temple HT, Smith WS, Smirniotopoulos JG. From the archives of the AFIP. Primary tumors of the spine: radiologic pathologic correlation. Radiographics 1996;16:1131–58 [DOI] [PubMed] [Google Scholar]
- 6.Sung MS, Lee GK, Kang HS, Kwon ST, Park JG, Suh JS, et al. Sacrococcygeal chordoma: MR imaging in 30 patients. Skeletal Radiol 2005;34:87–94 [DOI] [PubMed] [Google Scholar]
- 7.Miyazawa N, Ishigame K, Kato S, Satoh Y, Shinohara T. Thoracic chordoma: review and role of FDG-PET. J Neurosurg Sci 2008;52:117–21; discussion 121–2 [PubMed] [Google Scholar]
- 8.Park SA, Kim HS. F-18 FDG PET/CT evaluation of sacrococcygeal chordoma. Clin Nucl Med 2008;33:906–8 [DOI] [PubMed] [Google Scholar]
- 9.Rodallec MH, Feydy A, Larousserie F, Anract P, Campagna R, Babinet A, et al. Diagnostic imaging of solitary tumors of the spine: what to do and say. Radiographics 2008;28:1019–41 [DOI] [PubMed] [Google Scholar]
- 10.Murphey MD, Walker EA, Wilson AJ, Kransdorf MJ, Temple HT, Gannon FH. From the archives of the AFIP: imaging of primary chondrosarcoma: radiologic-pathologic correlation. Radiographics 2003;23:1245–78 [DOI] [PubMed] [Google Scholar]
- 11.Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, et al. The clinical approach towards chondrosarcoma. Oncologist 2008;13:320–9 [DOI] [PubMed] [Google Scholar]
- 12.Manaster BJ, Graham T. Imaging of sacral tumors. Neurosurg Focus 2003;15:E2. [DOI] [PubMed] [Google Scholar]
- 13.Llauger J, Palmer J, Amores S, Bague S, Camins A. Primary tumors of the sacrum: diagnostic imaging. AJR Am J Roentgenol 2000;174:417–24 [DOI] [PubMed] [Google Scholar]
- 14.Schulte M, Brecht-Krauss D, Werner M, Hartwig E, Sarkar MR, Keppler P, et al. Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J Nucl Med 1999;40:1637–43 [PubMed] [Google Scholar]
- 15.Hogendoorn PC, Athanasou N, Bielack S, De Alava E, Dei Tos AP, Ferrari S, et al. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 (Suppl. 5)v204–13 [DOI] [PubMed] [Google Scholar]
- 16.Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: clear cell sarcoma (malignant melanoma of soft parts). Cancer Genet Cytogenet 2001;130:1–7 [DOI] [PubMed] [Google Scholar]
- 17.Disler DG, Miklic D. Imaging findings in tumors of the sacrum. AJR Am J Roentgenol 1999;173:1699–706 [DOI] [PubMed] [Google Scholar]
- 18.Becker S, Babisch J, Venbrocks R, Katenkamp D, Wurdinger S. Primary non-Hodgkin lymphoma of the spine. Arch Orthop Trauma Surg 1998;117:399–401 [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Taoka T, Nakamine H. Superior clinical impact of FDG-PET compared to MRI for the follow-up of a patient with sacral lymphoma. J Clin Exp Hematop 2009;49:109–15 [DOI] [PubMed] [Google Scholar]
- 20.Fogelman I, Cook G, Israel O, Van derWall H. Positron emission tomography and bone metastases. Semin Nucl Med 2005;35:135–42 [DOI] [PubMed] [Google Scholar]
- 21.Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology 1996;199:751–6 [DOI] [PubMed] [Google Scholar]
- 22.Kosaka N, Maeda M, Uematsu H, Matsumine A, Koshimoto Y, Itoh H. Solitary plasmacytoma of the sacrum. Radiologic findings of three cases. Clin Imaging 2005;29:426–9 [DOI] [PubMed] [Google Scholar]
- 23.Puri A, Agarwal MG, Shah M, Srinivas CH, Shukla PJ, Shrikhande SV, et al. Decision making in primary sacral tumors. Spine J 2009;9:396–403 [DOI] [PubMed] [Google Scholar]
- 24.Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine 2005;3:111–22 [DOI] [PubMed] [Google Scholar]
- 25.Sciubba DM, Petteys RJ, Garces-Ambrossi GL, Noggle JC, McGirt MJ, Wolinsky JP, et al. Diagnosis and management of sacral tumors. J Neurosurg Spine 2009;10:244–56 [DOI] [PubMed] [Google Scholar]