Abstract
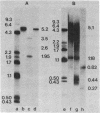
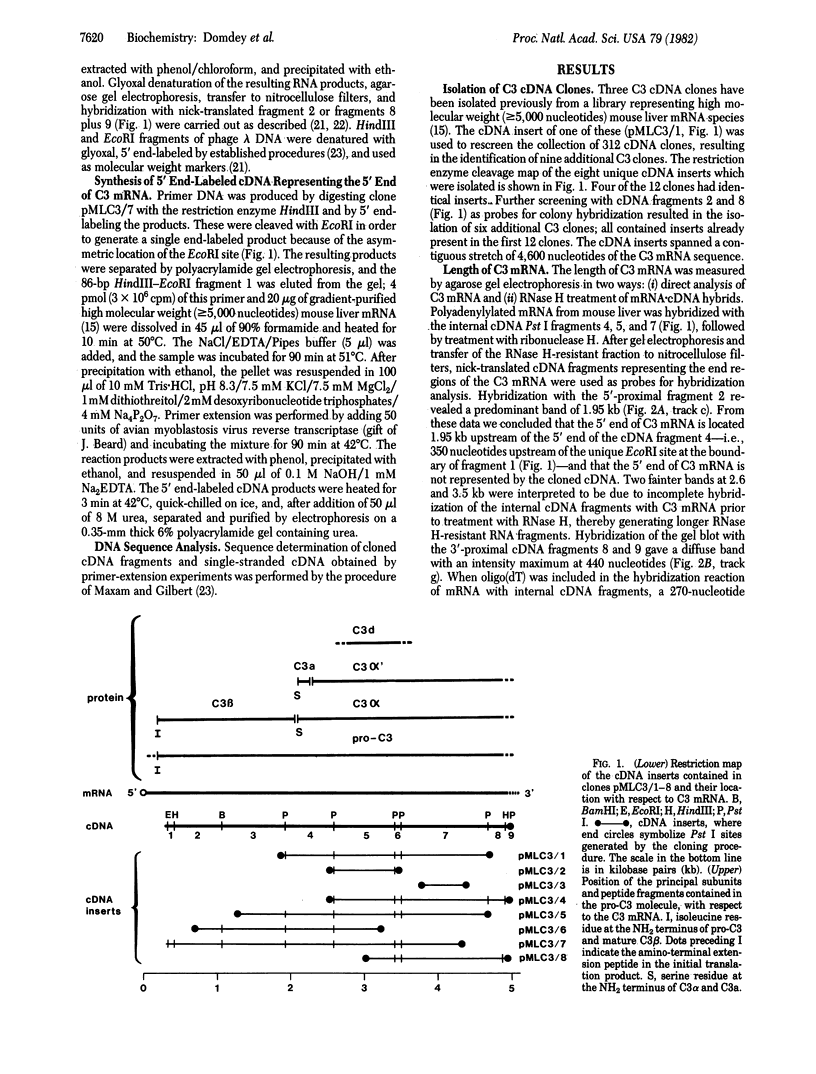
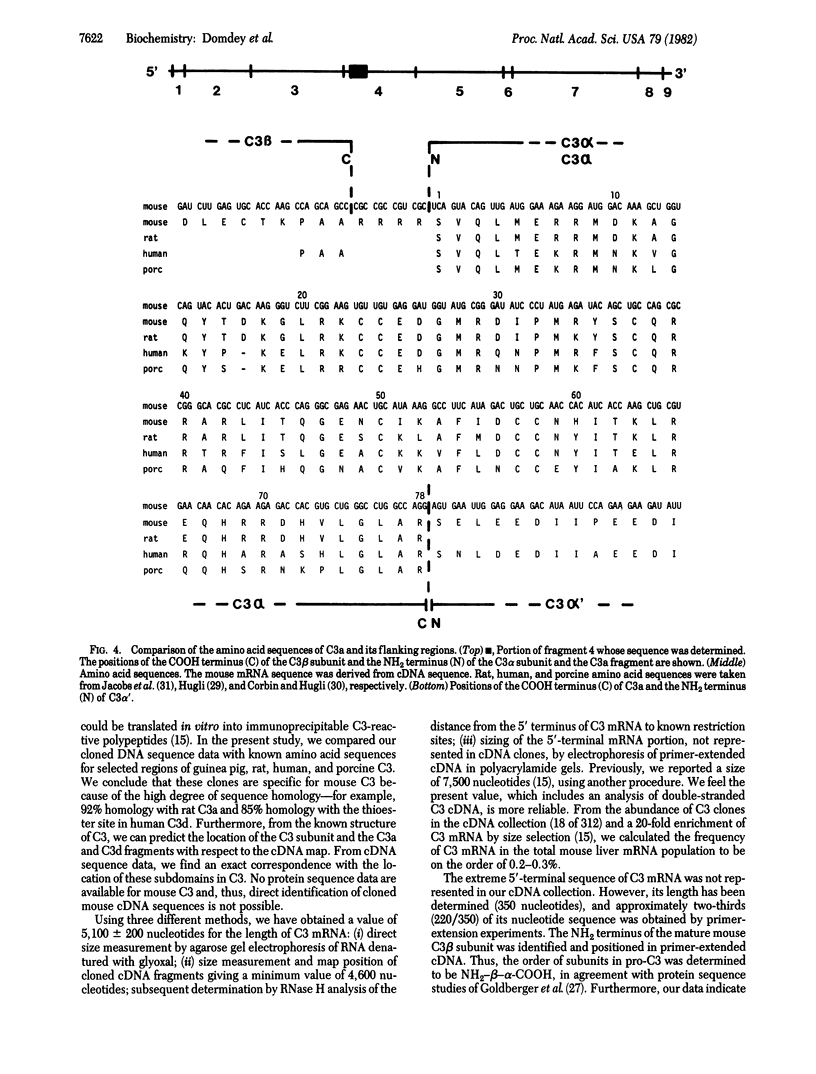
Eighteen cDNA clones containing inserts specific for the third component of complement (C3) have been derived from high molecular weight mouse liver mRNA. The inserts span 4,600 nucleotides of the C3 coding sequence, including the 3' end of C3 mRNA. The length of C3 mRNA was determined to be 5,100 +/- 200 nucleotides, including a poly(A)-containing tail of mean length 170 nucleotides. From cDNA sequence analysis of the 5'-proximal region of C3 mRNA, the NH2-terminal amino acid sequence of the mature C3 beta chain was predicted to be Ile-Pro-Met-Tyr-Ser-Ile-Ile-Thr-Pro-Asn-Val-Leu-Arg-Leu-Glu. This sequence is in good agreement with the reported amino acid sequences of human and guinea pig C3 beta chains. These data position the C3 beta subunit to the NH2-terminal portion of the precursor C3 molecule (pro-C3) and establish the order of subunits in pro-C3 to be NH2-beta-alpha-COOH. In addition, the cDNA sequence indicates that an NH2-terminal extension peptide precedes the beta chain in pro-C3. The amino acid sequence of the mouse C3a fragment and its flanking regions was determined. The data indicate the presence of four arginine residues located between the COOH terminus of the C3 beta and the NH2 terminus of the C3 alpha subunits in pro-C3. The coding sequences of the amino acids that constitute the internal thioester domain in C3 were determined. Unexpectedly, the glutamyl residue that has been shown to participate in the thioester bond in native C3 was found to be encoded as a glutamine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade V., Hall R. E., Colten H. R. Biosynthesis of pro-C3, a precursor of the third component of complement. J Exp Med. 1977 Sep 1;146(3):759–765. doi: 10.1084/jem.146.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Gagnon J., Porter R. R. Amino acid sequence around the thiol and reactive acyl groups of human complement component C4. Biochem J. 1981 Nov 1;199(2):359–370. doi: 10.1042/bj1990359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Corbin N. C., Hugli T. E. The primary structure of porcine C3a anaphylatoxin. J Immunol. 1976 Sep;117(3):990–995. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger G., Thomas M. L., Tack B. F., Williams J., Colten H. R., Abraham G. N. NH2-terminal structure and cleavage of guinea pig pro-C3, the precursor of the third complement component. J Biol Chem. 1981 Dec 25;256(24):12617–12619. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. E., Colten H. R. Cell-free synthesis of the fourth component of guinea pig complement (C4): identification of a precursor of serum C4 (pro-C4). Proc Natl Acad Sci U S A. 1977 Apr;74(4):1707–1710. doi: 10.1073/pnas.74.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A., Thomas M. L., Tack B. F. Sequence determination of the thiolester site of the fourth component of human complement. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7388–7392. doi: 10.1073/pnas.78.12.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter M. K., Thomas M. L., Rosen F. S., Tack B. F. Binding of C3b proceeds by a transesterification reaction at the thiolester site. Nature. 1982 Jul 1;298(5869):72–75. doi: 10.1038/298072b0. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. Human anaphylatoxin (C3a) from the third component of complement. Primary structure. J Biol Chem. 1975 Nov 10;250(21):8293–8301. [PubMed] [Google Scholar]
- Jacobs J. W., Rubin J. S., Hugli T. E., Bogardt R. A., Mariz I. K., Daniels J. S., Daughaday W. H., Bradshaw R. A. Purification, characterization, and amino acid sequence of rat anaphylatoxin (C3a). Biochemistry. 1978 Nov 14;17(23):5031–5038. doi: 10.1021/bi00616a027. [DOI] [PubMed] [Google Scholar]
- Lambris J. D., Dobson N. J., Ross G. D. Isolation of lymphocyte membrane complement receptor type two (the C3d receptor) and preparation of receptor-specific antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1828–1832. doi: 10.1073/pnas.78.3.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Fearon D. T., Levine R. P. Action of the C3b-inactivator on the cell-bound C3b. J Immunol. 1979 Mar;122(3):759–765. [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- Odink K. G., Fey G., Wiebauer K., Diggelmann H. Mouse complement components C3 and C4. Characterization of their messenger RNA and molecular cloning of complementary DNA for C3. J Biol Chem. 1981 Feb 10;256(3):1453–1458. [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Schreiber R. D., Müller-Eberhard H. J. Interaction of target cell-bound C3bi and C3d with human lymphocyte receptors. Enhancement of antibody-mediated cellular cytotoxicity. J Exp Med. 1981 Jun 1;153(6):1592–1603. doi: 10.1084/jem.153.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Morris S. C., Prahl J. W. Third component of human complement: structural analysis of the polypeptide chains of C3 and C3b. Biochemistry. 1979 Apr 17;18(8):1497–1503. doi: 10.1021/bi00575a017. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Janatova J., Gray W. R., Tack B. F. Third component of human complement: localization of the internal thiolester bond. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1054–1058. doi: 10.1073/pnas.79.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebauer K., Domdey H., Diggelmann H., Fey G. Isolation and analysis of genomic DNA clones encoding the third component of mouse complement. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7077–7081. doi: 10.1073/pnas.79.23.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]