Abstract
Objective
Standard tangential radiotherapy techniques after breast conservative surgery (BCS) often results in the irradiation of the tip of the left ventricle and the left anterior descending coronary artery (LAD), potentially increasing cardiovascular morbidity. The importance of minimising radiation dose to these structures has attracted increased interest in recent years. We tested a hypothesis that in some cases, by manipulating beam angles and accepting lower-than-prescribed doses of radiation in small parts of the breast distant from the surgical excision site, significant cardiac sparing can be achieved compared with more standard plans.
Methods
A sample of 12 consecutive patients undergoing radiotherapy after left-sided BCS was studied. All patients were planned with a 6 MV tangential beam, beam angles were manipulated carefully and if necessary lower doses were given to small parts of the breast distant from the surgical excision site to minimise cardiac irradiation (“institutional” plan). Separate “hypothetical standard” plans were generated for seven patients using set field margins that met published guidelines.
Results
In seven patients, the institutional plans resulted in lower doses to the LAD and myocardium than the hypothetical standard plans. In the other five patients, LAD and myocardial doses were deemed minimal using the hypothetical standard plan, which in these patients corresponded to the institutional plan (the patients were actually treated using the institutional plans).
Conclusion
Much attention has been devoted to ways of minimising cardiac radiation dose. This small sample demonstrates that careful manipulation of beam angles can often be a simple, but effective technique to achieve this.
Late cardiovascular morbidity associated with breast irradiation has received considerable attention recently, especially as diagnostic and therapeutic advances have translated into improvements in long-term survival [1].
Most invasive breast cancers are discovered at an early localised stage and can be treated with breast conservation surgery (BCS) and adjuvant radiotherapy with equivalent survival rates to mastectomy [2-4]. Whole-breast radiation therapy conventionally uses tangential beam arrangements, which include the entire breast, a portion of the chest wall and some contents of the anterior thoracic cavity. In left-sided breast irradiation, the field can include a significant volume of the heart. The mean cardiac dose from left-sided breast irradiation can be two or three times that of right-sided breast irradiation. In women treated in the 1950s to 1990s, it has been estimated that the mean cardiac dose was 0.9–14 Gy and 0.4–6 Gy for left and right breast/chest wall irradiation, respectively [5]. Using 6 MV tangential radiotherapy, the mean cardiac doses were 4.7 Gy and 1.5 Gy, and the mean left anterior descending coronary artery (LAD) doses were 21.9 Gy and 1.4 Gy for left and right breast irradiation, respectively [5].
In a meta-analysis by the Early Breast Cancer Trialists' Collaborative Group that included 78 randomised trials of breast or chest wall irradiation after surgery, there was an excess of non-breast cancer deaths owing to heart disease and lung cancer [6]. A population study showed that the cardiac mortality is 25% higher among women treated for left-sided breast tumours than those treated for right-sided tumours 15 years after treatment [7]. In another study of women irradiated between 1973 and 1982, the cardiac mortality ratio (left vs right breast cancer) was 1.42 after 10–14 years and 1.58 after 15 years [8].
Cardiovascular toxicity associated with radiation includes coronary artery disease, valvular disease, chronic pericardial disease, arrhythmia and conduction disturbances, and cardiomyopathy [9,10]. The exact mechanism of radiation-related heart disease and the threshold dose at which damage to the heart caused by radiation occurs is still unclear, which stresses the importance of striving to minimise the dose to the heart and the volume irradiated whenever possible. A review of some of the experiments investigating coronary artery disease after radiation has suggested that radiation increases myocardial infarction (MI) frequency by interacting with the pathological pathway of age-related coronary artery atherosclerosis resulting in accelerated atherosclerosis [10]. Radiation could also increase lethality of age-related MI by reducing the heart's tolerance to acute infarctions as a result of microvascular myocardial damage [10].
There is a correlation between cardiac perfusion defects and the volume of irradiated left ventricle; the defects becoming evident when 6% of the ventricle is irradiated by greater than 23–25 Gy [11]. A cardiac catheterisation study showed that there was an excess of cardiac stress test abnormalities among left-side irradiated patients; these were located in the anterior heart, which is most at risk in the tangential field and with 85% of abnormalities occur as stenoses of the LAD [12]. In a study of 50 patients treated with left tangential irradiation, the mean heart dose was 2.3 Gy and the mean LAD dose was 7.6 Gy [13]. A dosimetric study of 20 patients who had left-sided breast radiotherapy found that standard tangential radiotherapy resulted in a mean dose of 2.9 Gy to the heart, 12.05 Gy to the proximal LAD, 31.52 Gy to the distal LAD and a V30 (the volume receiving more than 30 Gy) of 23.09±28.37% for the proximal LAD and 45.43±42.5% for the distal LAD [14].
As patients with early breast cancer have an increasingly good prognosis, consideration of long-term effects such as cardiac toxicity and resulting complications is necessary when planning post-operative radiotherapy. It usually takes 10 years for radiation-related coronary artery disease and cardiac deaths to become apparent after breast irradiation [15]. However, it has been highlighted that delineation of anatomical subregions of the irradiated heart and LAD is challenging because it is difficult to accurately visualise these structures using current imaging modalities used in treatment planning [16].
Since the 1970s, it has been estimated that left chest wall/breast tangential radiotherapy-associated heart dose has reduced from 14 Gy with 250 kV to 4.7 Gy with cobalt-60, to 2.3 Gy with CT planned 6 MV photons [13,17]. However, with the increased use of anthracyclines, taxanes and trastuzumab there may be a potential increase in cardiac toxicity in the future. The literature review on radiation dose–volume effect on the heart did not show a clear quantitative dose and/or volume dependence for cardiac toxicity owing to scarcity of data [16]. As mentioned previously, this highlights the importance of minimising both the dose to these structures and the volume being irradiated as much as possible. There has been considerable interest in developing modern technology for radiotherapy planning to avoid excess cardiac irradiation, by modulation of the dose around organs at risk (OAR) using intensity modulated radiotherapy (IMRT) [18,19], IMRT with simultaneously integrated boost [20], placement of heart blocks [21] and deep inspiratory breath-holding (DIBH) and gated techniques [22-25]. Beam angle modulation remains a very simple, and, up until now, rather neglected way of achieving this outcome.
We hypothesised that in some cases, in comparison with “standard” plans, beam angle manipulation to reduce the dose to the LAD and the heart, while accepting lower-than-prescribed doses in small parts of the breast distant from the site of surgical excision, could lead to significant cardiac and LAD sparing without compromising the dose delivered to the “high-risk” part of the breast. This was done using dose–volume histograms (DVHs) of breast tissue, myocardium and LAD, taken from actually used plans, and “hypothetical standard” plans (see Methods and Materials for definition) in a series of our patients.
Methods and materials
Patient population
The study group comprised of 12 consecutive patients who had undergone CT-planned whole breast radiotherapy (WBRT) for left-sided breast cancer after BCS during 2009 at Nottingham City Hospital. Patients with documented ipsilateral or contralateral axillary, supraclavicular, infraclavicular or internal mammary nodal disease or right-sided breast cancer were excluded.
Analysis performed for this study consisted purely of retrospective dosimetric modelling and was fully independent of care delivered to each patient, so, after discussion with the Chair, full Ethics Committee permission was not deemed necessary.
Simulation: image acquisition
At simulation, the patients were placed in the supine position, supported on a breast bed tilted up to bring the sternum to an approximately horizontal position, with the ipsilateral arm abducted above the head and the torso straightened. Radio-opaque flexible wire markers were placed around the palpable extent of the breast tissue and the surgical scar (unless this had been an oncoplastic procedure).
For treatment planning purposes, all patients underwent CT scanning at 10 mm intervals from the angle of the mandible to 30 mm beyond the caudal limit of the breast skin, encompassing both breasts and the whole thoracic cavity. The CT data were transferred to the ProSoma® (MedCom GmbH, Darmstadt, Germany) virtual simulation system and the field was positioned with a fixed focus-to-skin distance (FSD) of 100 cm, and the medial and lateral tangential beams were defined to encompass the breast clinical target volume (CTV). Collimator and gantry angles and position of field borders were adjusted to achieve a degree of cardiac sparing (see beam arrangement). Our institution's traditional use of a fixed FSD-based technique rather than an isocentric one has, in our experience, made such adjustments during virtual simulation very easy to implement and to do so equally on both institutional and hypothetical standard plans (this includes such movements as “foot-end-up” adjustments to collimator angles to avoid the ventricular apex). However, it is beyond the scope of this paper to formally compare fixed FSD set-ups with isocentric ones.
Definition of treatment volumes and organs at risk
The CT data for the 12 treated patients were retrieved and the original datasets were used to outline the breast (CTV) and OAR (heart and LAD) on ProSoma. The CTV and heart was delineated by one of the authors (SV), the LAD was delineated by another (JM) and all volumes were checked by another author (DALM) to obtain consistent definitions of the volumes. The CTV, heart and LAD outlines were delineated using the methodology described in earlier publications [13,17,18,26].
Clinical target volume
The CTV was defined as all glandular breast tissue including fatty degenerated ducts of the breast down to the deep fascia extending from the pectoralis major muscle to the skin, but excluding the pectoralis muscle, ribs and 5 mm of skin. Delineation of CTV was performed on each transverse slice using the soft-tissue window setting on ProSoma. CTV was assumed to begin 5 mm below the skin surface and 5 mm anterior to the lung–chest wall interface. The planned target volume (PTV) was defined as the CTV plus an expansion of 10 mm in all directions except for the skin surface and lung interface to compensate for breathing, set-up errors, patient position variation and breast swelling [18].
Heart
The heart volume was defined as the entire visible myocardium excluding the pericardium, from the apex to the infundibulum of the right ventricle, the right atrium and auricle, the root of the ascending aorta and pulmonary trunk. The ascending aorta and superior vena cava were excluded.
Left anterior descending artery
The LAD was delineated on CT images obtained during normal respiration by a specialist cardiothoracic radiologist (JM) as it courses anteriorly along the interventricular septum on the CT images. As the planning study was obtained with a CT scanner without contrast enhancement, the LAD could not be delineated with certainty and its location was inferred using visible reliable landmarks, such as the anterior interventricular groove in some instances.
Margins
It has been highlighted that delineation of anatomical subregions and the heart and LAD is challenging as it is difficult to visualise these structures with the current imaging modalities used in treatment planning [16], but the degree of motion of the heart is modest with normal breathing [27]. To compensate for lung, heart, chest wall movement and any uncertainty regarding the position of the LAD, and to ease the difficulty encountered by the planning algorithms when dealing with narrow LAD volume, a 1 cm radial margin was added around the LAD, as described in another study [13].
Beam arrangement and treatment plan
The treatment field margins for the standard plan were 2 cm from the palpated ipsilateral breast tissue and approximately at the midline for the medial field edge; at the mid-axillary line or 2 cm lateral to breast tissue for the lateral field edge; 2 cm superior to breast tissue for the superior field edge; and 2 cm below the infra-mammary fold for the inferior field edge.
Two tangentially opposed 6 MV photons beams were used. Beam divergence into the lung at the posterior edge was reduced by changing gantry and collimator angles.
Two plans were generated for each patient:
-
Hypothetical standard plan (Figure 1)
The medial entry point was defined at the mid-line of the sternum and the lateral entry point was defined at the mid-axillary line, with slight modification to ensure that no more than 1.5 cm of myocardium was included at the “maximum heart depth”, but that the whole breast tissue was included.
-
Institutional plan (Figure 2)
These plans were generated with the beam angles chosen to minimise exposure to the heart field while accepting, if necessary, poor coverage of the breast tissue at parts of the breast distant from the initial tumour site but ensuring adequate coverage in the area around the original tumour site. Entry points and beams similar to those described for the hypothetical standard plan were used, but the beam angles and entry points were then altered carefully as deemed appropriate using gantry, collimator and bed movements to minimise cardiac dose, accepting that doing so might compromise the coverage of breast tissue distant from the tumour bed. Good dose distribution to breast tissue in the quadrant of the original tumour was maintained. In practice, this usually varies from the hypothetical standard plan by using beams at angles slightly more vertical for laterally placed tumours, with some consequent lower dosing at the medial edge of the breast, and using more horizontally inclined beams for medial tumours. This was the plan that was actually used to treat the patients.
Figure 1.
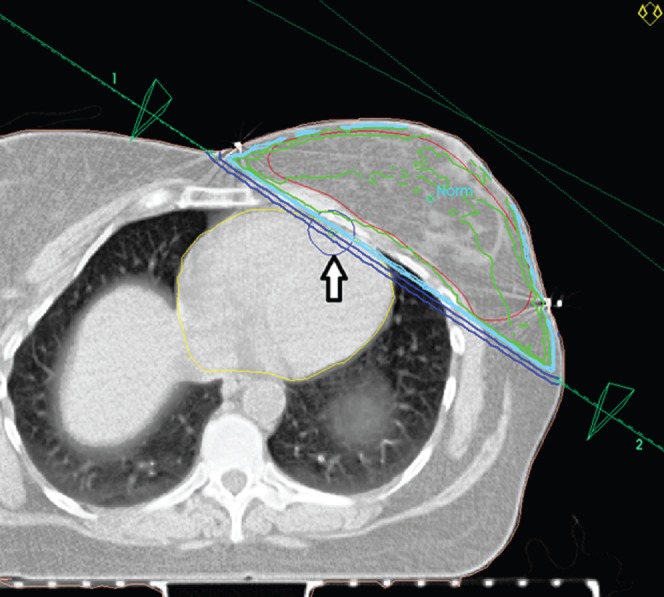
The hypothetical standard plan: beam set-up with planned target volume (PTV) (red line), myocardium (yellow line) and left anterior descending artery plus 1 cm margin (blue circle indicated with an arrow) marked. This set-up accords with published guidelines to fully encompass the PTV.
Figure 2.
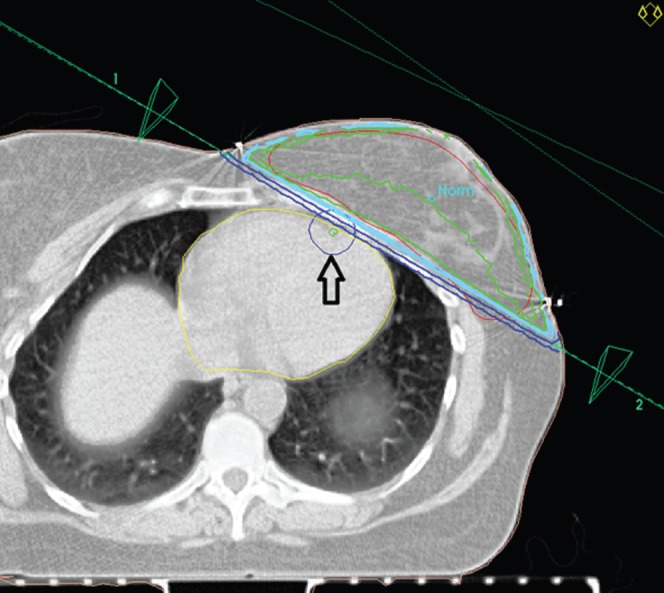
The institutional plan: beam set-up with planned target volume (PTV) (red line), myocardium (yellow line) and left anterior descending artery plus 1 cm margin (blue circle indicated with an arrow) marked. This set-up minimises cardiac volume irradiated by accepting poor coverage of PTV at sites distant from the tumour bed (the tumour was in upper breast so no seroma is seen on this CT section). Geometrically, the main difference between this and the hypothetical standard plan is the more horizontal angulation of these beams, with a more anterior lateral entrance point.
Prescribed dose
The prescribed dose was 50 Gy in 25 fractions for women under the age of 50 years and 42.5 Gy in 16 fractions for women aged over 50 years.
Quality assurance/verification
All treatment plans were checked through a quality control process by medical physicists and the dose was prescribed by a single clinical oncologist.
Dosimetric parameters for plan comparison
For each patient's hypothetical standard plan and institutional plan DVHs were generated for PTV, heart and LAD with 1 cm radial margin, and these DVHs were used to derive the parameters for plan comparison.
Results
The dose to OARs was already low using hypothetical standard plans for 5 of the 12 patients in the study group; hence formal comparison with the institutional plans was not carried out for these. The institutional plans resulted in a lower dose to the heart and LAD than the hypothetical standard plans in seven patients in the group. A typical example of the DVHs obtained for PTV and OAR is demonstrated for one of the seven patients. As the volume definitions are arbitrary to a certain degree, we felt there would be little scientific value gained in aggregating data from all seven patients. Owing to the arbitrary component being the same for each plan for any one individual, we have taken a single case to demonstrate the obvious overall effect, and the principle we are attempting to illustrate, without trying to accurately quantify it.
Planned target volume coverage
The DVHs for the PTV using each plan are shown in Figure 3. The apparent inferiority of the institutional plan is accounted for by lower doses of radiation being absorbed in parts of the breast distant from the tumour bed.
Figure 3.
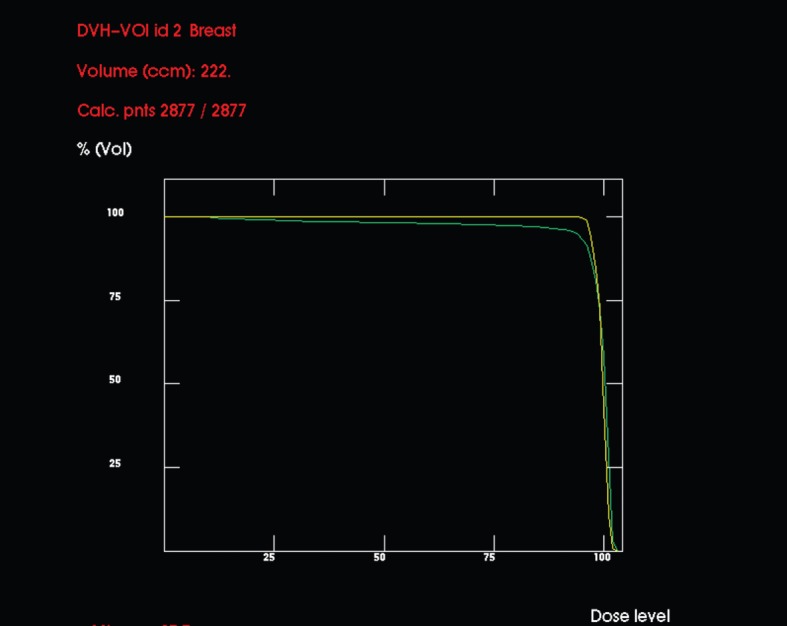
Comparative dose–volume histograms (DVHs) (in dose percentages) for planned target volume (PTV) using the hypothetical standard (upper, yellow line) and institutional (lower, green line) set-ups. By conventional criteria, the distribution in the latter is poor, but the low-dose areas are well away from the tumour bed, so not clinically important.
Organs at risk
Heart
Figure 4 shows the DVHs for myocardium for each plan, and demonstrates considerably less cardiac irradiation using the institutional plan compared with the hypothetical standard plan. With the latter, small areas receive over 75% of the prescribed dose, but with the former no point exceeds 25% of the prescribed dose.
Figure 4.
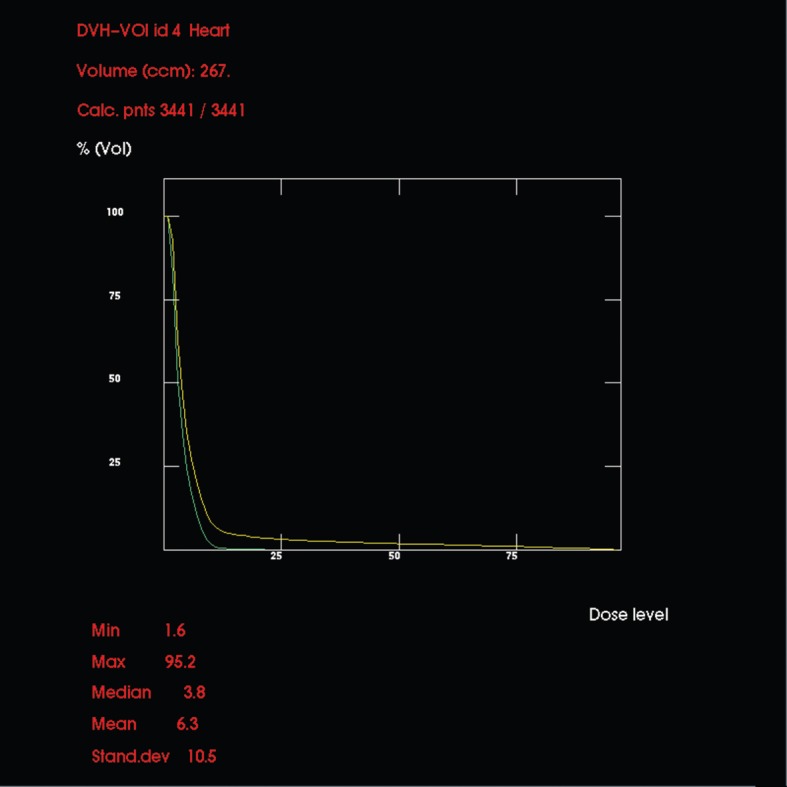
Comparative dose–volume histograms (in dose percentages) for myocardium using the hypothetical standard (upper, yellow line) and institutional (lower, green line) set-ups. With the former, small areas receive over 75% of prescribed dose whereas the latter receives no more than 25%.
Left anterior descending artery with 1 cm radial margin
Figure 5 shows DVHs for LAD with a 1 cm radial margin for each plan, and demonstrates considerably less LAD irradiation using the institutional plan compared with the hypothetical standard plan. The proportion of the LAD volume receiving more than 5 Gy (V5) for the hypothetical standard plan was 90% and for the institutional plan was 48%. The proportion of the LAD volume receiving more than 30 Gy (V30) for the hypothetical standard plan was 43% and for the institutional plan was 6%.
Figure 5.
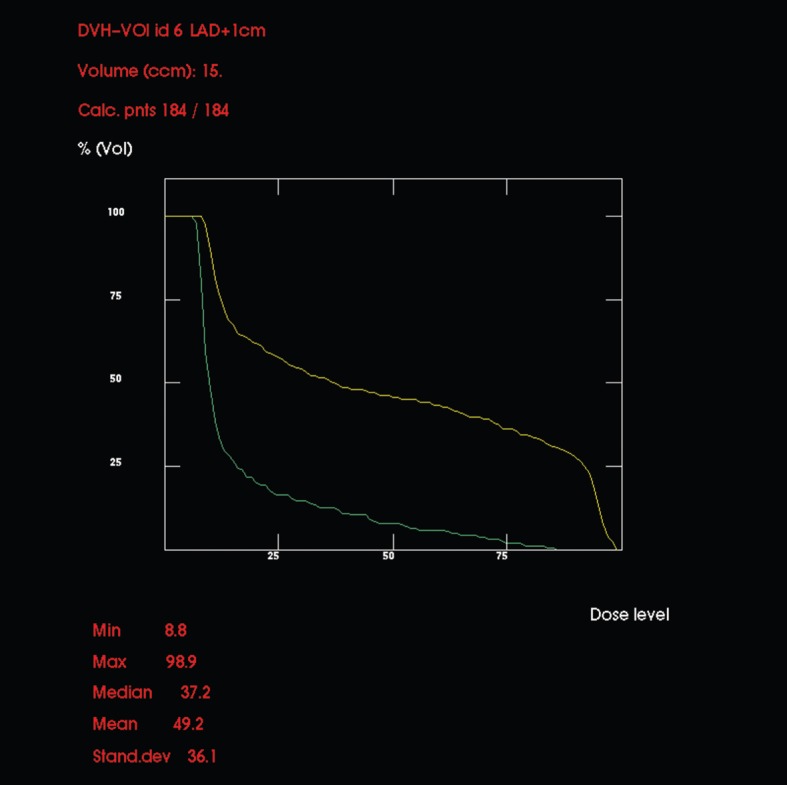
Comparative dose–volume histograms (in dose percentages) for left anterior descending coronary artery (LAD) using the hypothetical standard (upper, yellow line) and the institutional (lower, green line) set-ups. Note that an arbitrary 1 cm margin was drawn around the LAD, so precise figures should be regarded with caution, but the relationship between the two lines would be similar regardless of the size of the margin used.
It is possible that the hypothetical standard plan we have used as a comparator might be improved by making changes to the reference point, wedge angle and other parameters, but any differences achieved would be very minor in comparison with the effect of the different beam positions used in our institutional plan.
Discussion
While standard tangential radiotherapy decreases risk of recurrence post-BCS for early breast cancer, it is well-established, especially for left-sided lesions, that such treatment can result in an increased risk of cardiac morbidity, yet there is no clear evidence to identify the different components of this risk in terms of structures, volumes and dose. It is therefore wise when using such radiotherapy to minimise both dose to cardiac tissue and volumes irradiated, and pay particular attention to the LAD, in view of both its position and its critical role: 85% of radiotherapy-related abnormalities involve the LAD territory [12].
Many strategies have been explored in an attempt to reduce the cardiac dose during breast irradiation. These include the use of cardiac blocks; breathing techniques, such as gating and breath holding; IMRT; and IMRT with simultaneously integrated boost. Patient positioning is a simple method of reducing cardiac dose by increasing the distance between chest wall tissue and the heart by placing the arm on the treated side over the head, gripping the contralateral arm of the “T” grip of the breast board, instead of the standard technique whereby the arm on the treated side is flexed at the elbow and abducted at 90° from the body [28,29]. Beam angle modulation is another simple and effective method of reducing cardiac dose as we have demonstrated in this study and can be readily combined with other techniques. To our knowledge, there are currently no universally accepted criteria for precise PTV definition and there is considerable variation between individual radiation oncologists in practice [30,31].
Should the method we describe here be avoided in patients with what might be considered high-risk tumours (patient aged under 40 years, those with high grade tumours or with definite lymphovascular invasion)? While we recognise that accepting a “lower than conventional” dose at some parts of the breast may theoretically increase recurrence risk, even in these patients we think the risk is likely to be small compared with the long-term benefits achieved with the reduction in cardiac dose. We think the recurrence risk is low for several reasons, but accept that none can be considered “high-level evidence”. Firstly, it is widely recognised that PTV definition in breast planning is an imprecise science with high interobserver variability [30,31]; yet this does not, as far as we can deduce, result in significantly worse interpractitioner recurrence rates. We suggest that this is because the areas that are treated by some clinicians, which may be considered to be “underdosed” when compared with PTVs defined by others, are likely to be the same peripheral areas, distant from the primary tumour, that are systematically underdosed by the technique we report. This indicates that the underdose does not greatly prejudice recurrence risk, if at all. Secondly, recurrences of the high-risk tumours described above certainly tend to occur early. However, in our experience, we have not seen many, if any, isolated local recurrences at distant peripheral breast sites in the years that we have been following the approach described here. This is supported by Struikmans et al [31] “one must realise that recurrences occur very rarely at the margin of conventionally irradiated volume in breast conserving therapy.”
Much attention has been paid to the dose delivered to the tumour bed, the importance of which has been well established [32], but the variation in dose distribution and definition of PTV away from the tumour bed has received scant attention. We suggest that considerable variation in this area exists in practice but has little impact on disease outcome. Strong support for this view can be derived from the increasing evidence of the efficacy of partial breast irradiation for certain groups of patients.
The one example we have given shows that reduced cardiac irradiation can be achieved by the simple expedient of beam position manipulation; this is clearly evident from inspection of DVHs, without needing a detailed analysis of exact figures, the precision of which is uncertain, for reasons previously described (the differences between the DVHs in overall shape would be the same in whichever direction the individual values varied, as this variation would be the same for both plans). This improvement, which might be considered a standard plan by published criteria, was achievable in 7 of 12 consecutive cases examined, implying that it has a high relevance in day-to-day clinical practice.
We suggest that the small risk of local recurrence, because of lower doses of radiotherapy at parts of the breast distant from the tumour bed, is likely to be counterbalanced by the reduction in long-term cardiac morbidity achieved by manipulation of beam position. In addition, modern CT planning is already ensuring more accurate target delineation so that the present techniques are already better than ever before, even if we adjust the fields to reduce cardiac exposure.
When planning left-sided breast irradiation, a critical review of beam placement (accepting if appropriate some compromise on PTV coverage in order to achieve minimal cardiac irradiation) should always be considered. A degree of flexibility should be used in applying ICRU recommendations on dose homogeneity within the PTV when this enables worthwhile reduction in doses to important structures at risk.
References
- 1.Westlake S, Cooper N.Office for National Statistics. Cancer incidence and mortality: trends in the United Kingdom and constituent countries, 1993 to 2004. [cited 8 November 2010] Available from: http://www.nos.gov.uk/ons/rel/hsq/health-statistics-quarterly/no--38--summer-2008/cancer-incidence-and-mortality--trends-in-the-united-kingdom-and-constituent-countries--1993-to-2004.pdf. [PubMed]
- 2.Lichter AS, Lippman ME, Danforth DN, d'Angelo T, Steinberg SM, deMoss E, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: A randomized trial at the National Cancer Institute. J Clin Oncol 1992;10:976–83 [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143–50 [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–41 [DOI] [PubMed] [Google Scholar]
- 5.Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s–1990s. Int J Radiat Oncol Biol Phys 2007;69:1484–95 [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of randomised trials. Lancet 2005;366:2087–106 [DOI] [PubMed] [Google Scholar]
- 7.Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Moller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer 2007;7:9 2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005;6:557–65 [DOI] [PubMed] [Google Scholar]
- 9.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007;25:3991–4008 [DOI] [PubMed] [Google Scholar]
- 10.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int. J Radiat Oncol Biol Phys 2010;76:656–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lind PA, Pagnanelli R, Marks LB, Borges-Neto S, Hu C, Zhou S, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 2003;55:914–20 [DOI] [PubMed] [Google Scholar]
- 12.Correa CR, Litt HI, Hwang W, Ferrari VA, Solin LJ, Harris EE. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol 2007;25:3031–7 [DOI] [PubMed] [Google Scholar]
- 13.Taylor CW, Povall JM, McGale P, Nisbet A, Dodwell D, Smith JT, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008;72:501–7 [DOI] [PubMed] [Google Scholar]
- 14.Krueger EA, Schipper MJ, Koelling T, Marsh RB, Butler JB, Pierce LJ. Cardiac chamber and coronary artery doses associated with postmastectomy radiotherapy techniques to the chest wall and regional nodes. Int J Radiat Oncol Biol Phys 2004;60:1195–203 [DOI] [PubMed] [Google Scholar]
- 15.Harris EE. Cardiac mortality and morbidity after breast cancer treatment. Cancer Control 2008;15:120–9 [DOI] [PubMed] [Google Scholar]
- 16.Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77–85 [DOI] [PubMed] [Google Scholar]
- 17.Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s–1990s. Int J Radiat Oncol Biol Phys 2007;69:1484–95 [DOI] [PubMed] [Google Scholar]
- 18.Fong A, Bromley R, Beat M, Vien D, Dineley J, Morgan G. Dosimetric comparison of intensity modulated radiotherapy techniques and standard wedged tangents for whole breast radiotherapy. J Med Imaging Radiat Oncol 2009;53:92–9 [DOI] [PubMed] [Google Scholar]
- 19.Lohr F, El-Haddad M, Dobler B, Grau R, Wertz H, Kraus-Tiefenbacher U, et al. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys 2009;74:73–80 [DOI] [PubMed] [Google Scholar]
- 20.Van derLaan HP, Dolsma WV, Schilstra C, Korevaar EW, de Bock GH, Maduro JH, et al. Limited benefit of inversely optimised intensity modulation in breast conserving radiotherapy with simultaneously integrated boost. Radiother Oncol 2010;94:307–12 [DOI] [PubMed] [Google Scholar]
- 21.Raj KA, Evans ES, Prosnitz RG, Quaranta BP, Hardenbergh PH, Hollis DR, et al. Is there an increased risk of local recurrence under the heart block in patients with left-sided breast cancer? Cancer J 2006;12:309–17 [DOI] [PubMed] [Google Scholar]
- 22.Chen MH, Cash EP, Danias EP, Kissinger KV, Bornstein BA, Rhodes LM, et al. Respiratory maneuvers decrease irradiated cardiac volume in patients with left-sided breast cancer. J Cardiovasc Magn Reson 2002;4:265–71 [DOI] [PubMed] [Google Scholar]
- 23.Nemoto K, Oguchi M, Nakajima M, Kozuka T, Nose T, Yamashita T. Cardiac-sparing radiotherapy for the left breast cancer with deep breath-holding. Jpn J Radiol 2009;27:259–63 [DOI] [PubMed] [Google Scholar]
- 24.Borst GR, Sonke J, Den Hollander S, Betgen A, Remeijer P, Van Giersbergen A, et al. Clinical results of image-guided deep inspiration breath hold breast irradiation. Int J Radiat Oncol Biol Phys 2010;78:1345–51 [DOI] [PubMed] [Google Scholar]
- 25.Remouchamps VM, Huyskens DP, Mertens I, Destine M, Van Esch A, Salamon E, et al. The use of magnetic sensors to monitor moderate deep inspiration breath hold during breast irradiation with dynamic MLC compensators. Radiother Oncol 2007;82:341–8 [DOI] [PubMed] [Google Scholar]
- 26.Hijal T, Fournier-Bidoz N, Castro-Pena P, Kirova YM, Zefkili S, Bollet MA, et al. Simultaneous integrated boost in breast conserving treatment of breast cancer: A dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiother Oncol 2010;94:300–6 [DOI] [PubMed] [Google Scholar]
- 27.Jagsi R, Moran JM, Kessler ML, Marsh RB, Balter JM, Pierce LJ. Respiratory motion of the heart and positional reproducibility under active breathing control. Int J Radiat Oncol Biol Phys 2007;68:253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canney PA, Deehan C, Glegg M, Dickson J. Reducing cardiac dose in post-operative irradiation of breast cancer patients: the relative importance of patient positioning and CT scan planning. Br J Radiol 1999;72:986–93 [DOI] [PubMed] [Google Scholar]
- 29.Canney PA, Sanderson R, Deehan C, Wheldon T. Variation in the probability of cardiac complications with radiation technique in early breast cancer. Br J Radiol 2001;74:262–5 [DOI] [PubMed] [Google Scholar]
- 30.Hurkmans CW, Borger JH, Pieters BR, Russell NS, Jansen EPM, Munheer BJ. Variability in target volume delineation on CT scans of the breast. Int J Radiat Oncol Biol Phys 2001;50:1366–72 [DOI] [PubMed] [Google Scholar]
- 31.Struikmans H, Walam-Rodenhuis C, Stam T, Stapper G, Tersteeg RJHA, Bol GH, et al. Interobserver variability of clinical target volume delineation of glandular breast tissue and of boost volume in tangential breast irradiation. Radiother Oncol 2005;76:293–9 [DOI] [PubMed] [Google Scholar]
- 32.Poortmans PM, Collette L, Bartelink H, Struikmans H, Van denBogaert WF, Fourquet A, et al. The addition of a boost dose on the primary tumour bed after lumpectomy in breast conserving treatment for breast cancer. A summary of the results of EORTC 22881–10882 “boost versus no boost” trial. Cancer Radiother 2008;12:565–70 [DOI] [PubMed] [Google Scholar]


