Abstract
Ozone is a major gaseous pollutant thought to contribute to forest decline. Although the physiological and morphological responses of forest trees to ozone have been well characterized, little is known about the molecular basis for these responses. Our studies compared the response to ozone of ozone-sensitive and ozone-tolerant clones of hybrid poplar (Populus maximowizii × Populus trichocarpa) at the physiological and molecular levels. Gas-exchange analyses demonstrated clear differences between the ozone-sensitive clone 388 and the ozone-tolerant clone 245. Although ozone induced a decrease in photosynthetic rate and stomatal conductance in both clones, the magnitude of the decrease in stomatal conductance was significantly greater in the ozone-tolerant clone. RNA-blot analysis established that ozone-induced mRNA levels for phenylalanine ammonia-lyase, O-methyltransferase, a pathogenesis-related protein, and a wound-inducible gene were significantly higher in the ozone-tolerant than in the ozone-sensitive plants. Wound- and pathogen-induced levels of these mRNAs were also higher in the ozone-tolerant compared with the ozone-sensitive plants. The different physiological and molecular responses to ozone exposure exhibited by clones 245 and 388 suggest that ozone tolerance involves the activation of salicylic-acid- and jasmonic-acid-mediated signaling pathways, which may be important in triggering defense responses against oxidative stress.
Ozone is believed to cause more damage to forest trees than any other gaseous pollutant. Ambient concentrations of ozone have increased 1% to 2% per year during the past 20 years in Europe and the United States (Stockwell et al., 1997) and show no indication of leveling, particularly in developing industrialized areas (Chameides et al., 1994). Acute stress from exposure to high concentrations of ozone, even for short periods of time, generally results in visible injury. Chronic ozone stress, resulting from exposure to low concentrations over a long period of time, generally produces little or no visible injury but results in biochemical and physiological changes that lead to reduced vigor and growth (Heath and Taylor, 1997). Ozone has been demonstrated to alter basic metabolic processes of trees, including reducing photosynthetic rate (Reich and Amundson, 1985; Coleman et al., 1995b), decreasing Rubisco quantity and activity, reducing foliar conductance (Pell et al., 1992; Farage and Long, 1995; Paakkonen et al., 1996), and accelerating leaf senescence (Coleman et al., 1995b). Altered patterns of carbon allocation have also been reported, resulting in a reduction in winter storage pools (Coleman et al., 1995a). These alterations of critical metabolic processes can lead to an increased susceptibility to biotic and abiotic stressors and contribute to forest decline (McLaughlin, 1985; Schmieden and Wild, 1995).
Although the physiological responses of forest trees to ozone have been well characterized, very little is known about the responses at the molecular level. It is believed that when ozone enters the mesophyll through the stomata, it is rapidly degraded, generating AOS (Kanofsky and Sima, 1995). The presence of AOS activates defense mechanisms that may operate by preventing the formation of AOS and/or by scavenging them once they are formed. Glutathione and superoxide dismutase, proposed components of these oxidative defense systems, have been shown to increase upon ozone exposure in poplar (Populus spp.) (Sen-Gupta et al., 1991). Total cellular activities of superoxide dismutase, guaiacol peroxidase, and glutathione reductase were shown to increase in birch as a result of ozone exposure (Tuomainen et al., 1996).
A variety of plant systems have exhibited ozone induction of mRNAs for defense-related genes known to be induced by pathogens and other stresses (for review, see Kangasjärvi et al., 1994; Sandermann, 1996; Sharma and Davis, 1997). This overlap of induced gene expression is likely because many of these stresses produce AOS. AOS trigger many different pathways, some of which require SA as a signal molecule. AOS-regulated pathways include SAR and the HR (Mehdy et al., 1996; Lamb and Dixon, 1997), which are characterized by the induction of PR proteins. By using cross-reacting antibodies to PR proteins from herbaceous plants, it was demonstrated that ozone caused increased PR protein levels in Norway spruce (Kärenlampi et al., 1994). This induction of PR proteins by ozone is also well documented in several herbaceous plant species (Sandermann, 1996; Sharma and Davis, 1997).
The phenylpropanoid-biosynthetic pathway, which produces a number of defense metabolites, is also induced by ozone. Transcript levels for enzymes involved in this pathway, including PAL in birch (Tuomainen et al., 1996) and cinnamoyl alcohol dehydrogenase in Norway spruce (Galliano et al., 1993), were shown to be induced by ozone. The induction of PAL gene expression by ozone has been shown not to require SA in Arabidopsis, suggesting that ozone activates a SA-independent signal transduction pathway as well (Sharma et al., 1996). Further evidence for a second signal transduction pathway in ozone responses was recently reported by Örvar et al. (1997), who demonstrated that mechanical wounding or the direct application of JA (a known mediator of wound responses) before ozone exposure decreased the amount of ozone injury in tobacco plants.
To further elucidate the molecular mechanisms of ozone responses in tree species, we have chosen hybrid poplar (Populus maximowizii × Populus trichocarpa) as a model forest tree. The advantages of using hybrid poplar for molecular studies include a small genome size, ease of vegetative propagation, well-developed transformation and regeneration protocols, and the availability of ozone-sensitive and ozone-tolerant clonal lines. In our current studies, comparisons of physiological and molecular responses to ozone were made between an ozone-sensitive and an ozone-tolerant clone. Expression patterns of genes representing a variety of stress-response pathways were examined, including PAL (the first enzyme in the phenylpropanoid biosynthesis pathway), OMT (an enzyme involved in lignin formation), PR-1 (a PR protein characteristic of SA-mediated responses, including SAR and HR), and WIN3.7 (a wound-inducible trypsin inhibitor; Bradshaw et al., 1989). Clear differences in the pattern of expression of these genes in the ozone-tolerant and ozone-sensitive plants were observed. To determine if the ozone-sensitive plants are more susceptible to other stresses, wounding and pathogen-infection experiments were performed. A similar difference in defense-gene expression was observed, suggesting an overlap not only between ozone- and SA-mediated pathways, but also between ozone-, pathogen-, and wound-induced pathways in hybrid poplar.
MATERIALS AND METHODS
Plant Growth
Initial greenwood cuttings of hybrid poplar (Populus maximowizii × Populus trichocarpa) that had previously been designated as ozone sensitive (clone 388) or ozone tolerant (clone 245), based on visible lesion formation in response to a single acute dose of ozone (Wood and Coppolino, 1972), were obtained from E. Pell (The Pennsylvania State University, University Park). Cuttings were placed in sand, kept under mist until roots were established, and then transplanted into 15-cm-diameter pots containing Metromix 500 (Hummert, St. Louis, MO), amended as described by Pell et al. (1995). A drip-irrigation system delivered approximately 300 mL of water to each cutting daily. All side shoots were pruned so that each plant consisted of a single stem. No pruning was performed for at least 1 week before ozone fumigation, wounding, or pathogen infection.
Ozone Fumigation
Six weeks after transplantation, cuttings were transferred to growth chambers modified for ozone fumigation and were acclimated for 2 to 3 d before treatment. Growth-chamber conditions averaged 25°C, 67% RH, and 370 ppm CO2, with a 14-h photoperiod averaging 200 μmol m−2 s−1 at the top of the canopy. The cuttings were then treated for 6 h per d (9 am to 3 pm) with ozone at 300 ± 50 ppb for 4 d or maintained in ambient air (<30 ppb ozone). Ozone was generated with an Orec ozone generator (model 03V10-0, Ozone Research and Equipment, Phoenix, AZ) and each treatment was replicated for a total of four chambers. At 3, 6, 12, 24, 30, 54, and 78 h after the start of the fumigation period, the second and fourth fully expanded leaves were collected from eight plants per clone per treatment, frozen in liquid nitrogen, and stored at −80°C until used for RNA isolation.
Gas-Exchange Analysis
Gas exchange was measured under controlled conditions after 2 and 4 d of fumigation by placing the fourth fully expanded leaf in a 1-L cuvette of a photosynthetic system (model 6200, Li-Cor, Lincoln, NE). Photosynthetic rates and stomatal conductance were measured at 225 ± 25 μmol m−2 s−1 using a cool-beam PAR lamp (model 300 PAR 56/2 WFL, General Electric) as the light source after a 30-min acclimation period. The Li-Cor cuvette was flushed with outside air for several minutes, and conditions were allowed to stabilize before measurements were taken. Cuvette conditions averaged 23°C, 350 ppm CO2, and 45% RH. Measurements were taken between 1 and 4 pm on four plants per chamber per clone. This experiment used a completely randomized design, and analysis of variance, with the chamber as the experimental unit and the plants as the subsamples, was used to determine differences in gas exchange attributable to ozone treatment, clone, and number of days of exposure. Statistical Analysis Software (SAS Institute, 1989) was used for all statistical analyses, and data presented include means ± se.
Wounding Experiments
Mechanical wounding of cuttings was performed by crimping the leaves with pliers. Each leaf from the fourth fully expanded leaf on down was wounded 20 times at 9 am, and this was repeated at 11 am, 1 pm, and 3 pm every day for 4 consecutive d. At 3, 6, 24, 36, 54, and 78 h after the initiation of wounding, the second and fourth fully expanded leaves were collected, frozen in liquid nitrogen, and stored at −80°C until used for RNA isolation.
Bacterial Infection Experiments
Overnight cultures of Pseudomonas syringae pv maculicola KD4326 (Wanner et al., 1993) were diluted in 10 mm MgCl2 to a final A600 of 0.1. The bacterial suspension was then hand infiltrated into the undersides of the second through the fifth fully expanded leaves using a plastic 1-mL syringe without a needle. The infiltrated area of the leaf was outlined with a marker and collected at 3, 6, 12, or 24 h after infiltration for RNA analysis. Mock inoculations were performed by infiltrating with 10 mm MgCl2 alone.
Gene Probes
Clones for poplar PAL, OMT, and WIN3.7 were kind gifts from C. Douglas (University of British Columbia, Vancouver), (Subramaniam et al., 1993); M. Van Montagu (University of Gent, Belgium), (Dumas et al., 1992); and M. Gordon (University of Washington, Seattle), (Bradshaw et al., 1989); respectively. A probe for PR-1 was generated by reverse-transcriptase PCR using total RNA isolated from ozone-treated clone 245 plants and degenerate nucleotide primers (5′-GCNCARAAYTCNCCNCARGAYTA-3′ and 5′-CCANACNACYTGNGTRTARTG-3′) corresponding to conserved amino acid sequences AQNSP/QDY and HYTQVVW, respectively. The 310-nucleotide PCR product was cloned into a TA-vector (Invitrogen, San Diego, CA), sequenced, and compared with other known plant PR-1 genes. This analysis demonstrated that the PCR product had approximately 68% nucleotide identity with other PR-1 genes.
RNA Extraction and Analysis
Total RNA was isolated as described by Parsons et al. (1989). Fifteen micrograms of total RNA per lane was fractionated on 1.5% formaldehyde agarose gels in Mops buffer, pH 7.0, and transferred by capillary blotting onto a Duralon membrane (Stratagene). RNA was cross-linked to membranes by UV irradiation and then prehybridized for 1 h at 42°C in 6× SSC, 1% SDS, 2× Denhardt's solution, 25 μg/mL denatured salmon-sperm DNA, and 50% formamide. DNA probes were labeled by random priming (RadPrime, BRL) and hybridized at 42°C for 16 to 18 h. Filters were washed with 2× SSC containing 1% SDS at 55°C for 15 min, followed by a 30-min wash in 0.5× SSC containing 1% SDS at 65°C. Filters were then exposed to phosphor imager screens (Molecular Dynamics, Sunnyvale, CA) and the signal intensity was quantified using ImageQuant software (Molecular Dynamics). The data shown have been corrected for loading differences by quantitating counts obtained by rehybridizing with a 28S ribosomal gene probe from pea (Wanner and Gruissem, 1991). All experiments were performed at least twice, with two replicates per test condition. The data shown are from representative experiments.
RESULTS
Lesion Development
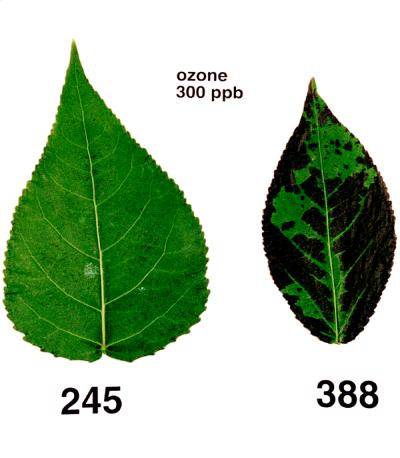
To confirm the different ozone sensitivities of the hybrid poplar clones 388 and 245, cuttings were exposed to 300 ppb ozone for 6 h daily for 5 d. Visible signs of ozone damage first appeared in both clones at 12 to 24 h after the start of the initial 6-h ozone fumigation and peaked at 36 h, with little additional injury occurring on subsequent days of exposure (Fig. 1). The vast majority of injury was observed on mid-aged and older leaves, with only a modest degree of injury occurring in leaves that were not yet fully expanded. The ozone-sensitive clone 388 plants displayed large necrotic regions on 30% to 80% of all leaves, and generally had at least one leaf that was covered with lesions over 50% or more of its area. Ninety percent of the clone 388 plants fumigated with ozone developed some type of injury, including stipple and large and small necrotic lesions (Environmental Protection Agency, 1976). The tolerant clone 245 plants developed stipple or flecks, but did not exhibit the extensive regions of necrosis observed in clone 388 plants. Only 30% of the clone 245 plants developed any lesions, and of those that did, 20% or less of the leaves had visible ozone damage.
Figure 1.
Comparison of ozone-induced leaf injury observed in the ozone-tolerant clone 245 (left) and the ozone-sensitive clone 388 (right). Cuttings were grown in the greenhouse for 6 to 8 weeks before being transferred to chambers, where they were allowed to acclimate for 2 to 3 d before treatment with 300 ppb ozone for 6 h daily. The photograph was taken 36 h after the start of the first 6 h of ozone fumigation.
Effects of Ozone on Photosynthesis and Gas Exchange
Before ozone treatment, 6-week-old cuttings of the two clones exhibited different patterns of growth and physiology. Clone 245 plants were on average 8.5 cm taller than clone 388 plants (59.5 ± 0.7 versus 51.1 ± 0.6 cm, respectively), and had about 1.5 times as much aboveground biomass (6.54 ± 0.44 versus 4.36 ± 0.16 g, respectively) and 1.8 times as much leaf area (1386 ± 87 versus 778 ± 30 cm2, respectively). Both clones averaged 20 leaves each, resulting in a larger average leaf size for clone 245. Light-response curves (data not shown) indicated that the photosynthetic capacity of clone 388 was significantly (P < 0.05) lower than that of clone 245 when measured under saturating light. However, the two clones demonstrated similar rates of photosynthesis when measured under low-light conditions, simulating the conditions used during ozone exposure.
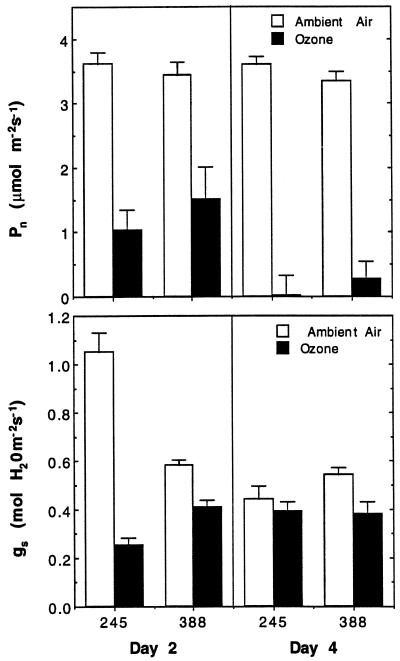
After 2 d of exposure, ozone significantly (P < 0.05) reduced photosynthetic rates in both clones (Fig. 2). Statistical analysis indicated that the absolute values of photosynthesis were not significantly different between the two clones. However, the percentage reduction was greater in clone 245 than in clone 388 (72% versus 56%). Ozone continued to reduce photosynthetic rates throughout the study, and by the 4th d of fumigation, all of the ozone-treated plants had photosynthetic rates that were less than those of the ambient-air-grown plants, and many had negative net photosynthetic rates, indicating that the leaf tissue was respiring more than photosynthesizing (Fig. 2).
Figure 2.
Effects of ozone on net photosynthesis rate (Pn) and stomatal conductance (gs). Measurements were made on the fourth fully expanded leaves of ozone-tolerant clone 245 plants or ozone-sensitive clone 388 plants after 2 and 4 d of exposure to ambient air or a 6-h daily treatment with 300 ppb ozone. Bars represent the mean of eight plants ±se. Results of analysis of variance produced the following P values for photosynthesis (ozone = 0.0035, clone = 0.7058, day = 0.0036, ozone × clone = 0.1477, ozone × day = 0.0081, and clone × day = 0.7137) and stomatal conductance (ozone = 0.0150, clone = 0.0906, day = 0.0001, ozone × clone = 0.0002, ozone × day = 0.0001, and clone × day = 0.0026).
Ozone exposure reduced stomatal conductance as well, and the magnitude of this reduction was significantly different (P < 0.05) between the clones (Fig. 2). After 2 d of exposure to ozone, stomatal conductance of the ozone-sensitive clone 388 was reduced by approximately 30% compared with a reduction of 80% in the ozone-tolerant clone 245. After 4 d of exposure, this same reduction of 30% was present in clone 388, but the difference between ozone-treated and ambient-air-grown plants for clone 245 was only 12%. The change in percentage reduction from d 2 to 4 in clone 245 was caused by a large reduction in stomatal conductance values in the ambient-air-grown plants combined with an increase in the stomatal conductance values in the ozone-fumigated plants. This change in ambient-air-grown clone 245 plants also accounted for the significant ozone-by-day and clone-by-day interactions.
Ozone-Induced Gene Expression
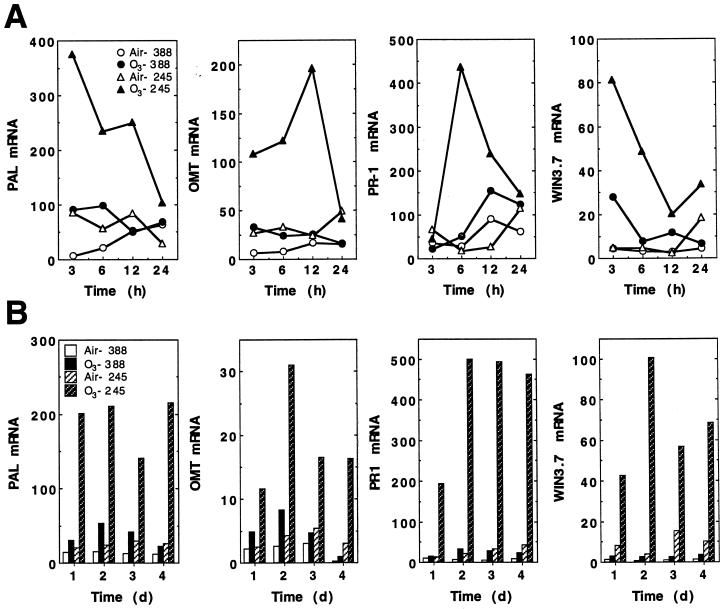
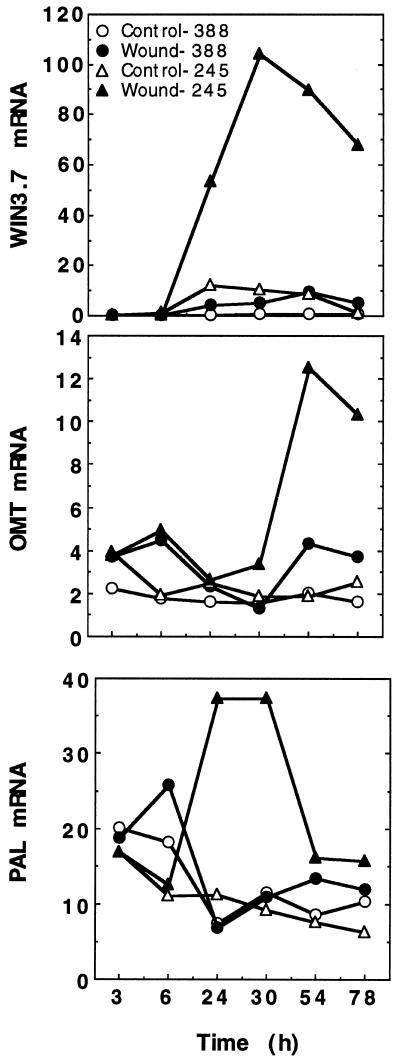
Because PAL induction is often a useful indicator of a general, coordinate plant defense response, we tested whether sensitive and tolerant clones differed in their accumulation of PAL transcripts in response to ozone. RNA-blot hybridization studies demonstrated ozone-induced accumulation of PAL transcripts in both the sensitive and tolerant clones (Fig. 3A). However, this increase was 3- to 5-fold greater in clone 245 than in clone 388. An early, transient induction of PAL mRNA was observed in both clones, with the highest levels observed at 3 h after the initiation of ozone exposure. Levels of PAL mRNA returned to those seen in the controls by 24 h. Trees that were treated with ozone for 4 consecutive d also showed induction of PAL mRNA each day (Fig. 3B). Transcript accumulation for OMT, an enzyme involved in lignin biosynthesis, was also induced by ozone. OMT mRNA reached the highest levels 12 h after the start of ozone treatment and, like PAL mRNA, returned to near control levels by 24 h (Fig. 3A). As was observed for PAL mRNA induction, OMT transcripts were induced each day during the 4-d treatment period (Fig. 3B).
Figure 3.
Comparison of the ozone-dependent induction of defense-related gene mRNA accumulation in the hybrid poplar clones 245 and 388. Total RNA was extracted from the combined second and fourth fully expanded leaves of 6- to 8-week-old cuttings that were exposed to either ambient air or 300 ppb ozone for 6 h. The amount of hybridizing radioactivity was quantitated using a phosphor imager and is expressed as relative counts. A, RNA accumulation during the first 24 h after the start of the first 6-h ozone treatment. B, RNA accumulation measured on sequential days after a 6-h ozone treatment.
Previous work in this laboratory has demonstrated that Arabidopsis has ozone-inducible defense mechanisms that provide some protection, and that the induction of this defense requires a SA-dependent signaling pathway (Sharma et al., 1996). We used PR-1 as a marker gene for a SA-mediated response and tested for mRNA accumulation in response to ozone to determine if induction of this pathway differed between the sensitive and tolerant hybrid poplar clones. In clone 388, very little (3-fold) induction of PR-1 by ozone was observed (Fig. 3A). However, the tolerant clone 245 demonstrated increased levels of PR-1 transcripts as early as 6 h after the initiation of ozone exposure, with the maximum level (24-fold) being reached after the 2nd d of fumigation. This high level of PR-1 transcript accumulation was attained on each of the subsequent days of exposure (Fig. 3B).
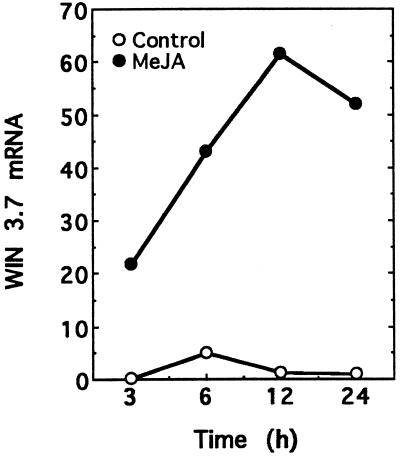
Recent work has indicated that in tobacco, ozone protection can be induced by wounding and treatment with jasmonates before ozone exposure (Örvar et al., 1997). To examine the role of JA-mediated gene expression in ozone responses, we measured the levels of WIN3.7 mRNAs in ozone-treated poplar plants. WIN3.7 is a wound-inducible gene from poplar that is homologous to the trypsin family of proteinase inhibitors (Bradshaw et al., 1989) that can also be induced by exogenous application of MeJA (Fig. 4). In clone 245, levels of WIN3.7 mRNA were induced up to 20-fold by ozone, whereas in clone 388, only a 6-fold induction was observed. Similar to the induction pattern of PAL, WIN3.7 transcript levels were induced maximally by 3 h, then returned to control levels by 24 h (Fig. 3A). This induction was evident every day during the 4 consecutive d of ozone exposure (Fig. 3B).
Figure 4.
Induction of WIN3.7 mRNA by MeJA. Clone 245 plants (6- to 8-week-old cuttings) were sprayed with an aqueous solution containing either 0.1% Triton X-100 (Control) or 0.1% Triton X-100 containing 100 mm MeJA until the leaves were completely wetted. At the indicated times after spraying, the second and fourth fully expanded leaves were harvested. Total RNA was extracted, subjected to RNA-blot hybridization analysis, and the amount of hybridizing radioactivity was quantitated using a phosphor imager. Time points represent the hours after the beginning of the treatments.
Wound- and Pathogen-Induced Gene Expression
Comparisons of the ozone-induced patterns of defense-gene expression in ozone-sensitive and -tolerant clones of hybrid poplar indicated that clone 388 has a greatly attenuated ozone response with respect to ozone-induced gene expression. To determine if this lack of defense-gene activation was specific to ozone treatment, we examined the patterns of gene expression in wounded leaves and in leaves infiltrated with an avirulent P. syringae pv maculicola strain. Wounding treatment appeared to induce PAL, OMT, and WIN3.7 expression in both clones (Fig. 5). However, the extent of this induction was far greater in the ozone-tolerant clone 245 than in the ozone-sensitive clone 388. In clone 245, wounding induced PAL mRNA levels 10-fold above unwounded controls, in contrast to ozone, which induced PAL levels by only 3- to 5-fold above ambient-air-grown controls (Fig. 5). Induction of PAL transcripts occurred as early as 6 h after wounding, peaked at 30 h, and remained elevated throughout the 4 d of wounding. In clone 388, wound-induced levels of PAL mRNA were barely detectable above the control levels. OMT transcript accumulation was also induced by wounding to a greater extent in clone 245 (Fig. 5). The highest expression level of OMT mRNA (3- to 5-fold above the controls) was not reached until 54 h after the start of the experiment. Expression of the wound- and MeJA-inducible WIN3.7 gene increased in both clones (Fig. 5). However, clone 245 showed an increase of WIN3.7 mRNA up to 15-fold greater than the levels seen in clone 388 after wounding. PR-1, as expected, was not induced in either clone 388 or 245 by wounding (data not shown).
Figure 5.
Comparison of wound-induced accumulation of PAL, OMT, and WIN3.7 mRNAs in hybrid poplar clones 245 and 388. Total RNA was extracted from the combined second and fourth fully expanded leaves of 6- to 8-week-old cuttings that had been wounded as described in the text. Total RNA was extracted, subjected to RNA-blot hybridization analysis, and the amount of hybridizing radioactivity was quantitated using a phosphor imager. Time points represent the hours after the initial wounding event.
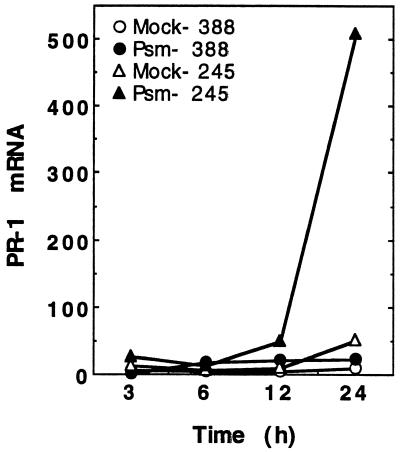
To determine if defense-gene expression via a SA-dependent pathway activated by pathogen infection was also reduced in the ozone-sensitive clone 388, we used the bacterial phytopathogen P. syringae pv maculicola KD4326 to induce a nonhost HR and monitored the induction of PR-1 mRNA accumulation. Infiltration of bacteria into leaves produced visible lesions by 12 h in both clones. Lesion formation was strictly limited to the site of infiltration, and the timing of lesion appearance was not significantly different between the clones. PR-1 transcripts were induced in both clones but, again, the magnitude of this induction was significantly less in the ozone-sensitive clone 388 (Fig. 6). At 24 h after infiltration, PR-1 transcript levels were induced only 2-fold over the mock-inoculated controls in clone 388 compared with a 10-fold induction in clone 245.
Figure 6.
Comparison of pathogen-induced PR-1 mRNA accumulation in hybrid poplar clones 245 and 388. Leaves of 8-week-old cuttings were infiltrated with either P. syringae pv maculicola KD4326 (Psm) in 10 mm MgCl2 or mock inoculated with 10 mm MgCl2 (Mock). Total RNA was extracted from the infiltrated area of the second through fifth fully expanded leaves, subjected to RNA-blot hybridization analysis, and the amount of hybridizing radioactivity was quantitated using a phosphor imager. Time points represent the hours after infiltration.
DISCUSSION
Ozone is known to induce a variety of stress responses in plants at both the physiological and molecular levels (for review, see Kangasjärvi et al., 1994; Iqbal et al., 1996; Sandermann, 1996; Heath and Taylor, 1997; Sharma and Davis, 1997). However, few studies have connected ozone-induced physiological responses to the underlying changes in gene expression, particularly in woody tree species. In our study, we compared changes in physiological and molecular responses in two hybrid poplar clones that exhibit different sensitivities to ozone. We found that specific differences in both physiological changes and gene-expression patterns observed in the two clones correlate with their ozone sensitivity.
The phenomenon of ozone causing a decrease in photosynthetic rate and stomatal conductance has been reported previously in hybrid poplar (Sen-Gupta et al., 1991; Pell et al., 1992) and other plant species (Dann and Pell, 1989; Darrall, 1989). We also found that ozone reduced stomatal conductance and photosynthesis in both of our poplar clones. However, after 2 d of ozone exposure the percentage reduction in stomatal conductance was significantly greater in the ozone-tolerant clone than in the ozone-sensitive clone. Because the stomata regulate the entry of gaseous pollutants into the plant, they may play an important role in determining plant sensitivity to ozone (Iqbal et al., 1996). Although both clones demonstrated an active avoidance response of stomatal closure during exposure to ozone, the ozone-tolerant clone responded more quickly. Thus, the tolerant clone may have excluded more ozone, resulting in less visible damage to the leaves.
After 4 d of ozone exposure, photosynthesis was reduced in both clones. However, stomatal conductance in ozone-treated plants actually increased slightly in clone 245 from d 2 to 4, but still remained below levels measured in ambient-air-treated plants. The percentage reduction in stomatal conductance in clone 245 decreased from 80% on d 2 to 12% on d 4. This is accounted for not only by the increase in stomatal conductance rate in ozone-treated clone 245 plants, but also by a 53% decrease in stomatal conductance in ambient-air-treated clone 245 plants. This decrease in stomatal conductance in ambient-air-treated clone 245 is likely related to the change in biomass within the chambers. Clone 245 is an extremely fast-growing variety and exhibited considerable growth and significant increases in biomass during the 4-d experimental period (data not shown). The increase in biomass was not accompanied by an increase in the rate of watering and may have led to a shortage of water to the plant, which could cause a decrease in stomatal conductance (Winner et al., 1988). However, even with the decrease in stomatal conductance observed in the control clone 245 plants, the ozone-induced reduction was greater.
Differences were also observed in defense-related gene expression between the ozone-tolerant and ozone-sensitive clones. A transient 5-fold induction of PAL in the ozone-tolerant clone 245 was observed, which reached a maximum level at 3 h after treatment. These results are consistent with those reported for ozone-treated herbaceous species (Eckey-Kaltenbach et al., 1994; Sharma and Davis, 1994) and a deciduous tree (Tuomainen et al., 1996). However, the level of ozone-induced PAL transcripts in the ozone-sensitive clone 388 was 4-fold less compared with the tolerant clone. During the 4 d of ozone exposure, the levels of ozone-induced PAL transcripts in the ozone-sensitive clone remained significantly below the levels attained in the ozone-tolerant clone. Transcripts for OMT, a phenylpropanoid-biosynthetic enzyme involved in lignin formation, were induced 5-fold by ozone in clone 245. This induction was transient, with maximum expression reached at 12 h after treatment. The difference in PAL and OMT transcript-induction kinetics is likely attributable to the fact that OMT is active downstream in the phenylpropanoid-biosynthetic pathway from PAL. As was observed with PAL mRNA, induction of OMT mRNA in clone 388 was only 2-fold above that in ambient-air-grown controls throughout the 4-d exposure.
The induction of phenylpropanoid biosynthesis by ozone is well documented; thus, the reported induction of PAL and OMT transcripts by ozone in hybrid poplar is not surprising. In addition to the ozone induction of PAL and OMT transcripts, ozone treatment has been shown to cause increased isoflavonoid and flavonoid biosynthesis in soybean (Keen and Taylor, 1975). Ozone-induced increases in the activities of phenylpropanoid and isoprenoid biosynthetic enzymes in pine have also been reported (Rosemann et al., 1991; Wegener et al., 1997). The observation that the phenylpropanoid pathway is induced in a variety of plants by ozone provides correlative evidence that synthesis of phenylpropanoid derivatives may play a protective role during ozone stress. This protective effect may be related to the ability of plant phenolics and flavonoid derivatives to function as antioxidants because of their ability to trap free radicals (Lewis, 1993). Thus, the increased ozone sensitivity of clone 388 may be attributable in part to the lack of sufficient induction of the phenylpropanoid pathway to provide protective levels of these antioxidant compounds.
Our results concerning the induction of PAL mRNA in hybrid poplar differ from those reported by Tuomainen et al. (1996), in which ozone-induced PAL transcript levels were the same in both ozone-sensitive and ozone-insensitive birch clones. That study also compared polyamine levels and enzyme activities of superoxide dismutase, peroxidase, and glutathione reductase and found that they reached higher levels in the ozone-sensitive birch clone compared with the ozone-insensitive clone. They concluded that higher levels of putrescine and AOS-scavenging enzymes correlated with the appearance of physical damage. The differences in PAL induction in ozone-sensitive and ozone-tolerant hybrid poplar and birch varieties may be related to different mechanisms of lesion formation in these two experimental systems. The hypothesis that different mechanisms of ozone sensitivity are important in different pairs of ozone-sensitive and ozone-tolerant plants is consistent with the results of Wellburn and Wellburn (1996). In their study it was found that changes in levels of polyamines, phenols, reduced glutathione, reduced ascorbate, and total ascorbate in pairs of tolerant or sensitive selections or cultivars of six different species were not correlated with either tolerance or sensitivity. Increased ethylene emission was consistently observed in the more ozone-sensitive plants.
Previous work in this laboratory has demonstrated that ozone activates at least two distinct signaling pathways in Arabidopsis, one of which overlaps with the HR and SAR activation pathways and is SA dependent (Sharma et al., 1996). To examine the potential role of this pathway in hybrid poplar, we used PR-1 as a marker gene. In clone 245, PR-1 was induced 24-fold over ambient-air-grown controls, reaching maximum levels 12 h after the start of ozone treatment. This result is consistent with the ozone-induced accumulation of the PR-1 transcripts in Arabidopsis reported by Sharma et al. (1996). Again, a difference between the ozone-tolerant and ozone-sensitive clones was observed. Induction of PR-1 in clone 388 reached levels only 2- to 3-fold over those of ambient-air-grown controls, about 10-fold less than the levels attained in the tolerant clone. Because activation of a SA-dependent pathway may provide some protection to ozone (Sharma et al., 1996), the apparent lack of a significant induction of SA-dependent gene expression may have enhanced the ozone sensitivity of clone 388. A significant difference in the induction of PR-1 mRNA accumulation was also observed in clones 245 and 388 in response to infection with an avirulent bacterial pathogen; PR-1 transcript levels were 20-fold higher in the ozone-tolerant clone 245 than in clone 388 (Fig. 6). These results demonstrate that attenuated PR-1 expression is not specific to ozone exposure and suggests that clone 388 may have generally diminished SA-mediated stress responses.
The overlap in the induction of SA-mediated responses by both ozone and pathogens is likely because both stresses result in the production of AOS. Increased production of AOS by ozone upon entering the plant could mimic the “oxidative burst,” which in plant-pathogen interactions is thought to trigger the signaling events that activate the HR and SAR, resulting in disease resistance (for review, see Mehdy et al., 1996; Lamb and Dixon, 1997). It has been proposed (Sharma and Davis, 1994; Sharma et al., 1996) that the necrotic lesions observed in some ozone-treated plants may be caused by the activation of the programmed cell death component of the HR (for review, see Dangl et al., 1996; Greenberg, 1997). Our results on PR-1 induction indicate that the ozone-sensitive hybrid poplar clone 388 has an attenuated SA-mediated response that would normally lead to the formation of these ozone-induced HR-like lesions. Because the sensitive clone 388 develops much larger regions of necrosis and the tolerant clone 245 develops smaller, HR-like lesions, it could be argued that these lesions develop via two distinct mechanisms. In the sensitive clone 388, the ozone-induced lesions may be caused by the toxic effects of AOS that accumulate because of the lack of any induced defense responses. In the ozone-tolerant clone 245, ozone may induce lesions by activating a cell-death pathway associated with HR. This interpretation is consistent with previous studies in tobacco, in which the ozone-sensitive cv Bel-W3 exhibited higher levels of PR gene expression compared with the more tolerant cv Bel-B (Ernst et al., 1992; Schraudner et al., 1992). Similar results were obtained in comparisons of tolerant and sensitive ecotypes of Arabidopsis (Sharma and Davis, 1997; I. Aguilar, Y. Sharma, and K. Davis, unpublished data). In cv Bel-W3 and the highly ozone-sensitive Arabidopsis ecotype, the apparent increased ozone sensitivity may be attributable to an enhanced HR in response to the ozone-induced production of AOS.
Evidence that a second, SA-independent pathway is also activated by ozone, resulting in the induction of PAL transcripts in Arabidopsis, was presented by Sharma et al. (1996). The possibility that JA may be involved in ozone-activated signal transduction pathways was addressed by Örvar et al. (1997), who demonstrated that pretreatment of tobacco by either mechanical wounding or JA decreased the amount of ozone injury in tobacco plants. We confirmed that the wound-induced WIN3.7 gene is induced by MeJA and used this gene as a marker for JA-mediated responses. We found that ozone induced WIN3.7 transcript accumulation in the ozone-tolerant clone 245 by 20-fold over ambient-air-grown controls. This same transcript was only induced 3-fold in the ozone-sensitive clone 388. The induction of WIN3.7 transcripts cannot be an indirect response to ozone-induced lesion formation because transcript accumulation was detected at 3 h after the start of ozone treatment, whereas lesions in clone 245 were not visible until between 12 and 24 h. Furthermore, if induction of this gene is a by-product of lesion formation, it would be expected to correlate with the level of injury development. However, in clone 388, which develops the most severe lesions, WIN3.7 transcript levels were less than 10% of the levels reached in clone 245.
To determine if the reduced levels of defense-gene expression in clone 388 were caused only by the inability to respond to ozone stress, we performed wounding experiments. Wounding induced transcript levels of PAL, OMT, and WIN3.7 in both the sensitive and tolerant clones. However, wound induction of these transcripts in clone 388 was significantly reduced compared with that in clone 245. Thus, the ozone-sensitive clone appears to have greatly attenuated ozone- and wound-induced responses, indicating that these responses may share at least a portion of the same signaling pathway. A potential link between ozone- and wound-induced responses is JA. JA is associated with a variety of physiological responses (for review, see Sembdner and Parthier, 1993; Reinbothe et al., 1994; Creelman and Mullet, 1997), many of which correlate with ozone responses. For example, exogenous application of JA has been reported to accelerate leaf senescence, promote stomatal closure, inhibit photosynthetic activity, inhibit Rubisco biosynthesis, and induce defense-gene expression.
We found that clone 245, upon exposure to ozone, displays inhibition of photosynthetic activity, enhanced stomatal closure, and induction of both PAL and a proteinase- inhibitor gene. Furthermore, studies by Landry and Pell (1993) on this same clone indicated that ozone caused accelerated leaf senescence and inhibited both Rubisco activity and photosynthesis. The ozone-sensitive clone 388 also exhibits reduced photosynthetic activity and stomatal conductance, confirming results by Eckardt et al. (1991) and Pell et al. (1992). However, the data reported by Landry and Pell (1993), Eckardt et al. (1991), and Pell et al. (1992) did not include direct comparisons between these two hybrid poplar clones. Our data include measurements of photosynthetic rate and stomatal conductance that were performed under identical conditions for both clones so that the responses may be compared. These results indicate that the ozone-induced reduction in stomatal conductance in clone 388 plants is significantly (P < 0.05) less than the reduction measured in clone 245 plants. The ozone-induced reduction in photosynthetic rate did not differ significantly between the clones, which may be attributable to high variability between individuals combined with a small sample size. In addition, when photosynthetic rates were measured under saturating light, the ozone-induced reduction in photosynthetic rate in clone 245 plants was significantly greater than the reduction measured in clone 388 plants (data not shown). This may indicate that the greater reduction in photosynthetic rate observed in clone 388 compared with clone 245, although not statistically significant, may have biological significance. Furthermore, we observed very little senescence attributable to ozone treatment (data not shown) and a reduced level of proteinase-inhibitor gene expression in clone 388 compared with clone 245. All of these results are consistent with JA having a role in mediating some ozone-induced responses in hybrid poplar.
The apparent attenuated JA-mediated responses observed in clone 388 may act as an important component of its increased ozone sensitivity by causing a slower rate of stomatal closure. Thus, in clone 388, it is possible that more ozone enters the mesophyll and generates more AOS, resulting in more tissue necrosis. The effects of increased ozone entering the mesophyll may be compounded by the lack of SA-dependent inducible antioxidant defense responses. Alternatively, the enhanced ozone sensitivity of clone 388 may not be the direct result of the reduced levels of defense-response induction but, rather, may be caused by the increased stomatal conductance and higher levels of AOS production that exceed the antioxidant capacity of the cells. This could result in sufficient cell damage to prevent the defense responses from being activated. This alternative seems less likely given the observation that the timing of lesion development was very similar in clones 388 and 245 and occurred well after early defense-gene activation was observed. It would be expected that more rapid and extensive cell damage would be associated with more rapid necrosis. Moreover, the defense responses of clone 388 were attenuated not only in response to ozone, but also in response to wounding and pathogen treatments. Thus, it is more likely that the increased ozone sensitivity of clone 388 is related to the reduced activation of defense signaling pathway(s) that are common for all three stresses, e.g. a reduced ability to generate or respond to stress signals such as ethylene, JA, and SA.
Further study of the differential ozone sensitivity of the hybrid poplar clones 245 and 388 should prove important in characterizing further the mechanisms of interaction between ethylene-, SA-, and JA-activated defense-response pathways during exposure to oxidative stress. The current study will allow a comparison of the ozone-induced responses in a woody tree species with the responses of herbaceous plants such as tobacco and Arabidopsis. In addition, this system will be a valuable tool in defining novel signaling pathways for defense-gene induction in trees and for the identification of specific genes that may be useful in increasing stress resistance.
ACKNOWLEDGMENTS
We thank Eva Pell (The Pennsylvania State University, University Park) for supplying the initial cuttings of clones 245 and 388 and for her expertise and advice. We also thank Carl Douglas, Marc Van Montagu, and Milton Gordon for supplying the cDNA probes used in this study, and Staci Putney (The Ohio State University) for maintaining the hybrid poplar plants.
Abbreviations:
- AOS
active oxygen species
- HR
hypersensitive response
- JA
jasmonic acid
- MeJA
methyl jasmonate, OMT, O-methyltransferase
- PAL
Phe ammonia-lyase
- PR
pathogenesis-related
- SA
salicylic acid
- SAR
systemic acquired resistance
Footnotes
This work was supported in part by funds provided by the U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station.
LITERATURE CITED
- Bradshaw HD, Hollick JB, Parsons TJ, Clarke HRG, Gordon MP. Systemically wound-responsive genes in poplar trees encode proteins similar to sweet potato sporamins and legume Kunitz trypsin inhibitors. Plant Mol Biol. 1989;14:51–59. doi: 10.1007/BF00015654. [DOI] [PubMed] [Google Scholar]
- Chameides WL, Kasibhatla PS, Yienger J, Levy H., II Growth of continental-scale metro-agro-plexes, regional ozone pollution, and world food production. Science. 1994;264:74–77. doi: 10.1126/science.264.5155.74. [DOI] [PubMed] [Google Scholar]
- Coleman MD, Dickson RE, Isebrands JG, Karnosky DF. Carbon allocation and partitioning in aspen clones varying in sensitivity to tropospheric ozone. Tree Physiol. 1995a;15:593–604. doi: 10.1093/treephys/15.9.593. [DOI] [PubMed] [Google Scholar]
- Coleman MD, Isebrands JG, Dickson RE, Karnosky DF. Photosynthetic productivity of aspen clones varying in sensitivity to tropospheric ozone. Tree Physiol. 1995b;15:585–592. doi: 10.1093/treephys/15.9.585. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann MS, Pell EJ. Decline of activity and quantity of ribulose bisphosphate carboxylase/oxygenase and net photosynthesis in ozone-treated potato foliage. Plant Physiol. 1989;91:427–432. doi: 10.1104/pp.91.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrall NM. The effect of air pollutants on physiological processes in plants. Plant Cell Environ. 1989;12:1–30. [Google Scholar]
- Dumas B, Van Doorsselaere J, Gielen J, Legrand M, Fritig B, Van Montagu M, Inzé D. Nucleotide sequence of a complementary DNA encoding O-methyltransferase from poplar. Plant Physiol. 1992;98:796–797. doi: 10.1104/pp.98.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt NA, Landry LG, Pell EJ (1991) Ozone-induced changes in RuBPCase from potato and hybrid poplar. In EJ Pell, K Steffen, eds, Active Oxygen/Oxidative Stress and Plant Metabolism. American Society of Plant Physiologists, Rockville, MD, pp 233–237
- Eckey-Kaltenbach H, Ernst D, Heller W, Sandermann H., Jr Biochemical plant responses to ozone. IV. Cross-induction of defensive pathways in parsley plants. Plant Physiol. 1994;104:67–74. doi: 10.1104/pp.104.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (1976) Diagnosing Vegetation Injury Caused by Air Pollution. Air Pollution Training Institute, Research Triangle Park, NC
- Ernst D, Schraudner M, Langebartels C, Sandermann H., Jr Ozone-induced changes of messenger RNA levels of beta-1,3-glucanase, chitinase and pathogenesis-related protein 1B in tobacco plants. Plant Mol Biol. 1992;20:673–682. doi: 10.1007/BF00046452. [DOI] [PubMed] [Google Scholar]
- Farage PK, Long SP. An in vivo analysis of photosynthesis during short-term ozone exposure in three contrasting species. Photosynth Res. 1995;43:11–18. doi: 10.1007/BF00029457. [DOI] [PubMed] [Google Scholar]
- Galliano H, Cabané M, Eckerskorn C, Lottspeich F, Sandermann H, Jr, Ernst D. Molecular cloning, sequence analysis and elicitor-/ozone-induced accumulation of cinnamyl alcohol dehydrogenase from Norway spruce (Picea abies L.) Plant Mol Biol. 1993;23:145–156. doi: 10.1007/BF00021427. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:525–545. doi: 10.1146/annurev.arplant.48.1.525. [DOI] [PubMed] [Google Scholar]
- Heath RL, Taylor GE. Physiological processes and plant responses to ozone exposure. In: Sandermann H, Wellburn AR, Heath RL, editors. Forest Decline and Ozone: A Comparison of Controlled Chamber and Field Experiments. Berlin: Springer-Verlag; 1997. pp. 317–368. [Google Scholar]
- Iqbal M, Abdin MZ, Mahmooduzzafar, Yunus M, Agrawal M. Resistance mechanisms in plants against air pollution. In: Yunus M, Iqbal M, editors. Plant Response to Air Pollution. New York: John Wiley & Sons; 1996. pp. 195–240. [Google Scholar]
- Kangasjärvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defence systems induced by ozone. Plant Cell Environ. 1994;17:783–794. [Google Scholar]
- Kanofsky JR, Sima PD. Singlet oxygen generation from the reaction of ozone with plant leaves. J Biol Chem. 1995;270:7850–7852. doi: 10.1074/jbc.270.14.7850. [DOI] [PubMed] [Google Scholar]
- Kärenlampi SO, Airaksinen K, Miettinen ATE, Kokko HI, Holopainen JK, Kärenlampi LV, Karjalainen RO. Pathogenesis-related proteins in ozone-exposed Norway spruce [Picea abies (Karst) L.] New Phytol. 1994;126:81–89. [Google Scholar]
- Keen NT, Taylor OC. Ozone injury in soybeans: isoflavonoid accumulation is related to necrosis. Plant Physiol. 1975;42:529–551. doi: 10.1104/pp.55.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Landry LG, Pell EJ. Modification of Rubisco and altered proteolytic activity in O3-stressed hybrid poplar. Plant Physiol. 1993;101:1355–1362. doi: 10.1104/pp.101.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NG. Plant phenolics. In: Alscher RG, Hess J, editors. Antioxidants in Higher Plants. Boca Raton, FL: CRC Press; 1993. pp. 135–170. [Google Scholar]
- McLaughlin SB. Effects of air pollution on forests. J Air Pollut Control Assoc. 1985;35:512–534. [Google Scholar]
- Mehdy MC, Sharma YK, Sathasivan K, Bays NW. The role of activated oxygen species in plant disease resistance. Physiol Plant. 1996;98:365–374. [Google Scholar]
- Örvar BL, McPherson J, Ellis BE. Pre-activating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant J. 1997;11:203–212. doi: 10.1046/j.1365-313x.1997.11020203.x. [DOI] [PubMed] [Google Scholar]
- Paakkonen E, Vahala J, Holopainen T, Karjalainen R, Kärenlampi L. Growth responses and related biochemical and ultrastructural changes of the photosynthetic apparatus in birch (Betula pendula) saplings exposed to low concentrations of ozone. Tree Physiol. 1996;16:597–605. doi: 10.1093/treephys/16.7.597. [DOI] [PubMed] [Google Scholar]
- Parsons TJ, Bradshaw HD, Gordon MP. Systemic accumulation of specific mRNAs in response to wounding in poplar trees. Proc Natl Acad Sci USA. 1989;86:7895–7899. doi: 10.1073/pnas.86.20.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell EJ, Eckardt N, Enyedi AJ. Timing of ozone stress and resulting status of ribulose bisphosphate carboxylase/oxygenase and associated net photosynthesis. New Phytol. 1992;120:397–405. [Google Scholar]
- Pell EJ, Sinn JP, Johansen CV. Nitrogen supply as a limiting factor determining the sensitivity of Populus tremuloides Michx. to ozone stress. New Phytol. 1995;130:437–446. [Google Scholar]
- Reich PB, Amundson RG. Ambient levels of ozone reduce net photosynthesis in tree and crop species. Science. 1985;230:566–570. doi: 10.1126/science.230.4725.566. [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Mollenhauer B, Reinbothe C. JIPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell. 1994;6:1197–1209. doi: 10.1105/tpc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemann D, Heller W, Sandermann H., Jr Biochemical plant responses to ozone. II. Induction of stilbene biosynthesis in Scots pine (Pinus sylvestris L.) seedlings. Plant Physiol. 1991;97:1280–1286. doi: 10.1104/pp.97.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr Ozone and plant health. Annu Rev Phytopathol. 1996;34:347–366. doi: 10.1146/annurev.phyto.34.1.347. [DOI] [PubMed] [Google Scholar]
- SAS Institute (1989) SAS/STAT Users Guide, Version 6, Ed 4, Vol 2. SAS Institute, Cary, NC
- Schmieden U, Wild A. The contribution of ozone to forest decline. Physiol Plant. 1995;94:371–378. [Google Scholar]
- Schraudner M, Ernst D, Langebartels C, Sandermann H., Jr Biochemical plant responses to ozone. III. Activation of the defense-related proteins β-1,3-glucanase and chitinase in tobacco leaves. Plant Physiol. 1992;99:1321–1328. doi: 10.1104/pp.99.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:569–589. [Google Scholar]
- Sen-Gupta A, Alscher RG, McCune D. Response of photosynthesis and cellular antioxidants to ozone in Populus leaves. Plant Physiol. 1991;96:650–655. doi: 10.1104/pp.96.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma YK, Davis KR. Ozone-induced expression of stress-related genes in Arabidopsis thaliana. Plant Physiol. 1994;105:1089–1096. doi: 10.1104/pp.105.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma YK, Davis KR. The effects of ozone on antioxidant responses in plants. Free Radical Biol Med. 1997;23:480–488. doi: 10.1016/s0891-5849(97)00108-1. [DOI] [PubMed] [Google Scholar]
- Sharma YK, Leon J, Raskin I, Davis KR. Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA. 1996;93:5099–5104. doi: 10.1073/pnas.93.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell WR, Kramm G, Scheel H-E, Mohnen VA, Seiler W. Ozone formation, destruction and exposure in Europe and the United States. In: Sandermann H, Wellburn AR, Heath RL, editors. Forest Decline and Ozone: A Comparison of Controlled Chamber and Field Experiments. Berlin: Springer-Verlag; 1997. pp. 227–315. [Google Scholar]
- Subramaniam R, Reinold S, Molitor EK, Douglas CJ. Structure, inheritance, and expression of hybrid poplar (Populus trichocarpa × Populus deltoides) phenylalanine ammonia-lyase genes. Plant Physiol. 1993;102:71–83. doi: 10.1104/pp.102.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomainen J, Pellinen R, Roy S, Kiiskinen M, Eloranta T, Karjalainen R, Kangasjärvi J. Ozone affects birch (Betula pendula Roth) phenylpropanoid, polyamine and active oxygen detoxifying pathways at biochemical and gene expression levels. J Plant Physiol. 1996;148:179–188. [Google Scholar]
- Wanner LA, Gruissem W. Expression dynamics of the tomato rbcS gene family during development. Plant Cell. 1991;3:1289–1303. doi: 10.1105/tpc.3.12.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner LA, Mittal S, Davis KR. Recognition of the avirulence gene avrB from Pseudomonas syringae pv. glycinea by Arabidopsis thaliana. Mol Plant Microbe Interact. 1993;6:582–591. doi: 10.1094/mpmi-6-582. [DOI] [PubMed] [Google Scholar]
- Wegener A, Gimbel W, Werner T, Hami J, Ernst D, Sandermann H., Jr Molecular cloning of ozone-inducible protein from Pinus sylvestris L. with high sequence similarity to vertebrate 3-hydroxy-3-methylglutaryl-CoA-synthase. Biochim Biophys Acta. 1997;1350:247–252. doi: 10.1016/s0005-2760(96)00161-0. [DOI] [PubMed] [Google Scholar]
- Wellburn FAM, Wellburn AR. Variable patterns of antioxidant protection but similar ethene emission differences in several ozone-sensitive and ozone-tolerant plant selections. Plant Cell Environ. 1996;19:754–760. [Google Scholar]
- Winner WE, Gillespie C, Shen WS, Mooney HA (1988) Stomatal responses in SO2 and O3. In S Schulte-Hostede, NM Darrall, LW Blank, AR Wellburn, eds, Air Pollution and Plant Metabolism. Elsevier Applied Science, New York, pp 255–271
- Wood FA, Coppolino JB. The response of eleven hybrid poplar clones to ozone. Phytopathology. 1972;62:501–502. [Google Scholar]