Abstract
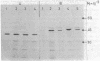
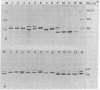
Subunits A and B of chloroplast glyceraldehyde-3-phosphate dehydrogenase are synthesized as higher molecular weight precursors when polyadenylylated mRNA from angiosperm seedlings is translated in vitro by wheat germ ribosomes. The in vivo levels of mRNA coding for these precursors are strongly light dependent, and the increase in translational activity stimulated by continuous white light, relative to dark-grown seedlings, is at least 5- to 10-fold for the seven plant species investigated. As opposed to this, light does not seem to change mRNA levels coding for cytosolic glyceraldehyde-3-phosphate dehydrogenase, and the polypeptides synthesized in vitro have the same size as the authentic subunits. In addition, precursors of the chloroplast enzyme were identified for 12 different angiosperm species and compared with their respective subunits synthesized in vivo. The patterns of the in vitro and in vivo products correlate in several major characteristics. They both display a remarkable interspecific heterogeneity with respect to size and number of polypeptides. The peptide extensions of the enzyme precursors calculated from these data vary between 4,000 and 12,000 daltons and seem to fall into three major size classes. The present data demonstrate that chloroplast glyceraldehyde-3-phosphate dehydrogenase, like its cytosolic counterpart, is encoded in the nucleus. Yet, the two dehydrogenases are controlled differently at both the ontogenetic and phylogenetic levels. They follow separate biosynthetic pathways with respect to light regulation, post-translational processing, and transport and also exhibit different evolutionary rates. The fast evolutionary change observed for the chloroplast enzyme contrasts sharply with the conservative structure and sequence of the cytosolic enzyme.
Keywords: polyadenylylated mRNA, immunoprecipitation of in vitro precursors, subunit compositions, transit peptides, enzyme evolution
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerff R., Chambers S. E. Subunit structure of higher plant glyceraldehyde-3-phosphate dehydrogenases (EC 1.2.1.12 and EC 1.2.1.13). J Biol Chem. 1979 Jul 10;254(13):6094–6098. [PubMed] [Google Scholar]
- Cerff R. Evolutionary divergence of chloroplast and cytosolic glyceraldehyde-3-phosphate dehydrogenases from angiosperms. Eur J Biochem. 1982 Sep 1;126(3):513–515. doi: 10.1111/j.1432-1033.1982.tb06810.x. [DOI] [PubMed] [Google Scholar]
- Cerff R. Glyceraldehyde 3-Phosphate Dehydrogenases and Glyoxylate Reductase: I. Their Regulation Under Continuous Red and Far Red Light in the Cotyledons of Sinapis alba L. Plant Physiol. 1973 Jan;51(1):76–81. doi: 10.1104/pp.51.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerff R., Quail P. H. Glyceraldehyde 3-Phosphate Dehydrogenases and Glyoxylate Reductase: II. Far Red Light-Dependent Development of Glyceraldehyde 3-Phosphate Dehydrogenase Isozyme Activities in Sinapis Alba Cotyledons. Plant Physiol. 1974 Jul;54(1):100–104. doi: 10.1104/pp.54.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerff R. Quaternary structure of higher plant glyceraldehyde-3-phosphate dehydrogenases. Eur J Biochem. 1979 Feb 15;94(1):243–247. doi: 10.1111/j.1432-1033.1979.tb12891.x. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. Critical values for testing the significance of amino acid composition indexes. Anal Biochem. 1980 Jul 1;105(2):233–238. doi: 10.1016/0003-2697(80)90450-9. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb L. D. Conservation and duplication of isozymes in plants. Science. 1982 Apr 23;216(4544):373–380. doi: 10.1126/science.216.4544.373. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- HAGEMAN R. H., ARNON D. I. Changes in glyceraldehyde phosphate dehydrogenase during the life cycle of a green plant. Arch Biochem Biophys. 1955 Aug;57(2):421–436. doi: 10.1016/0003-9861(55)90304-0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Link D. P., Lantz B. M. Vasodilator response in the lower extremity induced by contrast medium. I Canine model. Acta Radiol Diagn (Stockh) 1982;23(2):81–86. doi: 10.1177/028418518202300201. [DOI] [PubMed] [Google Scholar]
- Marcus A. Photocontrol of Formation of Red Kidney Bean Leaf Triphosphopyridine Nucleotide Linked Triosephosphate Dehydrogenase. Plant Physiol. 1960 Jan;35(1):126–128. doi: 10.1104/pp.35.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ogawa K., Kaji A. Ribosome run through of the termination codon in the absence of the ribosome releasing factor. Biochim Biophys Acta. 1975 Sep 1;402(3):288–296. doi: 10.1016/0005-2787(75)90266-x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H., Terenna B. Synthesis of the Small Subunit of Ribulose-1,5-bisphosphate Carboxylase by Soluble Fraction Polyribosomes of Pea Leaves. Plant Physiol. 1977 Oct;60(4):532–537. doi: 10.1104/pp.60.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Devillers-Thiery A., Desruisseaux H., Blobel G., Chua N. H. NH2-terminal amino acid sequences of precursor and mature forms of the ribulose-1,5-bisphosphate carboxylase small subunit from Chlamydomonas reinhardtii. J Cell Biol. 1979 Dec;83(3):615–622. doi: 10.1083/jcb.83.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Ellis R. J. Light-stimulated accumulation of transcripts of nuclear and chloroplast genes for ribulosebisphosphate carboxylase. J Mol Appl Genet. 1981;1(2):127–137. [PubMed] [Google Scholar]
- Tobin E. M. Light regulation of specific mRNA species in Lemna gibba L. G-3. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4749–4753. doi: 10.1073/pnas.75.10.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden R., Leaver C. J. Synthesis of Chloroplast Proteins during Germination and Early Development of Cucumber. Plant Physiol. 1981 Jun;67(6):1090–1096. doi: 10.1104/pp.67.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]