Abstract
Objective
Differentiating between malignant and benign lesions on the basis of MR images depends on the experience of the radiologist. For non-experts, we aimed to develop a simplified systematic MRI approach that uses depth, size and heterogeneity on T2 weighted MR images (T2WI) to differentiate between malignant and benign lesions, and evaluated its diagnostic accuracy.
Methods
MR images of 266 patients with histologically proven soft-tissue tumours of the extremities (102 malignant, 164 benign) were analysed according to depth (superficial or deep), size (<50, ≥50 mm) and signal intensity (homogeneous or heterogeneous) on T2WI, to determine the ability of each to predict benign and malignant tumours. These three parameters were categorised into systematic combinations of different orders of application, and each combination was assessed for its ability to differentiate between benign and malignant lesions.
Results
Univariate analysis showed that depth, size and heterogeneity on T2WI differed significantly between benign and malignant masses (p<0.0001 each). Multiple logistic regression analysis, however, showed that depth was not helpful in distinguishing benign from malignant lesions. The systematic combination of signal intensity, size and depth, in that order, was superior to other combinations, resulting in higher diagnostic values for malignancy, with a sensitivity of 64%, a specificity of 85%, a positive predictive value of 32%, a negative predictive value of 59% and an accuracy of 77%.
Conclusion
A simplified systematic imaging approach, in the order signal intensity, size and depth, would be a reference to distinguish between benign and malignant soft-tissue tumours for non-experts.
Soft-tissue sarcomas of the extremities are rare neoplasms of mesenchymal origin, accounting for approximately 1% of all malignant tumours [1]. The incidence of benign soft-tissue tumours is much higher, although their exact incidence is not known [2]. Survival of patients with malignant soft-tissue tumours depends mainly on adequate and timely resection, and/or adjuvant or neoadjuvant radiotherapy, and/or chemotherapy, whereas benign tumours require less aggressive treatment. Imaging is used not only for local staging but also to differentiate between benign and malignant lesions. MRI is the preferred imaging modality for the evaluation of soft-tissue masses in clinical practice. Generally, the major criteria used to diagnose malignant soft-tissue tumours include deep location, large size and heterogeneous signal intensity (SI), particularly on T2 weighted MR images (T2WI), although other criteria are also evaluated, including margins, shape, degree and pattern of enhancement, and evidence of necrosis [3-5].
Despite the superiority of MRI in delineating soft-tissue tumours, its ability is limited because most of these tumours have a non-specific appearance on MR images. Thus, it is often impossible using MR to determine whether the lesion is benign or malignant [5-8]. Indeed, MRI has been found to have low diagnostic value in such differentiation [5,6]. When experienced radiologists evaluated images, MRI was found to have better diagnostic values [9], demonstrating the importance of experts in differentiating benign from malignant lesions.
Therefore, for non-experienced radiologists, we have developed a simplified systematic imaging approach to differentiate between malignant and benign lesions. We assessed three MR findings—depth, size and SI on T2WI—of soft-tissue masses, and compared these findings with those of histological diagnoses. We also evaluated the diagnostic values of our simplified system. This approach constitutes an easier initial evaluation system that non-expert radiologists can use to differentiate between malignant and benign lesions.
Methods and materials
Patients
Between October 2005 and February 2010, 373 consecutive patients underwent MRI followed by biopsy or surgical excision at our institution for histopathologically diagnosed soft-tissue masses of the extremities. Final diagnoses were based on pathology reports of surgical or core needle biopsy specimens, as determined by an experienced pathologist. The study protocol was approved by our institutional review board, which waived informed consent because the study required only the retrospective review of MR images and pathology reports. We excluded 12 patients with recurrent soft-tissue tumours and 97 with soft-tissue lesions originating from bones. The remaining 266 patients consisted of 134 males (median age 46 years; range 3–86 years) and 132 females (median age 46 years; range 14–83 years), with no significant difference in age distribution between the sexes. Among these 266 soft-tissue tumours, 164 (62%) were benign and 102 (38%) were malignant. Specific diagnoses and their numbers of cases are listed in Table 1. The three most common types of benign lesions were lipoma (39/164, 24%), schwannoma (27/164, 16%) and fibromatosis (14/164, 9%), and the three most common types of malignant lesions were liposarcoma (18/102, 18%), myxofibrosarcoma (11/102, 11%) and undifferentiated pleomorphic sarcoma (UPS; 9/102, 9%).
Table 1. List of specific diagnoses and number of cases of each.
| Benign | Number of cases | Malignant | Number of cases |
| Lipoma | 39 | Liposarcoma | 18 |
| Schwannoma | 27 | Myxofibrosarcoma | 11 |
| Fibromatosis | 14 | UPS | 9 |
| Haemangioma | 13 | Melanoma | 7 |
| Epidermal cyst | 10 | Dermatofibrosarcoma protuberance | 7 |
| Neurofibroma | 7 | Metastasis | 6 |
| Angiomyolipoma | 5 | Spindle cell sarcoma | 6 |
| Nodular fasciitis | 5 | Rhabdomyosarcoma | 6 |
| GCT of tendon sheath | 5 | Synovial sarcoma | 5 |
| Ganglion cyst | 4 | Ewing's sarcoma | 4 |
| Myxoma | 4 | Myxoid chondrosarcoma | 3 |
| Pilomatricoma | 3 | Lymphoma | 3 |
| PVNS | 2 | Leiomyosarcoma | 3 |
| Hamartoma | 2 | MPNST | 3 |
| Hibernoma | 2 | Epithelioid sarcoma | 2 |
| AVM | 2 | Malignant granular cell tumour | 2 |
| Dermatofibroma | 1 | Squamous cell carcinoma | 2 |
| Kimura disease | 1 | Eccrine porocarcinoma | 1 |
| Glomangioma | 1 | Merkel cell carcinoma | 1 |
| Angiokeratoma | 1 | Others | 3 |
| Others | 16 | ||
| Total | 164 | Total | 102 |
AVM, arteriovenous malformation; GCT, giant cell tumour; MPNST, malignant peripheral nerve sheath tumour; PVNS, pigmented villonodular synovitis; UPS, undifferentiated pleomorphic sarcoma.
MRI technique
MRI of all patients was performed with 1.5 or 3 T magnets using commercially available transmit–receive coils when appropriate. The MRI scanners used were Achieva 1.5 and 3 T and Gyroscan Intera 1.5 T (Philips Medical Systems, Best, Netherlands), and Magnetom® Avanto 1.5 T (Siemens Medical Solutions, Erlangen, Germany). Standardised imaging protocols were used according to the anatomical locations, but, in general, they included at least two planes of spin echo T1, fast spin echo T2, short tau inversion–recovery (STIR) T2 and fat-suppressed and contrast-enhanced T1 weighted images.
MRI interpretation
MRI findings were retrospectively reviewed by two radiologists—one with 15 years of experience in musculoskeletal radiology and the other a trainee—in consensus. Parameters analysed included: (1) depth (superficial or deep), (2) size (<50 or ≥50 mm diameter) and (3) SI on T2WI (homogeneous or heterogeneous). Depth of a lesion was defined as superficial or deep relative to the superficial investing fascia on MR images (Figure 1). Lesion size was measured in the longitudinal, anteroposterior and transverse dimensions, and classified into two categories: <50 mm and ≥50 mm, based on maximal diameter, with 50 mm chosen for comparison with existing guidelines [2,6,10]. The degree of SI heterogeneity on T2WI was graded subjectively, with a lesion showing heterogeneous SI over 30% of its volume regarded as heterogeneous. Representative examples of cases were demonstrated for these three MRI parameters in benign and malignant tumours (Figure 2). These three parameters were categorised into systematic combinations of different orders of application and each combination was assessed for its ability to differentiate between benign and malignant lesions (Table 2).
Figure 1.
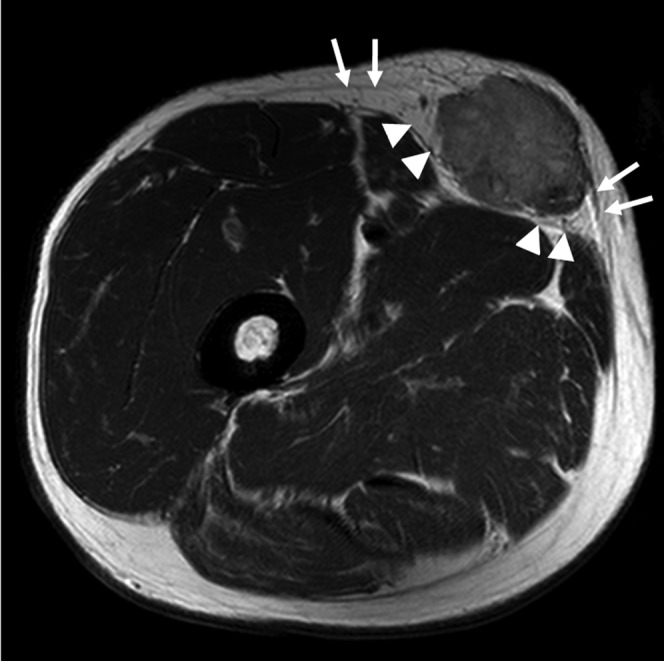
Superficial investing fascia on an MR image. Axial T2 weighted MR image (repetition time=2291 ms, echo time=80 ms) of a 75-year-old male shows a soft-tissue tumour confirmed as leiomyosarcoma. Superficial investing fascia is clearly visualised deep to this soft-tissue mass (arrow heads), as well as superficial fascia of subcutaneous tissue (arrows).
Figure 2.
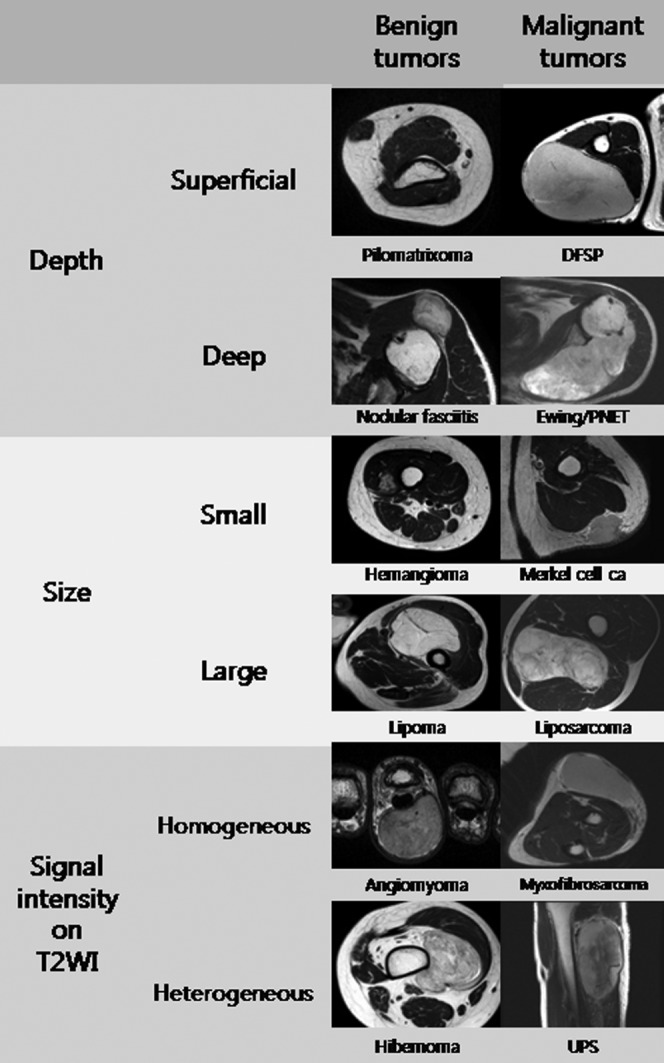
Representative examples of cases for three MRI parameters in benign and malignant tumours. ca, carcinoma; DFSP, dermatofibrosarcoma protuberance; PNET, primitive neuroectodermal tumour; T2WI, T2 weighted MR image; UPS, undifferentiated pleomorphic sarcoma.
Table 2. Systematic combination arranged in the order signal intensity–size–depth.
| Signal intensity | Size | Depth | Groups | Numbers of tumours |
|
| Benign | Malignant | ||||
| Homogeneous | <50 mm | Superficial | A | 28 | 4 |
| Deep | B | 12 | 5 | ||
| ≥50 mm | Superficial | C | 13 | 3 | |
| Deep | D | 20 | 1 | ||
| Heterogeneous | <50 mm | Superficial | E | 23 | 12 |
| Deep | F | 31 | 10 | ||
| ≥50 mm | Superficial | G | 6 | 8 | |
| Deep | H | 31 | 59 | ||
Statistical analysis
Individual findings of depth, size and SI on T2WI were evaluated by univariate and multivariate logistic regression analysis to determine the significance of each for differentiating between benign and malignant tumours. To determine the optimal simplified systematic imaging approach, the diagnostic values of each systematic combination were analysed using multivariate logistic regression and Kendall's tau-c statistics. Kendall's tau-c statistic is a measure of ordinal association that considers whether the column variable Y tends to increase as the row variable X increases. It classifies pairs of observations as concordant or discordant. Tau-c equals 0 under statistical independence for non-square tables.
Results
The location, size and SI of the 266 soft-tissue tumours, both benign and malignant, are shown in Table 3. Of these 266 lesions, 102 were malignant and 164 were benign; on MRI, 169 were deep and 97 were superficial. We found that 27 of the 102 malignant lesions (27%) were superficial, and that 27 of the 97 superficial tumours (28%) were malignant. In contrast, 94 of the 164 benign lesions (56%) were classified as deep.
Table 3. Location, size and signal intensity (SI) of lesions on MRI relative to benign and malignant tumours.
| Parameter | Numbers of tumours |
p-value | |||
| Subtotal | Benign | Malignant | |||
| Location | Superficial | 97 | 70 | 27 | 0.0076 |
| Deep | 169 | 94 | 75 | ||
| Size | <50 mm | 125 | 94 | 31 | <0.0001 |
| ≥50 mm | 141 | 70 | 71 | ||
| SI on MRI | Homogeneous | 86 | 73 | 13 | <0.0001 |
| Heterogeneous | 180 | 91 | 89 | ||
| Total | 266 | 164 | 102 | ||
MRI showed that 125 lesions were small (<50 mm) and 141 were large (≥50 mm). Of the 125 small lesions, 31 (25%) were malignant and 94 (75%) were benign. Of the 141 large lesions, 71 (50%) were malignant and 70 (50%) were benign. There was a statistically significant correlation between larger lesion size and the probability of malignancy (p<0.0001). SI on T2WI was homogeneous for 86 lesions and heterogeneous for 108. 13 of the 102 malignant lesions (13%) had homogeneous SI, and 13 of the 86 homogeneous tumours (15%) were malignant. In contrast, 91 of the 164 benign lesions (56%) were heterogeneous on T2WI.
Univariate analysis showed that depth, size and heterogeneity on T2WI differed significantly between benign and malignant masses. In multivariate logistic regression analysis, however, depth was not an independent predictor of benign and malignant masses (adjusted odds ratio=1.11; Table 4). Despite this, however, depth may be important in clinical practice and was therefore included in the Breslow–Day test, a multivariate analysis test. When we adjusted for depth using the Breslow–Day test, we found that the possibility of malignancy was 2.48 and 4.76 times greater for large lesions and those with heterogeneous SI, respectively (Table 4).
Table 4. Multivariate logistic regression analysis of the association between individual imaging findings and the differentiation of benign and malignant masses.
| Parameter | Crude OR (95% CI) | Adjusted OR (95% CI)a |
| Depth | 2.07 (1.21–3.54) | 1.11 (0.58–2.13) |
| Size (<50, ≥50 mm) | 3.08 (1.82–5.19) | 2.48 (1.36–4.53) |
| Signal intensity on MRI | 5.49 (2.84–10.61) | 4.76 (2.34–9.66) |
CI, confidence interval; OR, odds ratio.
aOdds ratio adjusted for age, sex and other variables.
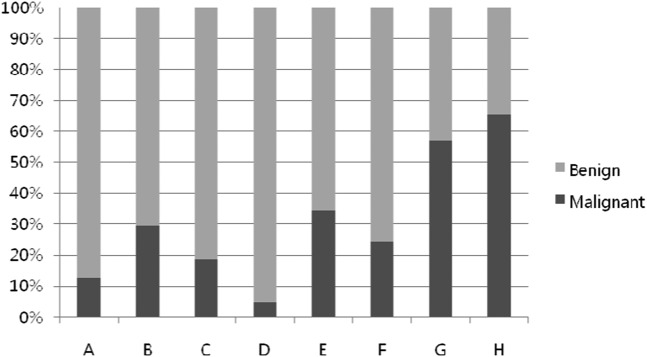
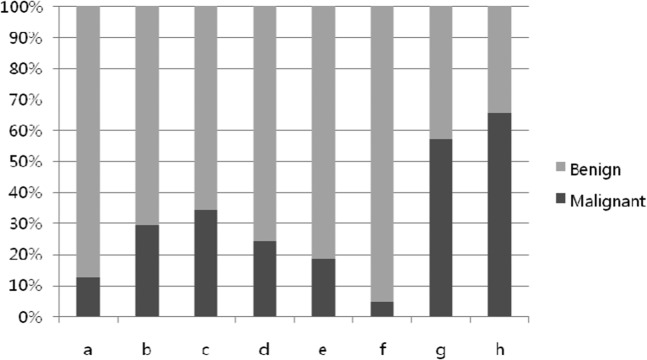
Since heterogeneity and size could differentiate between benign and malignant tumours, we used systematic combinations of the three parameters in different orders of importance to determine the best simplified systematic imaging approach: SI–size–depth (Table 2) and size–SI–depth. Using the first combination, in the order SI–size–depth, we observed an upward tendency of malignancy from A to H (Kendall's tau-c coefficient=0.452; Figure 3). Group D, consisting of homogeneous, large and deeply located lesions, contained the highest proportion of benign lesions, and Groups G and H, containing heterogeneous and large lesions, each had >50% malignant lesions, regardless of depth. Using the second systematic combination, arranged in the order size–SI–depth, we observed a greater tendency of malignancy from a to h (Figure 4). However, its Kendall's tau-c coefficient was 0.394, which was lower than that of the first combination. Therefore the combination SI–size–depth, in that order, resulted in higher diagnostic values for malignancy, with a sensitivity of 64%, a specificity of 85%, a positive predictive value of 32%, a negative predictive value of 59% and an accuracy of 77%.
Figure 3.
Tendency of malignancy from A to H (signal intensity–size–depth; Kendall's tau-c coefficient=0.452).
Figure 4.
Tendency of malignancy from a to h (size–signal intensity-depth; Kendall's tau-c coefficient=0.394).
Discussion
MRI is a well-established tool for the detection and local staging of soft-tissue tumours. However, its ability to differentiate between benign and malignant soft-tissue lesions has been found to vary widely [6-8,10-12]. Using morphological criteria for benign lesions such as smooth well-defined margins, small size and homogeneous SI, particularly on T2WI, MRI was reported to be able to differentiate >90% of benign from malignant masses [10]. Another study, however, noted that malignant lesions may appear as smoothly margined homogeneous masses and that MRI could therefore not reliably distinguish benign from malignant processes [11].
MR findings have been evaluated individually or together for their ability to differentiate benign from malignant lesions. For example, larger size has been associated with greater heterogeneity and a higher likelihood of malignancy [13,14], with only 5% of benign soft-tissue tumours >5 cm in diameter. In addition, most malignant tumours are deeply located, compared with only about 1% of all benign soft-tissue tumours. Our results are not consistent with these reports. In our cases, 43% of benign soft-tissue tumours were >5 cm in diameter and, likewise, 57% of benign soft-tissue tumours were deeply located.
A multivariate statistical analysis of 10 imaging parameters, individually and in combination, showed that high SI on T2WI, diameter >33 mm and heterogeneous SI on T1 weighted MR images predicted malignancy with the highest sensitivity [3]. Signs having the greatest specificity for malignancy included tumour necrosis, bone or neurovascular involvement and mean diameter >66 mm. Although many MRI findings are considered important criteria for the diagnosis of malignant soft-tissue tumours, tumour margin, shape, and the degree and pattern of enhancement are less useful in clinical practice. Most soft-tissue tumours have well-defined margins, are oval or globular in shape and have variable patterns of enhancement, regardless of whether they are benign or malignant.
Evaluation of MR images by experienced radiologists with a centralised approach has been found to yield better diagnoses of soft-tissue tumours [9]. However, many radiologists or clinicians responsible for treating patients with soft-tissue lesions in initial practice may be non-experts in the diagnosis of soft-tissue tumours. Sometimes they erroneously excise a mass without either considering the possibility of malignancy or performing pre-excisional biopsy. In that case, those radiologists and clinicians require a simplified approach to differentiate between benign and malignant soft-tissue tumours. We therefore selected only three major parameters—deep location, large size and heterogeneous SI on T2WI—all of which showed statistically significant differences between benign and malignant masses on univariate analysis. On multivariate analysis, however, depth was not an independent factor in distinguishing benign from malignant lesions. This was somewhat surprising because, generally, deep location with respect to superficial investing fascia has been diagnostic for malignant soft-tissue tumours, as well being prognostic for patient outcomes [13-16]. Similar findings, showing no significant association between lesion depth and diagnostic subgroup, have been reported previously [17].
To determine the optimal simplified systematic imaging approach, we tested two systematic combinations, arranged in order of importance among these three parameters. We found that one, arranged in the order SI–size–depth, was superior to the other, arranged in the order size–SI–depth, resulting in higher diagnostic values for malignancy. Using this simplified systematic approach, we observed comparable specificity and accuracy, and acceptable sensitivity, to initial screening, although positive and negative predictive values were not as high. We found that Group D, consisting of large, homogeneous lesions of deep location, contained the highest proportion of benign lesions, because this group contained many large lipomas and fibromatoses, ranging in size from 5.5 to 21.0 cm diameter. Groups G and H, consisting of large, heterogeneous lesions, contained the highest proportion of malignant tumours (67/102), regardless of depth. Most of these large, heterogeneous lesions were liposarcomas (n=16); other sarcomas were malignant fibrous histiocytoma (n=7), myxofibrosarcoma (n=5), synovial sarcoma (n=5), and rhabdomyosarcoma (n=3); and metastases (n=3).
This study had several limitations. Owing to its retrospective design, there was some variability in MRI parameters. The sample size was modest because the sample of patients was from a regional centre for oncology, and there was a possibility of selection bias in that we did not exclude tumours originating from the skin, such as melanomas. Enrolment of histologically confirmed cases was another selection bias, because obviously benign lesions that did not need biopsy or surgical excision might be omitted. Also, we included lipomas, which, although large and sometimes deeply located, are benign. Thus, this simplified systematic imaging approach could be applied to large or deeply located lipomas together with large or deeply located malignant tumours, even though lipomas usually do not cause a diagnostic dilemma with proper use of T1, T2 and fat-suppressed sequences. In addition, other MRI findings may help differentiate between benign and malignant lesions, including tumour enhancement pattern and invasion of surrounding structures such as bone or vessels. Although the latter is an important predictor of malignancy, it is also rare, and we therefore did not include it in our analysis. We used heterogeneity only on T2WI, but heterogeneity on other sequences would be an important predictor of malignancy. Since our purpose was to provide an easy and realistic system to differentiate benign and malignant tumours in clinical practice, we considered only three major characteristics. In addition, the findings of this study may not necessarily reflect the data that may be encountered in the general population: because the sample of patients was from a regional centre of oncology, only limited findings (especially on T2WI) were used, and enrolled cases were retrospective histologically proven cases. Therefore, great care must be used in interpreting soft-tissue masses on MRI. The findings of this study are not definitive conclusions or recommendations.
In conclusion, the proposed simplified systematic imaging approach may help predict the benign or malignant nature of soft-tissue tumours for non-expert radiologists or clinicians. This approach may provide a basis for further developmental studies of MRI characteristics for differentiating between benign and malignant soft-tissue tumours.
References
- 1.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin 1996;46:5–27 [DOI] [PubMed] [Google Scholar]
- 2.Kransdorf MJ. Benign soft-tissue tumors in a large referral population: distribution of specific diagnoses by age, sex, and location. AJR Am J Roentgenol 1995;164:395–402 [DOI] [PubMed] [Google Scholar]
- 3.De Schepper AM, Ramon FA, Degryse HR. Statistical analysis of MRI parameters predicting malignancy in 141 soft tissue masses. Rofo 1992;156:587–91 [DOI] [PubMed] [Google Scholar]
- 4.De Schepper AM, De Beuckeleer L, Vandevenne J, Somville J. Magnetic resonance imaging of soft tissue tumors. Eur Radiol 2000;10:213–23 [DOI] [PubMed] [Google Scholar]
- 5.Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol 2000;175:575–87 [DOI] [PubMed] [Google Scholar]
- 6.Kransdorf MJ, Jelinek JS, Moser RP, Utz JA, Brower AC, Hudson TM, et al. Soft-tissue masses: diagnosis using MR imaging. AJR Am J Roentgenol 1989;153:541–7 [DOI] [PubMed] [Google Scholar]
- 7.Petasnick JP, Turner DA, Charters JR, Gitelis S, Zacharias CE. Soft-tissue masses of the locomotor system: comparison of MR imaging with CT. Radiology 1986;160:125–33 [DOI] [PubMed] [Google Scholar]
- 8.Sundaram M, McGuire MH, Herbold DR. Magnetic resonance imaging of soft tissue masses: an evaluation of fifty-three histologically proven tumors. Magn Reson Imaging 1988;6:237–48 [DOI] [PubMed] [Google Scholar]
- 9.Gielen JL, De Schepper AM, Vanhoenacker F, Parizel PM, Wang XL, Sciot R, et al. Accuracy of MRI in characterization of soft tissue tumors and tumor-like lesions. A prospective study in 548 patients. Eur Radiol 2004;14:2320–30 [DOI] [PubMed] [Google Scholar]
- 10.Berquist TH, Ehman RL, King BF, Hodgman CG, Ilstrup DM. Value of MR imaging in differentiating benign from malignant soft-tissue masses: study of 95 lesions. AJR Am J Roentgenol 1990;155:1251–5 [DOI] [PubMed] [Google Scholar]
- 11.Crim JR, Seeger LL, Yao L, Chandnani V, Eckardt JJ. Diagnosis of soft-tissue masses with MR imaging: can benign masses be differentiated from malignant ones? Radiology 1992;185:581–6 [DOI] [PubMed] [Google Scholar]
- 12.Moulton JS, Blebea JS, Dunco DM, Braley SE, Bisset GS, Emery KH. MR imaging of soft-tissue masses: diagnostic efficacy and value of distinguishing between benign and malignant lesions. AJR Am J Roentgenol 1995;164:1191–9 [DOI] [PubMed] [Google Scholar]
- 13.Myhre-Jensen O. A consecutive 7-year series of 1331 benign soft tissue tumours. Clinicopathologic data. Comparison with sarcomas. Acta Orthop Scand 1981;52:287–93 [DOI] [PubMed] [Google Scholar]
- 14.Rydholm A. Management of patients with soft-tissue tumors. Strategy developed at a regional oncology center. Acta Orthop Scand Suppl 1983;203:13–77 [PubMed] [Google Scholar]
- 15.Hussein R, Smith MA. Soft tissue sarcomas: are current referral guidelines sufficient? Ann R Coll Surg Engl 2005;87:171–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson BM, Rydholm A. Soft-tissue masses of the locomotor system. A guide to the clinical diagnosis of malignancy. Acta Orthop Scand 1986;57:216–19 [DOI] [PubMed] [Google Scholar]
- 17.Datir A, James SL, Ali K, Lee J, Ahmad M, Saifuddin A. MRI of soft-tissue masses: the relationship between lesion size, depth, and diagnosis. Clin Radiol 2008;63:373–8; discussion 379–80 [DOI] [PubMed] [Google Scholar]